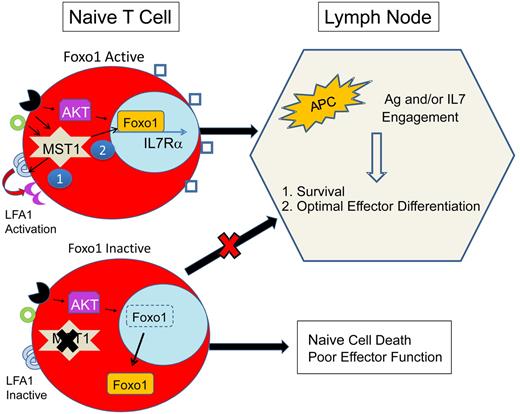
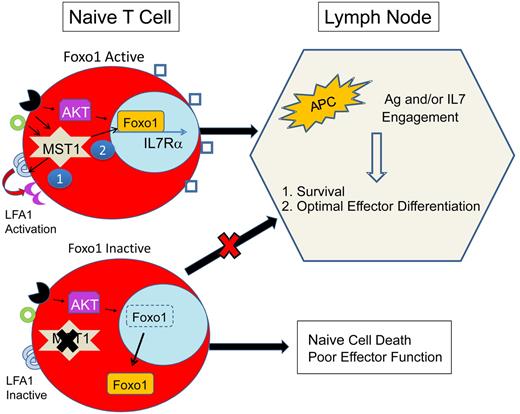
Proposed mechanism for immunodeficiency because of MST1 defects. MST1-deficient humans and mice exhibit significantly restricted populations of naive T cells. This is likely due to impairment of at least 2 primary roles of MST1: (1) mediation of TCR (black receptor) and chemokine (green circle) driven integrin (LFA1) activation (purple crescent), necessary for T-cell adhesion and (2) activation of Foxo1, necessary for IL-7Rα transcription. In MST1 deficiency, unopposed AKT activity leads to phosphorylation and cytoplasmic shuttling of Foxo1 and impaired transcription of IL-7Rα, decreasing the sensitivity of naive T cells to IL-7. Naive T-cell survival is dependent on TCR and IL-7 exposure in the lymph node. In MST1 deficiency, lymph node entry is impaired because of poorly activated LFA1 (blue semicircles); IL-7R (blue squares) signaling is diminished; and T-cell stimulation by antigen-presenting cells (APCs) is suboptimal (also due to impaired LFA1 activation). Therefore, naive T cells die and effector cells undergo suboptimal activation and proliferation.
Proposed mechanism for immunodeficiency because of MST1 defects. MST1-deficient humans and mice exhibit significantly restricted populations of naive T cells. This is likely due to impairment of at least 2 primary roles of MST1: (1) mediation of TCR (black receptor) and chemokine (green circle) driven integrin (LFA1) activation (purple crescent), necessary for T-cell adhesion and (2) activation of Foxo1, necessary for IL-7Rα transcription. In MST1 deficiency, unopposed AKT activity leads to phosphorylation and cytoplasmic shuttling of Foxo1 and impaired transcription of IL-7Rα, decreasing the sensitivity of naive T cells to IL-7. Naive T-cell survival is dependent on TCR and IL-7 exposure in the lymph node. In MST1 deficiency, lymph node entry is impaired because of poorly activated LFA1 (blue semicircles); IL-7R (blue squares) signaling is diminished; and T-cell stimulation by antigen-presenting cells (APCs) is suboptimal (also due to impaired LFA1 activation). Therefore, naive T cells die and effector cells undergo suboptimal activation and proliferation.
STK4 encodes the Mammalian Ste20-like Kinase (MST) 1, a protein that is ubiquitously expressed in mammalian cells. The functions of homologous kinases Hippo in Drosophila and Ste20 Kinase in Yeast have been well described, largely as regulators of proliferation, apoptosis, morphology, and cell shape, but the function of the Ste20 mammalian kinases in the immune system and other organs is less well explored.
Mammalian MST1 was first conceptualized as an enhancer of apoptosis in neuronal cells, in the setting of oxidative stress. MST1 activation was shown to phosphorylate and retain nuclear localization of Foxo1, a transcriptional activator of pro-death genes.3 Subsequently, MST1 was identified as a regulator of leukocyte adhesion in mammals. Knockdown of MST1 cDNA in T cells prevented the activation of LFA1-induced T-cell adhesion after T-cell receptor (TCR) or chemokine stimulation of lymphocytes.4
Studies in MST1-deficient mice confirmed the dual role of MST1 in both LFA1-mediated adhesion and apoptosis.5,–7 MST1-deficient mice exhibit decreased numbers of lymphocytes in the blood, lymph nodes, and spleen, resulting from both reduced homing to the lymph tissues and, paradoxically, from reduced survival of naive T cells. Induction of oxidative stress by whole body γ irradiation led to a further reduction of CD4 T cells in the peripheral blood of MST1-deficient mice, suggesting MST1 modulates stress-induced lymphocyte apoptosis.
Two manuscripts in this issue describe a phenotype of recurrent infections (bacterial and viral), EBV-driven lymphoproliferative disease, dermatitis, subclinical cardiac anomalies, autoimmune cytopenias, and significant lymphopenia in 7 children (from 3 unrelated families) diagnosed with biallelic MST1 mutations. The clinical phenotype is characteristic of a growing group of primary immunodeficiencies marked by chronic viral infections, autoimmunity, and malignancy.8 The immunologic phenotype is distinct: instead of hypogammaglobulinemia and absent serologic titers typically seen in severe combined immune-deficiency and other combined immunodeficiencies, MST1-deficient patients demonstrated hypergammaglobulinemia and a humoral response to viral infection (HSV, EBV) and vaccines. However, the B-cell phenotype was not entirely normal. In the report from Abdollahpour et al, B-cell numbers were significantly reduced, especially memory B cells.2 Further studies are needed to dissect if the B-cell phenotype is simply related to defective T-cell function or an intrinsic B-cell defect.
Humoral responses depend on a T cell–dependent, germinal center reaction. Thus, with evidence of serologic titers and hypergammaglobulinemia, one would expect intact T-cell proliferation in response to TCR stimulation. Paradoxically, peripheral blood T cells from MST1-deficient patients displayed markedly impaired thymidine uptake in response to mitogens (phytohaemagglutinin) and anti-CD3 antibody. The mechanism proposed for this apparent contradiction is intriguing—T cells from affected individuals were remarkably sensitive to mitogen and/or anti-CD3–induced death. Instead of proliferating, the cells underwent apoptosis. The circulating T cells were also phenotypically abnormal, with decreased CD4/8 ratios, restricted T cell–receptor repertoire, and a severe reduction of circulating naive (CD45RA+) cells. The CD45RA+ T cells were phenotypically abnormal, with decreased IL-7 receptor α and CD62L and up-regulated Fas on the cell surface, more typical for an antigen-experienced cell.
A loss of naive CD4 T cells is seen in many primary immunodeficiencies, such as DiGeorge Syndrome, common variable immunodeficiency, ITK deficiency, and XLP. However, T cells generally exhibit intact response to mitogens in these disorders. In fact, the immunophenotype that MST1 deficiency most closely resembles is HIV-induced AIDS. AIDS is manifested by hypergammaglobulinemia, severe lymphopenia, and propensity to herpes viral infections because of a progressive loss of functional CD4 T cells.9 MST1 deficiency may also involve a progressive loss of functional T cells, illustrated most vividly in the report from Nehme et al by the normal proliferation of T cells in response to Candida and tetanus in the youngest patient of the series and a relatively late onset of symptoms (1 year and beyond).1 Thus, the T-cell defect may be less severe in early infancy and worsen with time.
Further studies are required to explain the progressive loss of T-cell function and the retained capacity for generation of T cell–dependent humoral responses. A number of potential mechanisms may contribute: (1) MST1-deficient naive T cells may develop normally and exit the thymus but undergo apoptosis in the periphery because of restricted entry into lymph nodes and reduced IL-7 signaling (see figure). According to this model, as thymic output decreases with age, lymphopenia would progress.10 (2) Conversely, thymic education may be abnormal, with expansion of a limited number of MST1-deficient T-cell clones contributing to severe lymphopenia. (3) Alternatively, antigenic stimulation of MST1-deficient T cells may lead to accelerated cell death in vivo. Thus, T-cell stimulation from additive pathogen exposures would lead to a progressive lymphopenia and immunodeficiency. (4) Finally, cumulative oxidative stress may contribute to a progressive lymphopenia.
In contrast to the expected role of MST1 in facilitating apoptosis, the immunologic phenotype suggests that MST1 deficiency leads to death of circulating T cells. Both manuscripts suggest a potential mechanism for MST1 to preserve the life of naive T cells via phosphorylation of Foxo proteins that are also required for naive T-cell survival.11 Indeed, detection of Foxo1 and Foxo3 proteins was reduced in mononuclear cell lysates from MST1-deficient patients. Further studies are needed to clarify if this is simply due to an expansion of effector/memory T cells that have reduced Foxo proteins. Interestingly, murine models that lead to diminished Foxo1 activity also lead to a progressive lymphopenia and severe loss of naive T cells, that is, Foxo1 and Gimap5 deficiency.11,12
Based on previous experimental models of the role of MST1 in LFA1 activation, one would also expect human MST1 deficiency to impair lymphocyte homing and, potentially, immune synapse formation. Evaluation of immune synapse formation in MST1 deficiency has not yet been tested. Alterations in T-cell surface homing receptors were documented in both reports. Further evaluation of lymph tissue from affected individuals may be informative.
The findings of viral infections and viral-associated malignancy in patients suggest a lack of cytotoxic lymphocyte function; however, neither CTL nor NK function was reported. Because cytotoxic lymphocytes rely on LFA1 activation to form a functional immune synapse, it may be useful to test for cytotoxic dysfunction in MST1 deficiency (NK or CTL).
The discovery of a novel, primary immunodeficiency because of homozygous mutations in MST1 confirms that MST1 is a critical regulator of T-cell function. According to a simplified model (see figure) in naive T cells, MST1 likely potentiates TCR- and/or chemokine-activated LFA1 activation. In addition, MST1 preserves the life of naive T cells by an unknown mechanism, theoretically involving Foxo1 activation, IL-7R maintenance, and lymph node trafficking. Future studies are needed to dissect the downstream targets of MST1 in T cells.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■