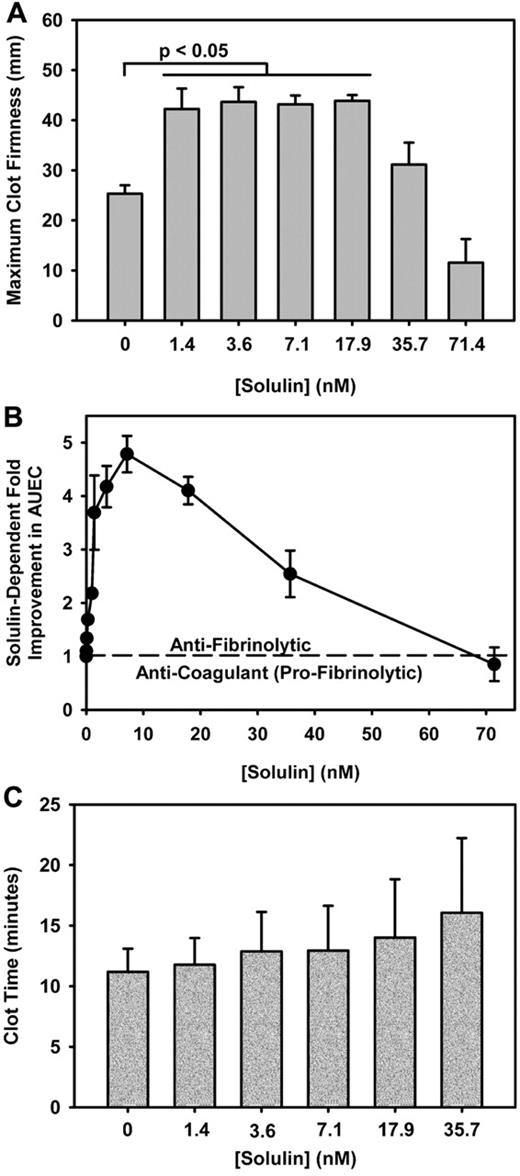
Solulin increases the MCF and AUEC in human hemophilic blood with minimal effect on CLT. (A) The maximum clot firmness (MCF) increased from 25.3 ± 4.5 mm to 43.2 ± 6.6 mm in the presence of 1.4-17.9nM Solulin (P < .01). At higher concentrations, the MCF decreased from peak values. (B) In severe hemophilia A, the area under the elasticity curve (AUEC) is increased by ∼ 4-fold in the presence of Solulin due in part to an increase in MCF but also to an increased clot lysis time (CLT). (C) Solulin marginally increases the CLT in hemophilia A blood. At the lowest concentrations of Solulin (< 7.1nM), the CLT increased marginally from 11.2 ± 1.9 to 12.9 ± 3.7 minutes. The increase in CLT with Solulin was not statistically significant even at 35.7nM (P = .07). This is Figure 2 from the article by Foley et al that begins on page 3622 of this issue.
Solulin increases the MCF and AUEC in human hemophilic blood with minimal effect on CLT. (A) The maximum clot firmness (MCF) increased from 25.3 ± 4.5 mm to 43.2 ± 6.6 mm in the presence of 1.4-17.9nM Solulin (P < .01). At higher concentrations, the MCF decreased from peak values. (B) In severe hemophilia A, the area under the elasticity curve (AUEC) is increased by ∼ 4-fold in the presence of Solulin due in part to an increase in MCF but also to an increased clot lysis time (CLT). (C) Solulin marginally increases the CLT in hemophilia A blood. At the lowest concentrations of Solulin (< 7.1nM), the CLT increased marginally from 11.2 ± 1.9 to 12.9 ± 3.7 minutes. The increase in CLT with Solulin was not statistically significant even at 35.7nM (P = .07). This is Figure 2 from the article by Foley et al that begins on page 3622 of this issue.
Since the original description of the importance of enhanced fibrinolysis in hemophilia,2 investigators have used antifibrinolytic agents as an adjunct to treatment.3 Various novel treatment modalities are being explored, with that of the recently reported molecule Solulin (recombinant soluble modified thrombomodulin)4 potentially holding therapeutic promise via its antifibrinolytic properties. This modified thrombomodulin has reduced affinity for thrombin with enhanced TAFI activation.
In this issue of Blood, Foley and colleagues are the first to demonstrate in both patient samples and in dogs that low concentrations of Solulin can enhance TAFI activation with concomitant reduced fibrinolysis and increased clot strength.5 Plasma from 17 subjects (15 with hemophilia A [12 severe] and 2 with moderately severe hemophilia B) were studied. Clot time and maximum clot firmness were determined by thromboelastography. There was a statistically significant increase in clot firmness (4- to 5-fold; area under the elasticity curve [AUEC]) at low concentrations of Solulin, little to reduced impact on clot firmness at higher concentrations, and no significant impact on the clot time with any concentration of Solulin (see figure). Low-dose Solulin also improved clot stability (AUEC) in 3 patients on prophylaxis. This improvement was most pronounced for individual patients at low (trough) levels of circulating factor VIII/IX, similar to the AUEC achieved for hemophilia patients administered exogenous factor correcting to normal (100%) levels. Kinetic studies were performed to demonstrate that the thrombin-Solulin complex activates TAFI more efficiently than protein C, suggesting a preference for reduced fibrinolysis over anticoagulation at low concentrations of Solulin. Finally, in vivo/ex vivo studies were performed with low-dose Solulin administration in 3 hemophilia A dogs. Similar to the in vitro experiments, low-dose Solulin administration demonstrated improvement in maximal clot strength, although due to equipment limitations, maximum amplitude and not AUEC was measured.
Strengths of this investigation include (1) demonstration that in vitro, low-dose Solulin improves clot strength with this effect being maintained during the factor level variations inherent in prophylaxis replacement therapy, (2) kinetic studies showing the predominant antifibrinolytic effect of low-dose Solulin, and (3) preliminary data suggesting that low-dose Solulin can improve ex vivo maximal clot strength in hemophilic dogs. This small study provides proof of principle for the therapeutic use of low-dose Solulin and its effect at down-regulating fibrinolysis, but further study is needed. In addition, there might be a narrow tipping point of Solulin dosing on the anticoagulation effects (and resultant increased bleeding) mediated through protein C activation. This is illustrated by the in vivo hemophilic dog studies where 2 of 3 animals showed a variation in Solulin-induced clot strength. Further, the potential enhanced antifibrinolytic and/or thrombotic complications with concomitant use of Solulin and factor replacement therapy have not been explored. Accordingly, it is essential that potential bleeding and clotting risk with this agent be assessed in well-designed studies in hemophilia dogs followed by appropriate phase 1/2 human studies.
Solulin has a long half-life and exerts its antifibrinolytic (and clot-sustaining) effect through TAFI. The molecule has been shown to have a relatively wide therapeutic window but potential bleeding concerns at higher dosing could be lessened by engineering additional mutations in the current molecule. Indeed, several constructs have been made that only minimally activate protein C.5 Factor replacement with accompanying thrombin generation is essential in the treatment of hemophilia. Now there appears promise that Solulin, or similar long-acting thrombomodulin-like molecules, can be engineered for enhanced fibrin formation.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■