Abstract
Activation-induced cytidine deaminase (AID) is essential for class switch recombination and somatic hypermutation. Its deregulated expression acts as a genomic mutator that can contribute to the development of various malignancies. During treatment with imatinib mesylate (IM), patients with chronic myeloid leukemia often develop hypogammaglobulinemia, the mechanism of which has not yet been clarified. Here, we provide evidence that class switch recombination on B-cell activation is apparently inhibited by IM through down-regulation of AID. Furthermore, expression of E2A, a key transcription factor for AID induction, was markedly suppressed by IM. These results elucidate not only the underlying mechanism of IM-induced hypogammaglobulinemia but also its potential efficacy as an AID suppressor.
Introduction
Activation-induced cytidine deaminase (AID) is essential for class switch recombination (CSR) and somatic hypermutation.1 Deregulated expression of AID acts as a genomic mutator and can contribute to tumorigenesis through genomic recombination and aberrant somatic hypermutation.2-4 E2A, which harbors 2 binding sites in the AID promoter, is the crucial transcription factor for induction of AID.5 Imatinib mesylate (IM) has diverse immunomodulatory effects,6,7 including reduction of T-cell proliferation and inhibition of T-cell effector functions.8,9 Previously, we reported that serum titers of IgG and IgA, but not IgM, were significantly lower in chronic myeloid leukemia patients treated with IM versus those treated with IFN-α,10 suggesting that IM impairs CSR. In the present study, we investigated the effects of IM on CSR both in vitro and in vivo. Here, we present evidence that IM inhibits CSR through down-regulation of AID expression in splenic B cells.
Methods
Mouse immunization
Eight-week-old mice were immunized as previously reported,1 with or without 50 mg/kg imatinib mesylate. The experiments were approved by the Committee of Animal Care at the Institute of Medical Science, University of Tokyo.
Immunohistochemistry
Immunostaining for AID was performed on frozen sections following the manufacturer's instructions using an AID antibody (H-80; Santa Cruz Biotechnology).
Primer sequences, reagents, and more detailed methods are shown in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Results and discussion
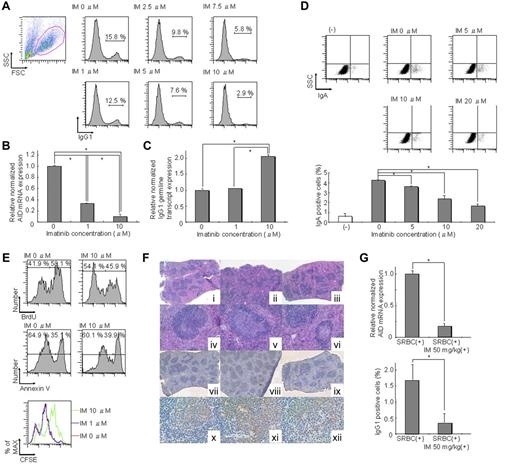
CSR is induced in splenic B cells by stimulation with IL-4 and lipopolysaccharide (LPS).11 After stimulation with IL-4 and LPS for 72 hours, IM decreased the proportion of IgG1-positive B cells dose-dependently. The proportion of B cells expressing surface IgG1 was approximately 16% without IM but was significantly reduced to approximately 3% with 10μM IM (Figure 1A). In the present culture system, only B cells can survive and proliferate,1 suggesting that IM may act directly on B cells and inhibit their CSR.
IM directly inhibits CSR in activated B cells through down-regulation of AID. (A) IgG1 expression levels in spleen cells cultured in conditioning medium containing 12.5 μg/mL LPS and 7.5 ng/mL IL-4 with 0, 1, 2.5, 5, 7.5, and 10μM IM for 72 hours were 15.8%, 12.5%, 9.8%, 7.6%, 5.8%, and 2.9% of untreated controls, respectively. Reduction of IgG1 expression was induced by IM dose-dependently. (B) Real-time RT-PCR in spleen cells cultured in conditioning medium containing IL-4 and LPS for 72 hours indicated that expression of AID was decreased by IM dose-dependently. Significant differences were found between 0 and 1μM or 10μM IM. *P < .05. The y-axis represents AID mRNA levels relative to the no-IM control. The levels of AID mRNA at each IM concentration were calculated relative to the internal control (GAPDH); n = 6. (C) The level of the germline transcript of IgG1 in spleen cells cultured in conditioning medium containing IL-4 and LPS for 72 hours was not decreased in contrast to AID mRNA expression levels, which were decreased by IM in a dose-dependent manner. Significant differences were found between 0, 1, and 10μM IM. *P < .05. The y-axis represents expression levels of the IgG1 germline transcript relative to the no-IM control in the same manner as that in panel B; n = 4. (D) IgA expression levels in CH12F3-2A cells cultured in conditioning medium containing 7.5 μg/mL IL-4, 0.3 ng/mL TGF-β1, and 40% CD40 ligand with 0, 5, 10, and 20μM IM for 72 hours were reduced in an IM dose-dependent manner. (E) Cell proliferation, division, and apoptosis in spleen cells cultured in conditioning medium containing IL-4 and LPS for 72 hours were investigated using BrdU, annexin V, and CFSE assays. The BrdU incorporation rate of 10μM IM was 45.9%, whereas that of 0μM IM was 58.1%. Cell fluorescence of 10μM IM using the CFSE assay was shifted, but that of 1μM IM was not shifted to the right compared with that of 0μM IM. Annexin V analysis of 10μM IM was 39.9%, whereas that of 0μM IM was 35.1%. These results indicate that IM affects cell proliferation but not apoptosis. (F) Immunohistochemical analysis of spleens from mice that were administered SRBC with or without IM. Serial sections of spleens were prepared from nonimmunized (i,iv,vii,x), SRBC-immunized (ii,v,viii,xi), or SRBC-immunized + IM (50 mg/kg; iii,vi,ix,xii) animals. (i-vi) H&E staining. (vii-xii) Immunohistochemical analysis of AID. Low-power fields are shown in panels i to iii and vii to ix. High-power fields are shown in subpanels iv to vi and x to xii. Individual germinal centers from SRBC-immunized IM (+) mice were significantly smaller than those from SRBC-immunized IM (−) mice and were comparable with those from nonimmunized mice. AID expression, which was induced in germinal center-activated B cells, was barely detectable in spleens of IM-treated mice but was strongly positive in those of nontreated mice. Moreover, IM significantly suppressed AID expression, even in the residual germinal centers. (G) Real-time RT-PCR analysis of AID and FACS analysis of IgG1 expression of spleen cells harvested from SRBC-immunized mice with or without 50 mg/kg IM. The top panel shows relative normalized AID mRNA expression, and the bottom panel shows surface IgG1 expression of total splenocytes. A significant difference was found between SRBC (+) and SRBC (+) IM 50 mg/kg (+) for both AID and IgG1. *P < .05. The y-axis represents the relative ratio of the relative expression level of AID mRNA (top panel) and the percentage of surface IgG1 expression of total splenocytes (bottom panel). Normalized values obtained for SRBC (+) IM 50 mg/kg (+) were derived from SRBC (+); n = 2.
IM directly inhibits CSR in activated B cells through down-regulation of AID. (A) IgG1 expression levels in spleen cells cultured in conditioning medium containing 12.5 μg/mL LPS and 7.5 ng/mL IL-4 with 0, 1, 2.5, 5, 7.5, and 10μM IM for 72 hours were 15.8%, 12.5%, 9.8%, 7.6%, 5.8%, and 2.9% of untreated controls, respectively. Reduction of IgG1 expression was induced by IM dose-dependently. (B) Real-time RT-PCR in spleen cells cultured in conditioning medium containing IL-4 and LPS for 72 hours indicated that expression of AID was decreased by IM dose-dependently. Significant differences were found between 0 and 1μM or 10μM IM. *P < .05. The y-axis represents AID mRNA levels relative to the no-IM control. The levels of AID mRNA at each IM concentration were calculated relative to the internal control (GAPDH); n = 6. (C) The level of the germline transcript of IgG1 in spleen cells cultured in conditioning medium containing IL-4 and LPS for 72 hours was not decreased in contrast to AID mRNA expression levels, which were decreased by IM in a dose-dependent manner. Significant differences were found between 0, 1, and 10μM IM. *P < .05. The y-axis represents expression levels of the IgG1 germline transcript relative to the no-IM control in the same manner as that in panel B; n = 4. (D) IgA expression levels in CH12F3-2A cells cultured in conditioning medium containing 7.5 μg/mL IL-4, 0.3 ng/mL TGF-β1, and 40% CD40 ligand with 0, 5, 10, and 20μM IM for 72 hours were reduced in an IM dose-dependent manner. (E) Cell proliferation, division, and apoptosis in spleen cells cultured in conditioning medium containing IL-4 and LPS for 72 hours were investigated using BrdU, annexin V, and CFSE assays. The BrdU incorporation rate of 10μM IM was 45.9%, whereas that of 0μM IM was 58.1%. Cell fluorescence of 10μM IM using the CFSE assay was shifted, but that of 1μM IM was not shifted to the right compared with that of 0μM IM. Annexin V analysis of 10μM IM was 39.9%, whereas that of 0μM IM was 35.1%. These results indicate that IM affects cell proliferation but not apoptosis. (F) Immunohistochemical analysis of spleens from mice that were administered SRBC with or without IM. Serial sections of spleens were prepared from nonimmunized (i,iv,vii,x), SRBC-immunized (ii,v,viii,xi), or SRBC-immunized + IM (50 mg/kg; iii,vi,ix,xii) animals. (i-vi) H&E staining. (vii-xii) Immunohistochemical analysis of AID. Low-power fields are shown in panels i to iii and vii to ix. High-power fields are shown in subpanels iv to vi and x to xii. Individual germinal centers from SRBC-immunized IM (+) mice were significantly smaller than those from SRBC-immunized IM (−) mice and were comparable with those from nonimmunized mice. AID expression, which was induced in germinal center-activated B cells, was barely detectable in spleens of IM-treated mice but was strongly positive in those of nontreated mice. Moreover, IM significantly suppressed AID expression, even in the residual germinal centers. (G) Real-time RT-PCR analysis of AID and FACS analysis of IgG1 expression of spleen cells harvested from SRBC-immunized mice with or without 50 mg/kg IM. The top panel shows relative normalized AID mRNA expression, and the bottom panel shows surface IgG1 expression of total splenocytes. A significant difference was found between SRBC (+) and SRBC (+) IM 50 mg/kg (+) for both AID and IgG1. *P < .05. The y-axis represents the relative ratio of the relative expression level of AID mRNA (top panel) and the percentage of surface IgG1 expression of total splenocytes (bottom panel). Normalized values obtained for SRBC (+) IM 50 mg/kg (+) were derived from SRBC (+); n = 2.
Next, we examined expression of the germline transcript directed by the I promoter of IgG1 and AID, both of which are essential for CSR after B-cell stimulation.12 Expression of AID was suppressed by IM dose-dependently (Figure 1B), whereas the IgG1 germline transcripts were not decreased by IM (Figure 1C). Likewise, IgA CSR in CH12F3-2A cells was impaired by IM in a dose-dependent manner (Figure 1D). These results showed that AID, but not the germline transcript, was responsible for inhibition of CSR by IM. BrdU, CFSE, and annexin V analysis revealed that IM affected proliferation but not apoptosis (Figure 1E). Importantly, 1μM of IM did not decrease proliferation but down-regulates AID (Figure 1B,E). In addition, 5-fluorouracil decreased proliferation but did not down-regulate AID (supplemental Figure 1), suggesting that proliferation is not necessarily coupled with expression of AID. Therefore, it is possible to differentiate the effect of IM on proliferation and AID expression.
To further confirm that CSR is impaired by IM through down-regulation of AID in vivo, immunohistochemical analysis was performed on splenic tissues from nonimmunized and sheep red blood cells–immunized C57BL/6 mice with or without IM treatment (Figure 1F). The individual germinal centers from SRBC-immunized IM (+) mice were significantly smaller than those from SRBC-immunized IM (−) mice and comparable with those from nonimmunized mice (Figure 1F). As expected from these findings, AID expression, which is induced in germinal center-activated B cells, was barely detectable in the spleens of IM-treated mice but was strongly positive in those of nontreated mice. In addition, IM significantly suppressed AID expression, even in the residual germinal centers. Expression of AID was confirmed by real-time RT-PCR analysis. The results of IgG1 expression did not conflict with these results (Figure 1G). Compatible with the results obtained by in vitro stimulation of spleen cells, IM down-regulated expression of IgG1 as well as AID. Although enlargement of germinal center formation has been reported in AID knockout mice,1 it is assumed that the immunomodulatory effects of IM on B cells, T cells, and dendritic cells6,7 resulted in impairment of germinal center formation in our system.
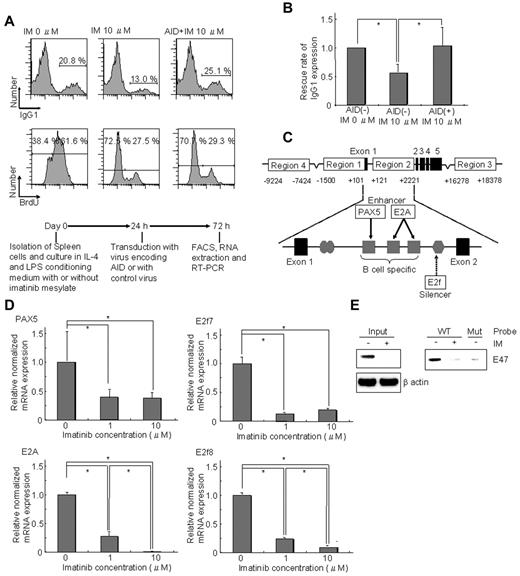
Furthermore, we investigated whether ectopic expression of AID could rescue inhibition of CSR by IM. IgG1 expression in spleen cells decreased with IM treatment, whereas ectopic expression of AID completely rescued impairment of CSR under the condition that cell proliferation was suppressed by IM (Figure 2A-B). The results indicated that impairment of CSR by IM was at least in part the result of down-regulation of AID.
Down-regulation of AID mediated by E2A, Pax5, E2f7, and E2f8 is responsible for CSR impairment by IM. (A) Ectopic expression of AID completely rescued reduction of IgG1 expression caused by IM. Spleen cells were cultured in IL-4 and LPS conditioning medium with or without IM. After 24 hours of prestimulation culture, cells were transduced with retrovirus encoding AID-eGFP or retrovirus encoding eGFP only (control). After a further 48 hours, IgG1 expression was analyzed (bottom panel). A mononuclear cell fraction based on forward scatter/side scatter profiles was gated and sequentially subdivided into an eGFP-positive fraction. This eGFP-positive fraction was analyzed. Ectopic AID expression with 10μM IM increased IgG1 expression from 13.0% to 25.1% versus 20.8% without IM. The BrdU assay revealed that DNA synthesis decreased in the 10μM IM culturing condition. Although the BrdU assay was similar with or without ectopic expression of AID, IgG1 expression was completely rescued by ectopic expression of AID. (B) Average of the IgG1 expression rescue rate among 4 rescue experiments. IgG1 expression of AID(+) IM at 10μM was completely rescued by ectopic expression of AID. (C) Schema illustrating transcriptional binding sites in the Aicda gene promoter region, focusing particularly on region 2 in the first intron. PAX5 and E2A activate the Aicda promoter, whereas E2f7 and E2f8 have silencing effects. (D) The expression levels of 4 transcriptional factors (PAX5, E2A, E2f7, and E2f8) in spleen cells cultured in conditioning medium containing IL-4 and LPS for 72 hours were determined by real-time RT-PCR. All were reduced by IM. E2A expression was most markedly reduced.*P < .05. The y-axis represents mRNA levels of the PAX5, E2A, E2f7, and E2f8 relative to the no-IM control. Levels of each transcriptional factor mRNA were calculated relative to the internal control (GAPDH); n = 2. (E) Protein expression and DNA-binding activity of E2A in spleen cells cultured in conditioning medium containing IL-4 and LPS for 72 hours. The E2A gene encodes 2 transcription factors: E12 and E47. Western blot analysis revealed that expression of E47 in splenocytes cultured in conditioning medium containing LPS and IL-4 was down-regulated by IM to a barely detectable level. DNA affinity precipitation analysis of the same cell extracts using biotinylated E-box probe and its mutant revealed that E-box binding activity of E47 in the extracts was similarly reduced by IM.
Down-regulation of AID mediated by E2A, Pax5, E2f7, and E2f8 is responsible for CSR impairment by IM. (A) Ectopic expression of AID completely rescued reduction of IgG1 expression caused by IM. Spleen cells were cultured in IL-4 and LPS conditioning medium with or without IM. After 24 hours of prestimulation culture, cells were transduced with retrovirus encoding AID-eGFP or retrovirus encoding eGFP only (control). After a further 48 hours, IgG1 expression was analyzed (bottom panel). A mononuclear cell fraction based on forward scatter/side scatter profiles was gated and sequentially subdivided into an eGFP-positive fraction. This eGFP-positive fraction was analyzed. Ectopic AID expression with 10μM IM increased IgG1 expression from 13.0% to 25.1% versus 20.8% without IM. The BrdU assay revealed that DNA synthesis decreased in the 10μM IM culturing condition. Although the BrdU assay was similar with or without ectopic expression of AID, IgG1 expression was completely rescued by ectopic expression of AID. (B) Average of the IgG1 expression rescue rate among 4 rescue experiments. IgG1 expression of AID(+) IM at 10μM was completely rescued by ectopic expression of AID. (C) Schema illustrating transcriptional binding sites in the Aicda gene promoter region, focusing particularly on region 2 in the first intron. PAX5 and E2A activate the Aicda promoter, whereas E2f7 and E2f8 have silencing effects. (D) The expression levels of 4 transcriptional factors (PAX5, E2A, E2f7, and E2f8) in spleen cells cultured in conditioning medium containing IL-4 and LPS for 72 hours were determined by real-time RT-PCR. All were reduced by IM. E2A expression was most markedly reduced.*P < .05. The y-axis represents mRNA levels of the PAX5, E2A, E2f7, and E2f8 relative to the no-IM control. Levels of each transcriptional factor mRNA were calculated relative to the internal control (GAPDH); n = 2. (E) Protein expression and DNA-binding activity of E2A in spleen cells cultured in conditioning medium containing IL-4 and LPS for 72 hours. The E2A gene encodes 2 transcription factors: E12 and E47. Western blot analysis revealed that expression of E47 in splenocytes cultured in conditioning medium containing LPS and IL-4 was down-regulated by IM to a barely detectable level. DNA affinity precipitation analysis of the same cell extracts using biotinylated E-box probe and its mutant revealed that E-box binding activity of E47 in the extracts was similarly reduced by IM.
Finally, we examined the mechanism of down-regulation of AID by IM. Recently, Tran et al reported that Aicda regulation involved derepression by several layers of positive regulatory elements in addition to the 5′-promoter region.5,13 Promoter region 2 in the first intron contains the functional binding elements for the ubiquitous silencers c-Myb and E2f and for the B cell–specific activators Pax5 and E2A (Figure 2C).5,13 Surprisingly, all of these transcription factors were down-regulated by IM. Among them, expression of E2A was most markedly reduced (to 1 of 500) by IM (Figure 2D). We further found that levels of E2A protein as well as E-box binding activity were markedly reduced by IM (Figure 2E), suggesting that down-regulation of E2A by IM causes significant suppression of AID.
For the first time, our findings elucidate a mechanism of hypogammaglobulinemia caused by IM, which has been observed frequently in IM-treated chronic myeloid leukemia patients.8,9 Its adverse effects as well as the immunomodulatory functions of each drug and their underlying mechanisms must be examined in more extensive studies.
AID was previously reported to be induced by BCR-ABL1 in Ph1+ pre B-ALL cell lines and inhibited by IM through ID2 up-regulation. Interestingly, neither PAX5 nor E2A showed changes in expression.14 In the present study using normal mature B cells, PAX5 and E2A levels were significantly decreased by IM, whereas ID2 was not increased (data not shown). PDGFR15 and c-kit,16 kinases that are also inhibited by IM, were not expressed in mature B cells. Together, these results could be induced by the off-target multikinase inhibitory effects of IM. The results of microarray analysis (supplemental Table 1; supplemental Figures 2-3) are consistent with this hypothesis. All microarray data are available for viewing on the Gene Expression Omnibus under accession number GSE35559.
Inappropriate expression of AID affects many diseases, such as malignancy and autoimmune diseases.17,18 It probably also affects allergic disorders because AID is also essential to CSR from IgM to IgE, deregulation of which is an important causative factor of allergic disorders. The results of the present study suggest that IM, which has been used safely for several decades in clinical settings, can be used for various diseases involving AID. Indeed, dramatic resolution by IM has been reported in several cases of rheumatoid arthritis or asthma complicated with chronic myeloid leukemia.19,20
In conclusion, suppression of AID by IM is responsible for CSR impairment, leading to the frequent adverse effects of IM. IM may also be clinically useful as an AID suppressor.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Tasuku Honjo, Dr Toru Nakano, Mrs Bidisha Chanda, Mr Haruo Onoda, and Mr Keisuke Takahashi for critical comments or technical assistance. The array analysis was performed by Mr Masayuki Tanaka and Hideki Hayashi (Education and research Support Center, Toaki University).
This work was supported by the Japan Society for the Promotion of Science, Takeda Science Foundation, Sagawa Cancer Foundation, The Foundation for Cancer Research, Kobayashi Cancer Research Foundation, Mochida Science Foundation, Sankyo Science Foundation, and Shiseido Science Grant for the female researcher (to A.K.).
Authorship
Contribution: T.K., J.L., T.S., A.K., and M.T. designed, performed, and analyzed the experiments and wrote the manuscript; T.T., H.N., Y.A., K.Y., N.O., and N.N. contributed vital reagents; K.A. collected the clinical samples; and A.T. supervised the research.
Conflict-of-interest disclosure: A.T. has received honoraria and research funding from Novartis. The remaining authors declare no competing financial interests.
Correspondence: Arinobu Tojo, Department of Hematology-Oncology, Research Hospital, Institute of Medical Science, University of Tokyo, 4-6-1 Shirokanedai, Minato-ku, Tokyo 108-8639, Japan; e-mail: a-tojo@ims.u-tokyo.ac.jp; and Ai Kotani, Tokai University Institute of Innovative Science and Technology, Medical Science Division, Shimokasuya 143, Isehara City, Kanagawa Prefecture 259-1193, Japan; e-mail: ka102009@tsc.tokai-u.ac.jp.
References
Author notes
T.K., J.L., and T.S. contributed equally to this study.
A.T. and A.K. contributed equally to this study as co–last authors.