Abstract
Monoclonal antibodies (mAbs) have revolutionized the treatment of B-cell malignancies. Although Fc-dependent mechanisms of mAb-mediated tumor clearance have been extensively studied, the ability of mAbs to directly evoke programmed cell death (PCD) in the target cell and the underlying mechanisms involved remain under-investigated. We recently demonstrated that certain mAbs (type II anti-CD20 and anti-HLA DR mAbs) potently evoked PCD through an actin-dependent, lysosome-mediated process. Here, we reveal that the induction of PCD by these mAbs, including the type II anti-CD20 mAb GA101 (obinutuzumab), directly correlates with their ability to produce reactive oxygen species (ROS) in human B-lymphoma cell lines and primary B-cell chronic lymphocytic leukemia cells. ROS scavengers abrogated mAb-induced PCD indicating that ROS are required for the execution of cell death. ROS were generated downstream of mAb-induced actin cytoskeletal reorganization and lysosome membrane permeabilization. ROS production was independent of mitochondria and unaffected by BCL-2 overexpression. Instead, ROS generation was mediated by nicotinamide adenine dinucleotide phosphate (NADPH) oxidase. These findings provide further insights into a previously unrecognized role for NADPH oxidase-derived ROS in mediating nonapoptotic PCD evoked by mAbs in B-cell malignancies. This newly characterized cell death pathway may potentially be exploited to eliminate malignant cells, which are refractory to conventional chemotherapy and immunotherapy.
Introduction
Monoclonal antibodies (mAbs) have revolutionized the treatment of cancer. The first mAb approved for this purpose, rituximab, which targets the CD20 antigen on B-lymphocytes, has substantially improved the clinical outcome of patients with B-cell malignancies in combination with chemotherapy.1,2 However, a substantial proportion of patients still relapse and acquire resistance to rituximab.3 In an attempt to further improve therapeutic outcomes and develop novel therapies for rituximab-refractory patients, many next-generation mAbs directed against CD20 or various other cell surface antigens have been developed by the pharmaceutical industry. An enhanced understanding of the mechanisms underlying mAb-induced tumor clearance is therefore pivotal for improving the therapeutic performance of mAbs.
In addition to classic Fc-dependent mechanisms such as antibody-dependent cellular cytotoxicity (ADCC), antibody-dependent cellular phagocytosis (ADCP), and complement-dependent cytotoxicity (CDC), certain mAbs can eliminate B cells by triggering intracellular signaling on antigen ligation to directly induce programmed cell death (PCD). Although Fc-dependent mechanisms of mAb-induced tumor clearance have been extensively studied, and many novel mAbs with optimized Fc properties are currently being developed, the role of direct PCD and its underlying mechanisms remain largely under-investigated. A significant advantage of exploiting the direct induction of PCD to augment mAb efficacy is that, unlike Fc-dependent mechanisms, it does not rely on host immune effector mechanisms which are amenable to saturation or exhaustion, such as in patients with high tumor burden, or immune effector cell failure secondary to depletion by chemotherapy regimes.4 Further investigation of the mechanism of mAb-induced PCD therefore provides the potential for improved therapeutic efficacy through the development of optimized next-generation mAbs and novel, mechanism-based combination therapies.
We have previously characterized anti-CD20 mAbs into 2 subtypes and observed that in contrast to type I rituximab-like anti-CD20 mAbs, type II (tositumomab-like) anti-CD20 mAbs more potently evoke direct PCD, and their capacity to induce PCD correlates with the induction of inter-cellular homotypic adhesion (HA).5 Type II anti-CD20 mAbs showed greater therapeutic efficacy compared with rituximab and other type I anti-CD20 mAbs in vivo, with F(ab)′2 fragments providing substantial immunotherapy in lymphoma xenograft models, indicating that direct PCD contributes toward the superior efficacy of type II anti-CD20 mAbs.6 On further exploration of the cellular mechanisms underlying this novel cell death phenomenon, we found that PCD was dependent on HA and the rearrangement of the actin cytoskeleton, which in turn triggered lysosome membrane permeabilization (LMP) and cathepsin-mediated cell death bearing the morphologic features of necrosis.7 Importantly, this lysosomal cell death pathway was independent of the cell's apoptotic and autophagic machinery, and could circumvent resistance to chemotherapy-induced apoptosis.7 We have also recently observed that this mode of cell death is potently elicited by GA101 (obinutuzumab), a glycoengineered and humanized type II anti-CD20 mAb which is currently being evaluated in phase 3 clinical trials in B-cell malignancies and showing considerable therapeutic activity in rituximab- refractory patients.8 A similar mode of nonapoptotic cell death triggered by actin reorganization can be induced by mAbs targeting various cell surface antigens, including HLA DR,7,9 CD47,10,11 CD74,12 and CD99,13 which suggests that mAbs may evoke a universal mode of PCD. However, the mechanism through which mAb-induced PCD ultimately leads to a cell's demise remains to be elucidated.
Several studies have demonstrated that mAbs which trigger direct PCD can stimulate the production of reactive oxygen species (ROS).9,12,14 ROS are highly reactive molecules that perform numerous essential functions in living organisms, including signaling cell growth and differentiation, stimulating cytokine production in inflammation, and eliminating pathogens in phagocytosis.15 However, excessive amounts of ROS can cause irreversible oxidative damage to lipids, proteins, and DNA, which lead to cell death.16 Although the production of ROS is closely associated with various apoptotic stimuli, its functional significance in executing the apoptotic program is poorly understood. It still remains unclear whether the production of ROS in apoptosis is an essential component of the apoptotic pathway primarily by inducing mitochondrial damage,17,18 or an epiphenomenon of mitochondrial dysfunction that is unrelated to the key signaling events leading to apoptotic cell death.19 In contrast, emerging evidence indicates that ROS play an important role in the execution of various forms of nonapoptotic PCD, such as the cyclophilin D–dependent mitochondrial necrosis occurring during mycocardial infarction,20 stroke,21 and Alzheimer disease,22 as well in autophagic cell death.23 ROS were also shown to be critical for the nonapoptotic, receptor-interacting protein (RIP) kinase–dependent PCD induced by activation of the cell-surface death receptors, tumor necrosis factor-receptor (TNF)24 and Fas (CD95).25 Therefore, we hypothesized that ROS play a pivotal role in the nonapoptotic, lysosome-mediated mAb-induced cell death which we have recently characterized.7,26
Here we demonstrate that ROS are critical for PCD evoked by a range of mAbs including type II anti-CD20 mAbs (tositumomab and GA101) and anti-HLA DR mAbs (L243 and 1D10/apolizumab) in human B-lymphoma cell lines and primary B-cell chronic lymphocytic leukemia (B-CLL) cells. ROS were generated downstream of HA, actin reorganization, LMP, and cathepsin B release. mAb-induced ROS production was independent of mitochondria, but rather was mediated by nicotinamide adenine dinucleotide phosphate (NADPH) oxidases. Given our most recent findings, we propose a model for mAb-induced PCD in which certain mAb-antigen interactions trigger actin reorganization, LMP, and release of lysosomal proteases into the cytosol culminating in the generation of ROS by NADPH oxidases, which ultimately results in the loss of plasma membrane integrity and nonapoptotic, cytoplasmic cell death.
Methods
Cell lines and primary B-CLL cells
Human lymphoma cell lines (Raji, Daudi, and SU-DHL4) were obtained from the European Collection of Cell Cultures (ECACC). Cells were maintained in antibiotic-free RPMI 1640 or DMEM media supplemented with 10% FCS and 2mM Glutamine (Gibco, Invitrogen) at 37°C, 5% CO2. Raji cells overexpressing the antiapoptotic protein BCL-2, Raji-BCL2, were previously described.27 The generation and culture conditions for the respiration-deficient Raji subclones with impaired mitochondrial respiration function were described by Pelicano et al.28 Primary B-CLL cells were isolated from whole blood by Ficoll gradient centrifugation and cryopreserved as previously detailed.26 Samples and ethical approval for use of B-CLL cells were obtained from the Manchester Cancer Research Center Biobank Ethics Committee, in accordance with the declaration of Helsinki.
Antibodies and reagents
GA101 and tositumomab were kindly provided by Roche Glycart AG and GlaxoSmithKline, respectively. The anti-HLA DR mAb 1D10 was a kind gift from Dr George Weiner (University of Iowa). Rituximab was obtained from the Christie Hospital pharmacy. AME-133V was a kind gift from Dr Shashidhara Marulappa (Mentrik Biotech). The anti-HER2 mAb trastuzumab was purchased commercially from Hoffmann La Roche AG. Ofatumumab and a GA101 derivative with a wild-type (WT), nonglycomodified huIgG1 Fc region, GA101 (NG) were produced in-house as previously described.26 Anti-HLA DR mAb (L243) and pan-HLA mAb (F3.3, A9-1, WR18) were provided by in-house from hybridoma supernatants. F(ab)′2 fragments of immunoglobulin (Ig)G were produced by standard pepsin digestion.29 The actin inhibitor latrunculin B, the vacuolar ATPase inhibitor concanamycin A and the cytotoxic drug mitoxantrone were purchased from Sigma-Aldrich. Cathepsin Inhibitor III (Z-FG-NHO-BzOME) was purchased from Calbiochem. The cell permeable ROS scavengers 4,5-dihydroxyl-1,3-benzededisulfonic acid (tiron) and 4-hydroxy-2,2,6,6-tetramethyl pierydine-1-oxyl (tempol), and the NADPH oxidase inhibitors mycophenolic acid (MPA) and diphenylamine iodoniumchloride (DPI) were all purchased from Sigma-Aldrich.
Antibody and inhibitor treatment of cells
Cells were seeded at 2.5 × 105/mL and treated with mAb at 10 μg/mL for 4 hours at 37°C 5% CO2 in plastic 24-well plates (Corning), and then harvested for analysis. Where appropriate, the inhibitors latrunculin B, concanamycin A, and cathepsin inhibitor III were added for 30 minutes; tempol and DPI for 1 hour; tiron for 2 hours; and MPA for 16 hours before mAb treatment. For guanosine repletion experiments, cells were incubated with MPA in the presence of excess guanosine (20μM) and treated with mAb as normal.
Detection of ROS by flow cytometry
The generation of ROS was detected using dihydroethidium (HE) staining (Sigma-Aldrich). HE is a cell-permeable fluorogenic probe that reacts with ROS to form ethidium which intercalates within double-stranded DNA in the nucleus and emits red fluorescence. Cells were resuspended in fresh media containing 2.5μM HE and incubated for 30 minutes in the dark at room temperature. Cells were then washed, resuspended in fresh media, and analyzed by flow cytometry. An alternative probe for ROS detection was also used: carboxy-H2DCFDA dye (Molecular Probes). Carboxy-H2DCFDA is oxidized in the presence of ROS to carboxy-DCF which emits green fluorescence. After treatment with mAbs, cells were labeled with 25μM carboxy-H2DCFDA in Hanks balanced salt solution/Ca/Mg (Gibco) for 30 minutes at 37°C, and then analyzed by flow cytometry to detect the increase in green fluorescence.
Detection of ROS by live cell imaging
For live cell imaging 5 × 105/mL Raji cells were preloaded with 50μM carboxy-H2DCFDA, incubated for 10 minutes at 37°C, and transferred into a glass-bottom dish (Iwaki), and then mAbs were added at 10 μg/mL. Live cell imaging was then performed in a closed, humidified chamber at 37°C in a CO2-enriched atmosphere using a Nikon A1R confocal microscope (488 nm excitation and 500-550 nm emission) with a Nikon PanFluor ×40 objective lens and a numerical aperture of 1.30. Images were acquired and processed using NIS-Elements AR3 Version 3 software.
Cell death assays
Cell death was quantified using a rapid propidium iodide (PI) inclusion assay. Cells were washed and resuspended in PBS containing 10 μg/mL PI and incubated for 15 minutes at room temperature, in the dark. Samples were then analyzed by flow cytometry. For cell death/ROS release correlation, cells were stained with HE as previously described, and then resuspended in annexin V binding buffer (10mM HEPES [N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid; pH 7.4], 140mM NaCl, and 2.5mM CaCl2) containing 1 μg/mL annexin V Cy5.5 and analyzed by dual-color flow cytometry. Chromium-51 (51Cr) release cytotoxicity assays were previously described.26 For primary B-CLL cells, cell death was detected by annexin V Cy5.5 and 7-aminoactinomycin (7-AAD) as previously described.26
Measurement of mitochondrial transmembrane potential (ΔΨm) loss
Mitochondrial depolarization was monitored with the potentiometric dye JC-1 using the Mitoprobe assay kit (Invitrogen) according to the manufacturer's instructions. JC-1 accumulates in polarized mitochondria with a resting membrane potential and fluoresces red. However, on loss of ΔΨm, JC-1 aggregates were released from the mitochondria and fluoresce green. Thus, to assess mitochondrial depolarization, mAb-treated cells were stained with JC-1 (2μM) for 30 minutes at 37°C 5% CO2, washed, resuspended in fresh media, and then green fluorescence was monitored using flow cytometry.
Assessment of HA and the actin cytoskeleton
HA was assessed by light microscopy as previously detailed.26 To assess actin reorganization during HA cells were cytospun on poly-L-lysine slides (VWR) then fixed in fresh 2% paraformaldehyde in PBS for 15 minutes, and then incubated with 0.1M glycine for 5 minutes. Cells were then permeabilized with 0.1% Triton X-100 for 5 minutes, and then incubated with Alexa Fluor 488–phalloidin (Molecular Probes) overnight at 4°C. Slides were then washed in PBS and mounted with ProLong Gold antifade reagent with DAPI (Invitrogen). Cells were then imaged using an Olympus BX51 microscope equipped with a CoolSnap HQ camera and Sedat excitation filters using an ×40 or ×100 lenses, and images were processed using Metamorph Version 7.5.6 and Imaris Version 7.2.1 software.
Assessment of LMP
LMP and the release of lysosomal proteases into the cytosol were assessed using acridine orange (AO) staining analyzed by flow cytometry and immunofluorescence staining of the lysosomal protease cathepsin B by fluorescence microscopy, respectively, as previously described.7
Western blotting
Cell lysates were prepared in lysis solution containing protease inhibitor cocktail (Cell Signaling). Samples were separated by SDS-PAGE and proteins transferred to polyvinylidene difluoride (PVDF) membrane using the Surelock Western blot Module (Invitrogen). Membranes were blocked with 5% nonfat dried milk, incubated with NOX2/gp91phox antibody (1:1000; Abcam) or anti-β actin (1:1000; Sigma-Aldrich), washed, and incubated with appropriate HRP-conjugated secondary antibodies (1:2500; Dako). Membranes were treated with ECL reagent (Amersham) and visualized using a Fujifilm image analyzer.
Knockdown of NOX2/gp91 phox using small interfering RNA
small interfering RNA (siRNA) reagents targeting NOX2 (or scrambled control siRNA) were purchased from Santa Cruz Biotechnology and delivered into 2 × 106 Raji cells at a final concentration of 300 nM using the Amaxa nucleofection device (Buffer V, Program M-13), according to the manufacturer's instructions. Knockdown efficiency was then assessed by Western blotting 48 hours after transfection.
Statistical analysis
Error bars represent the SEM of 3 independent experiments unless otherwise stated. To compare the difference between the experimental groups an unpaired, 2-tailed t test was calculated. The number of asterisks displayed on the figures represents the degree of statistical significance as determined by P values as follows: * < .05, ** < .01, and *** < .001.
Results
mAb-induced cell death correlates with the generation of ROS
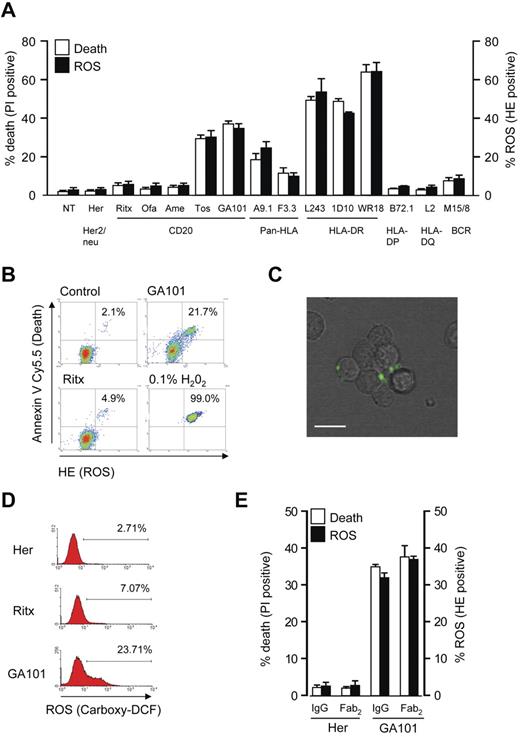
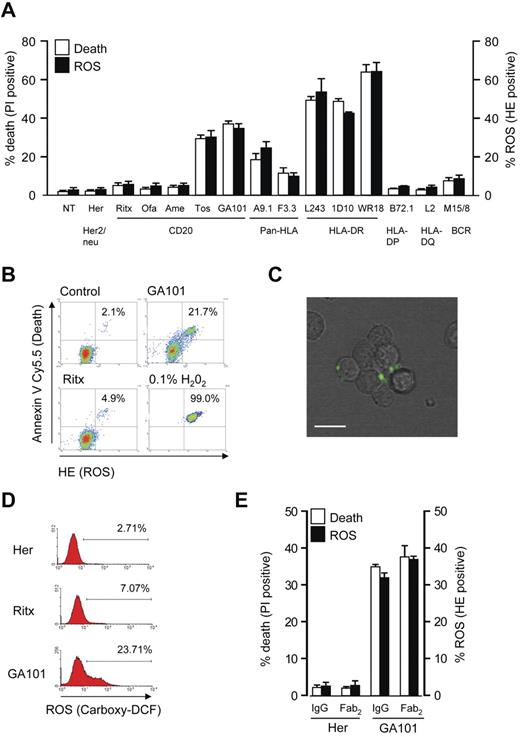
Based on our earlier observation that certain mAb specificities targeting CD20 and HLA DR induce cell death,7 we investigated the ability of these mAbs to stimulate the production of ROS. We found a direct correlation between mAb-induced cell death and ROS generation in Raji cells monitored using propidium iodide (PI) and dihydroethidium (HE) staining, respectively, and analyzed using flow cytometry. mAbs that potently induced cell death such as type II anti-CD20 mAbs (tositumomab and GA101) and anti-HLA DR mAbs (L243, 1D10, and WR18) produced high levels of ROS, whereas mAbs targeting HLA-DP, HLA-DQ, and type I anti-CD20 mAbs including rituximab and the next-generation reagents ofatumumab and AME-133V induced minimal levels (< 10%) of ROS and cell death. Intermediate levels of cell death and ROS were evoked by the pan-HLA mAbs F3.3 and A9-1 (Figure 1A). To link the induction of ROS to cell death, cells were labeled with HE and then stained with annexin V–Cy5.5 to identify dead cells. These experiments revealed that ROS production coincides with cell death, which confirms that these 2 processes are directly related (Figure 1B). ROS production was further assessed by an alternative probe in GA101-treated cells, using 5-(and-6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (carboxy-H2DCFDA) staining and live cell imaging. In the presence of ROS, carboxy-H2DCFDA is oxidized to carboxy-DCF which emits green fluorescence. After treatment with GA101, carboxy-DCF green fluorescence was detected within cellular aggregates at the cellular periphery, indicating that ROS were produced in close proximity to the plasma membrane (Figure 1C; supplemental Video 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The increase in carboxy-DCF staining on mAb stimulation was then quantified by flow cytometry, demonstrating a robust increase in cells treated with GA101, but not rituximab (Figure 1D) in keeping with their ability to trigger cell death. Finally, because we and others have previously shown that direct mAb-induced cell death is independent of the mAb Fc domain,6,30 we aimed to confirm that ROS production is also Fc-independent. Using F(ab)′2 fragments of GA101, we verified that the induction of both ROS and cell death are independent of the Fc domain, in keeping with previous work (Figure 1E).
Cell death induced by mAbs correlates with generation of ROS. (A) Raji cells were incubated with a panel of mAbs (10 μg/mL) for 4 hours, and assessed for the induction of cell death (PI) and ROS (HE) by flow cytometry. Bars represent mean + SEM from at least 3 separate experiments. (B) mAb induced cell death was directly correlated with production of ROS by flow cytometric analysis of Raji cells treated with anti-CD20 mAbs as before and dual-stained with HE (to label ROS) and annexin V Cy5.5 (to label dead cells). H2O2 (0.1%) was used as a positive control. Representative data are shown. (C) Live cell imaging to asses the production of ROS after mAb treatment of Raji cells. Cells were prelabeled with carboxy-H2DCFDA, and then treated with GA101 (10 μg/mL). Phase-contrast and green fluorescence images were captured and overlaid with a representative image captured 40 minutes after mAb treatment shown. Scale bar: 20 μm. mAb-induced ROS is generated within cellular aggregates toward the cell periphery (D) The amount of ROS production as measured by carboxy-H2DCFDA staining was quantified by flow cytometry. Representative histogram data are shown. (E) Cell death (PI) and ROS (HE) induced by GA101 IgG and F(ab)′2 fragments were compared in Raji cells treated as before. Bars represent mean + SEM from at least 3 separate experiments. Together these data demonstrate that cell death induced by mAb to CD20 and HLA-DR correlates with the generation of ROS.
Cell death induced by mAbs correlates with generation of ROS. (A) Raji cells were incubated with a panel of mAbs (10 μg/mL) for 4 hours, and assessed for the induction of cell death (PI) and ROS (HE) by flow cytometry. Bars represent mean + SEM from at least 3 separate experiments. (B) mAb induced cell death was directly correlated with production of ROS by flow cytometric analysis of Raji cells treated with anti-CD20 mAbs as before and dual-stained with HE (to label ROS) and annexin V Cy5.5 (to label dead cells). H2O2 (0.1%) was used as a positive control. Representative data are shown. (C) Live cell imaging to asses the production of ROS after mAb treatment of Raji cells. Cells were prelabeled with carboxy-H2DCFDA, and then treated with GA101 (10 μg/mL). Phase-contrast and green fluorescence images were captured and overlaid with a representative image captured 40 minutes after mAb treatment shown. Scale bar: 20 μm. mAb-induced ROS is generated within cellular aggregates toward the cell periphery (D) The amount of ROS production as measured by carboxy-H2DCFDA staining was quantified by flow cytometry. Representative histogram data are shown. (E) Cell death (PI) and ROS (HE) induced by GA101 IgG and F(ab)′2 fragments were compared in Raji cells treated as before. Bars represent mean + SEM from at least 3 separate experiments. Together these data demonstrate that cell death induced by mAb to CD20 and HLA-DR correlates with the generation of ROS.
ROS are required for mAb-induced cell death
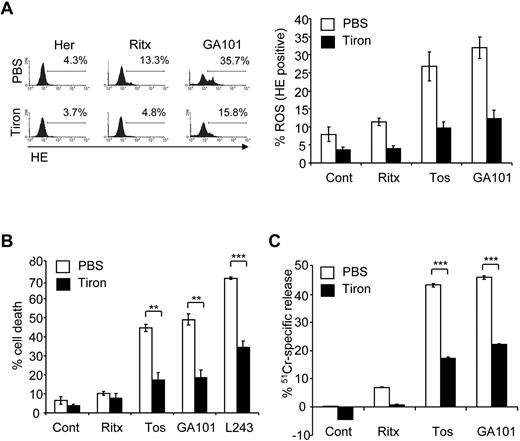
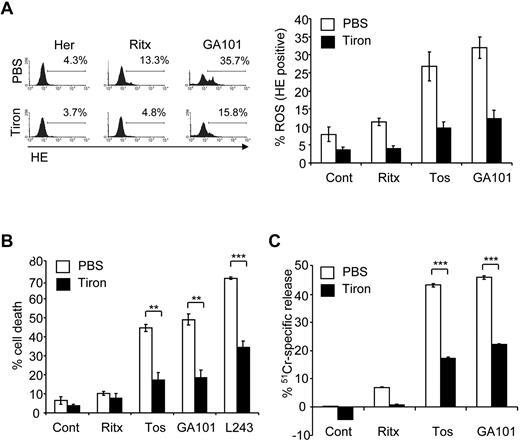
Having established a direct correlation between mAb-induced cell death and ROS production, we investigated whether ROS play a functional role in mAb-induced cell death using ROS scavengers. Tiron, a well characterized superoxide dismutase (SOD) mimetic,31 was shown to efficiently deplete ROS after mAb treatment in Raji cells (Figure 2A). Importantly, pretreatment of Raji cells with tiron significantly inhibited mAb-induced cell death (Figure 2B). Tiron inhibited mAb-induced cell death in a concentration-dependent manner, with concentrations as low as 1mM exhibiting an inhibitory effect (not shown). Tiron also significantly inhibited GA101-induced ROS and cell death in 2 additional lymphoma cell lines, Daudi (Burkitt lymphoma) and SUDHL-4 (transformed follicular lymphoma, supplemental Figure 1). The requirement of ROS for mAb-induced cell death was further validated using 51Cr-release killing assays, in which tiron significantly attenuated 51Cr-release induced by the type II anti-CD20 mAbs tositumomab and GA101 (Figure 2C). Moreover, pretreatment of Raji cells with a second ROS scavenger tempol, a nitroxide radical which can also function as a SOD mimetic,32 significantly inhibited mAb-induced Raji cell death (supplemental Figure 2). In contrast to the superoxide (O2−) scavengers tiron and tempol, ROS scavengers that operate downstream of O2− production, such as glutathione peroxidase which is selective for hydrogen peroxide (H2O2), failed to inhibit mAb-induced cell death (data not shown), suggesting that the form of ROS involved in mAb-induced cell death is specifically O2−.
ROS are required for mAb-induced cell death. (A) Before the addition of mAbs, cells were treated with PBS or the ROS scavenger, 4,5-dihydroxyl-1,3-benzededisulfonic acid (tiron; 10mM) for 2 hours. ROS production was then determined 4 hours later using HE staining, and analyzed by flow cytometry. Representative data from at least 3 independent experiments are shown. Bars represent mean ROS production ± SEM. (B) Cells were treated with tiron as before and the amount of mAb-induced cell death quantified 4 hours later using PI staining analyzed by flow cytometry. Bars represent mean cell death ± SEM from at least 3 separate experiments. (C) Cells were prelabeled with 51Cr for 1 hour at 37°C before treatment with tiron (10mM) for 2 hours. Cells were the treated with mAbs as before and 51Cr-release determined after 4 hours as described in “Cell death assays.” Bars represent mean + SEM from quintuplicate samples representative of 3 independent experiments.
ROS are required for mAb-induced cell death. (A) Before the addition of mAbs, cells were treated with PBS or the ROS scavenger, 4,5-dihydroxyl-1,3-benzededisulfonic acid (tiron; 10mM) for 2 hours. ROS production was then determined 4 hours later using HE staining, and analyzed by flow cytometry. Representative data from at least 3 independent experiments are shown. Bars represent mean ROS production ± SEM. (B) Cells were treated with tiron as before and the amount of mAb-induced cell death quantified 4 hours later using PI staining analyzed by flow cytometry. Bars represent mean cell death ± SEM from at least 3 separate experiments. (C) Cells were prelabeled with 51Cr for 1 hour at 37°C before treatment with tiron (10mM) for 2 hours. Cells were the treated with mAbs as before and 51Cr-release determined after 4 hours as described in “Cell death assays.” Bars represent mean + SEM from quintuplicate samples representative of 3 independent experiments.
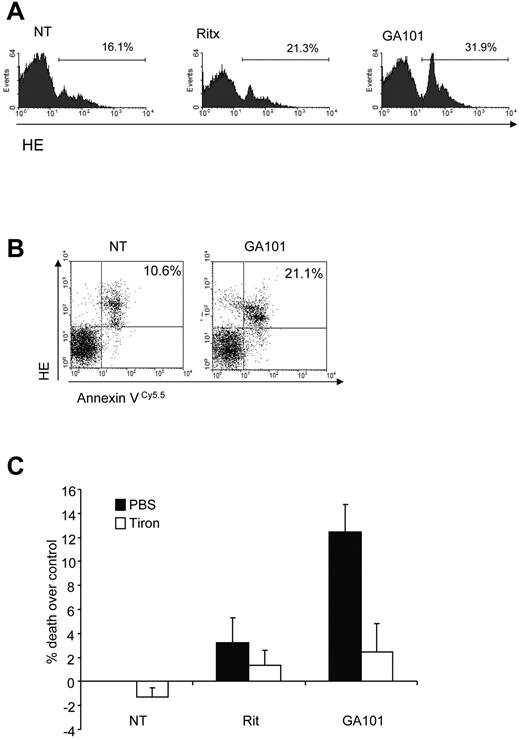
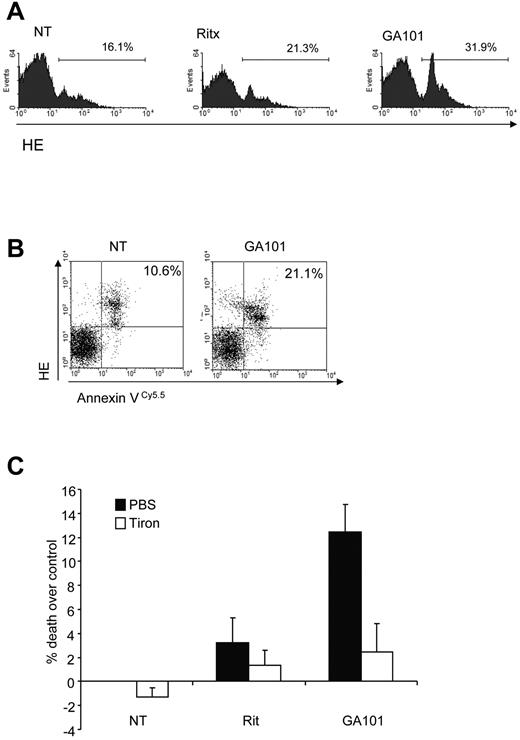
To examine the role of ROS in mAb-induced PCD in primary tumor cells, purified B-CLL samples were treated with anti-CD20 mAbs. The type II anti-CD20 mAb GA101 induced ROS more potently than the type I anti-CD20 mAb rituximab in B-CLL cells (Figure 3A), consistent with our earlier report on the superior ability of GA101 to induce direct PCD in B-CLL.26 GA101-induced ROS production was also seen to coincide with cell death in B-CLL cells (Figure 3B), as demonstrated earlier in our Raji cell line model (Figure 1D). Importantly, the ROS scavenger tiron significantly inhibited cell death induced by GA101 (Figure 3C). Taken together, these data indicate that ROS play an important functional role in mAb-induced cell death.
mAb-induced ROS triggers cell death in primary CLL samples. (A) Primary CLL cells were treated with mAbs (10 μg/mL) and 4 hours later stained with HE to assess ROS generation by flow cytometry. Representative data from 1 patient sample is shown. GA101 induces greater ROS than rituximab in primary B-CLL cells. (B) Dual staining of GA101-treated primary CLL cells with HE and annexin V Cy 5.5 to detect ROS and cell death, respectively confirms that ROS generation coincides with cell death in CLL cells. (C) CLL cells from 3 different patients were treated with mAb in the presence or absence of tiron and cell death was assessed after 4 hours by annexin V–Cy5.5 and 7-AAD costaining. Mean + SEM of cell death in the 3 independent patient samples are shown. Because of the heterogeneous levels of basal apoptosis in primary CLL samples, data are expressed as percentage cell death over control.
mAb-induced ROS triggers cell death in primary CLL samples. (A) Primary CLL cells were treated with mAbs (10 μg/mL) and 4 hours later stained with HE to assess ROS generation by flow cytometry. Representative data from 1 patient sample is shown. GA101 induces greater ROS than rituximab in primary B-CLL cells. (B) Dual staining of GA101-treated primary CLL cells with HE and annexin V Cy 5.5 to detect ROS and cell death, respectively confirms that ROS generation coincides with cell death in CLL cells. (C) CLL cells from 3 different patients were treated with mAb in the presence or absence of tiron and cell death was assessed after 4 hours by annexin V–Cy5.5 and 7-AAD costaining. Mean + SEM of cell death in the 3 independent patient samples are shown. Because of the heterogeneous levels of basal apoptosis in primary CLL samples, data are expressed as percentage cell death over control.
ROS production in mAb-induced cell death lies downstream of HA, actin relocalization, and LMP
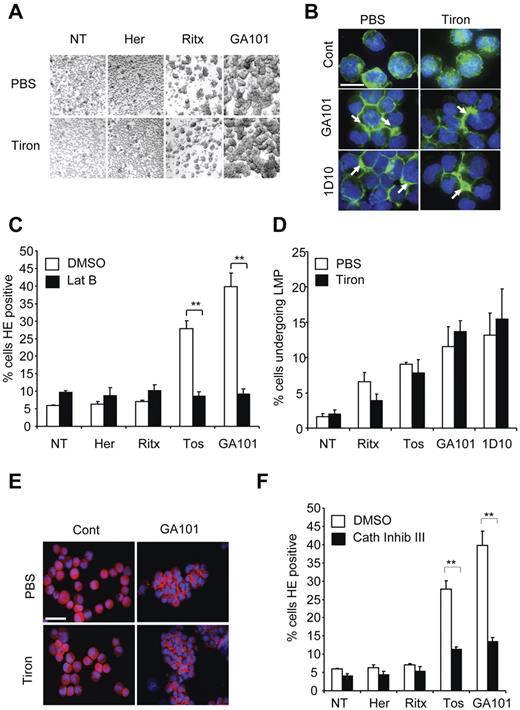
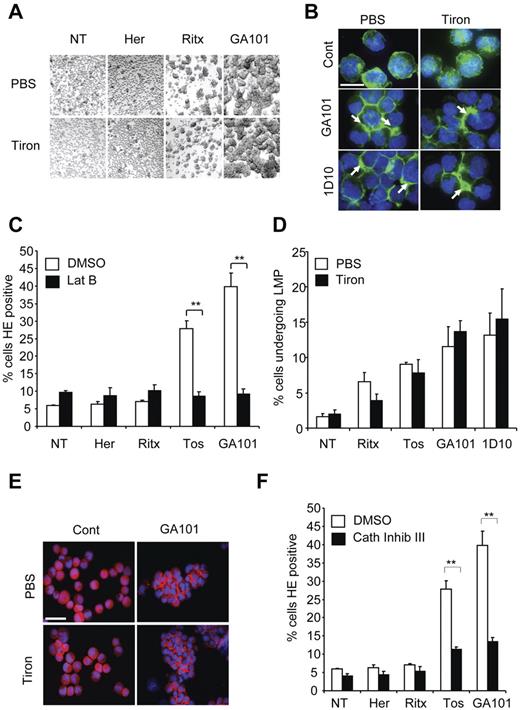
Given the functional significance of ROS production in mAb-induced cell death, we next aimed to delineate where the production of ROS lies within the nonapoptotic mAb-induced cell death pathway that we have previously characterized, involving HA, actin cytoskeletal reorganization, and LMP.7,26 First, we assessed the effect of ROS depletion on mAb-induced HA in Raji cells and found that tiron and tempol treatment did not inhibit cellular aggregation induced by GA101 (Figure 4A and not shown). Similarly, tiron had no effect on the redistribution of actin to areas of cell-cell contact underlying mAb-induced HA, as assessed by phalloidin staining and fluorescence microscopy (Figure 4B). Conversely, pharmacologic inhibition of actin polymerization using latrunculin B, which inhibits mAb-induced HA, and peripheral relocalization of actin7,26 abrogated the production of ROS (Figure 4C). Therefore, mAb-induced ROS generation occurs downstream of HA and the reorganization of the actin cytoskeleton.
Production of ROS occurs downstream of mAb-induced HA, actin-reorganization and lysosome permeabilization. (A) Raji cells were treated with PBS or tiron (10mM) for 2 hours before addition of mAb (10 μg/mL). Four hours later cells were assessed for HA by light microscopy. Original magnification ×20. (B) Cells were treated as in panel A, cytospun on poly-L-lysine coated slides and stained with Alexa Fluor 488–Phalloidin to label the actin filaments and slides were assessed by fluorescence microscopy. Representative images are shown. Scale bar: 25 μm. Tiron does not affect mAb-induced relocalization of actin filaments to the cell-cell junctions. (C) Raji cells were treated with the actin inhibitor latrunculin B (Lat B; 10μM) for 30 minutes before the addition of mAb, as before. Samples were assessed for ROS production after HE staining and flow cytometry. Bars represent mean + SEM from 3 separate experiments. (D) Cells were labeled with AO, and then treated with PBS or tiron and mAbs as before. The relative increase in green fluorescence, indicative of leakage of AO from the acidic lysosomes into the more pH neutral cytosol was assessed by flow cytometry 4 hours after addition of mAbs. Bars represent mean + SEM from 3 separate experiments. (E) Immunofluorescence staining of the lysosomal protease cathepsin B after mAb treatment in the presence or absence of tiron. Cells were treated and stained as described in panels A and B. Representative images are shown. Scale bar: 50 μm. Tiron does not block mAb-induced cathepsin B release into the cytoplasm and areas of cell-cell contact. (F) Cells were treated with cathepsin inhibitor III (100μM) 30 minutes before addition of mAbs (10 μg/mL). Samples were assessed for ROS production 4 hours later after HE staining and flow cytometry. Bars represent mean + SEM from 3 separate experiments. Together these data indicate that production of ROS occurs downstream of mAb-induced HA, actin reorganization and lysosomal membrane permeabilization.
Production of ROS occurs downstream of mAb-induced HA, actin-reorganization and lysosome permeabilization. (A) Raji cells were treated with PBS or tiron (10mM) for 2 hours before addition of mAb (10 μg/mL). Four hours later cells were assessed for HA by light microscopy. Original magnification ×20. (B) Cells were treated as in panel A, cytospun on poly-L-lysine coated slides and stained with Alexa Fluor 488–Phalloidin to label the actin filaments and slides were assessed by fluorescence microscopy. Representative images are shown. Scale bar: 25 μm. Tiron does not affect mAb-induced relocalization of actin filaments to the cell-cell junctions. (C) Raji cells were treated with the actin inhibitor latrunculin B (Lat B; 10μM) for 30 minutes before the addition of mAb, as before. Samples were assessed for ROS production after HE staining and flow cytometry. Bars represent mean + SEM from 3 separate experiments. (D) Cells were labeled with AO, and then treated with PBS or tiron and mAbs as before. The relative increase in green fluorescence, indicative of leakage of AO from the acidic lysosomes into the more pH neutral cytosol was assessed by flow cytometry 4 hours after addition of mAbs. Bars represent mean + SEM from 3 separate experiments. (E) Immunofluorescence staining of the lysosomal protease cathepsin B after mAb treatment in the presence or absence of tiron. Cells were treated and stained as described in panels A and B. Representative images are shown. Scale bar: 50 μm. Tiron does not block mAb-induced cathepsin B release into the cytoplasm and areas of cell-cell contact. (F) Cells were treated with cathepsin inhibitor III (100μM) 30 minutes before addition of mAbs (10 μg/mL). Samples were assessed for ROS production 4 hours later after HE staining and flow cytometry. Bars represent mean + SEM from 3 separate experiments. Together these data indicate that production of ROS occurs downstream of mAb-induced HA, actin reorganization and lysosomal membrane permeabilization.
An important component of mAb-induced cell death is the induction of LMP and release of lysosomal contents into the cytosol after HA; thus, we explored the role of ROS in relation to LMP in mAb-induced cell death. We have previously demonstrated the induction of LMP by mAb using AO, a metachromatic fluorochrome that concentrates in acidic lysosomes emitting red fluorescence. When LMP is triggered, AO is released into the more pH neutral cytosol and emits green fluorescence that can be detected by flow cytometry.7,26 ROS depletion with tiron had no effect on the mAb-induced increase in AO green fluorescence (Figure 4D). Tiron also had no effect on the mAb-induced release of the lysosomal protease cathepsin B into the cytosol adjacent to points of cell-cell contact, as assessed by fluorescence microscopy (Figure 4E). However, both the inhibition of lysosomal activation using the vacuolar ATPase concanamycin A (not shown), and the inhibition of cathepsin activity (using cathepsin inhibitor III) significantly blocked ROS production by tositumomab and GA101 (Figure 4F). Taken together, these data indicate that ROS production occurs downstream of LMP and the subsequent release of lysosomal cathepsins into the cytosol triggered during mAb-induced cell death.
Mitochondria are not essential for mAb-induced ROS production and cell death
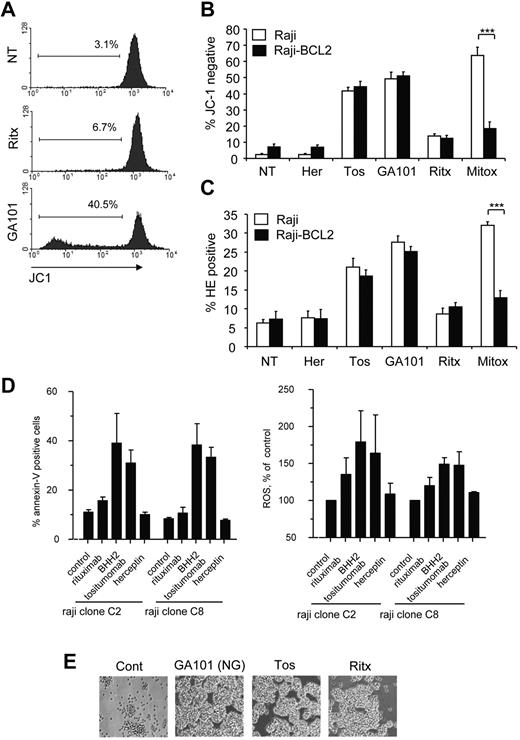
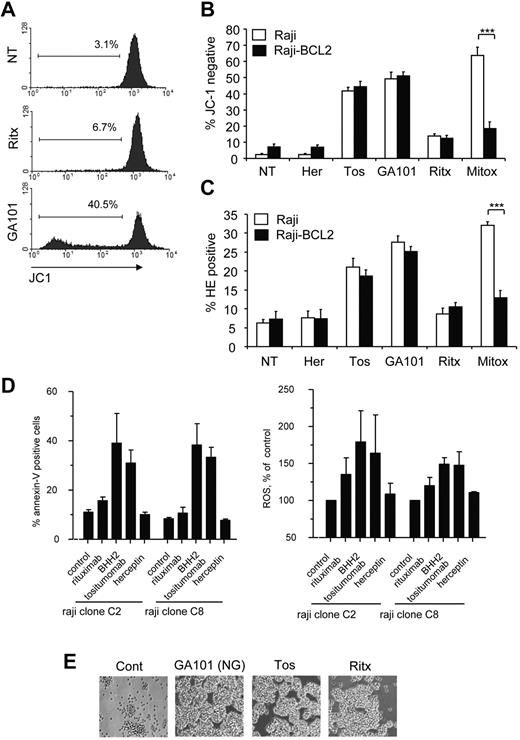
Mitochondrial oxygen metabolism is a major source of ROS in the cell, secondary to incomplete coupling of electrons and H+ with oxygen in the electron transport chain.16 During apoptosis, mitochondrial membrane permeabilization (MMP) and the accompanying loss of ΔΨm exacerbate the uncoupling of the electron transport chain, which results in the excessive production of ROS.33 Therefore, we asked whether the increase in ROS observed in mAb-induced cell death was a result of mitochondrial impairment. First, we assessed the ability of mAbs to induce the loss of ΔΨm, and using the potentiometric dye JC-1, we noted that mAbs promoted the loss of ΔΨm in Raji cells, which directly correlated with the ability of mAbs to induce cell death and ROS (Figure 5A). The loss of ΔΨm was further validated using an alternative probe, DiOC6,3 a green fluorochrome that incorporates into polarized mitochondria and is excluded on loss of ΔΨm (data not shown). Next we evaluated the role of the antiapoptotic oncoprotein BCL-2 in modulating mAb-induced loss of ΔΨm and ROS production. BCL-2 is an important inhibitor of MMP and consequent ROS production during apoptosis.34 However, we have previously demonstrated that mAb-induced cell death is independent of BCL-2 overexpression.5,7 We therefore wished to establish whether BCL-2 overexpression inhibits mAb-induced loss of ΔΨm and ROS production. Indeed, overexpression of BCL-2 in Raji cells had no effect on the loss of ΔΨm induced by tositumomab, GA101 (Figure 5B) or 1D10 (not shown), despite significantly inhibiting the loss of ΔΨm induced by the chemotherapeutic drug mitoxantrone, used here as a positive control for apoptosis (Figure 5B). Similarly, BCL-2 overexpression did not inhibit mAb-induced ROS production, whereas it significantly attenuated ROS production by mitoxantrone (Figure 5C). It has been previously shown that caspases can also directly damage mitochondria, inducing MMP and subsequent ROS production.35 However, we found that caspase inhibition using the pan-caspase inhibitor Q-VD-OPH had no impact on both mAb-induced loss of ΔΨm and ROS production (data not shown). Therefore, mAb-induced loss of ΔΨm and ROS production are independent of the apoptosis in agreement with our previous findings.7,26
Mitochondria are not essential for mAb-induced ROS production and cell death. (A) To detect mitochondrial membrane depolarization (loss of ΔΨm) Raji cells were prelabeled with 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide (JC-1; 2μM) for 30 minutes at 37°C. Cells were then treated with mAb (10 μg/mL) and assessed 4 hours later by flow cytometry. Representative histograms are shown. (B) The antiapoptotic oncoprotein BCL2 does not protect against mAb-induced loss of ΔΨm. WT Raji or a Raji cells transfected to overexpress BCL2 (Raji-BCL2) were labeled with JC-1 and treated as in panel A. Mitoxantrone (1 μg/mL) was used as positive control for apoptosis. Bars represent mean + SEM from 3 separate experiments. (C) Production of ROS by mAb is independent of BCL2 overexpression. Raji or Raji-BCL2 cells were treated with mAbs as before and assessed for production of ROS using HE analyzed by flow cytometry. Bars represent mean + SEM from 3 separate experiments. (D) Cell death and generation of ROS are independent of mitochondrial respiration. Parental Raji or respiratory deficient sub-clones were treated with mAb (10 μg/mL) then assessed for cell death (annexin V/PI) and ROS generation (HE) after 4 hours by flow cytometry. Bars represent mean + SEM from 3 separate experiments. (E) Respiratory deficient Raji subclone C2 was treated with mAb as before and the degree of HA visualized after 4 hours by light microscopy. Original magnification ×20.
Mitochondria are not essential for mAb-induced ROS production and cell death. (A) To detect mitochondrial membrane depolarization (loss of ΔΨm) Raji cells were prelabeled with 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide (JC-1; 2μM) for 30 minutes at 37°C. Cells were then treated with mAb (10 μg/mL) and assessed 4 hours later by flow cytometry. Representative histograms are shown. (B) The antiapoptotic oncoprotein BCL2 does not protect against mAb-induced loss of ΔΨm. WT Raji or a Raji cells transfected to overexpress BCL2 (Raji-BCL2) were labeled with JC-1 and treated as in panel A. Mitoxantrone (1 μg/mL) was used as positive control for apoptosis. Bars represent mean + SEM from 3 separate experiments. (C) Production of ROS by mAb is independent of BCL2 overexpression. Raji or Raji-BCL2 cells were treated with mAbs as before and assessed for production of ROS using HE analyzed by flow cytometry. Bars represent mean + SEM from 3 separate experiments. (D) Cell death and generation of ROS are independent of mitochondrial respiration. Parental Raji or respiratory deficient sub-clones were treated with mAb (10 μg/mL) then assessed for cell death (annexin V/PI) and ROS generation (HE) after 4 hours by flow cytometry. Bars represent mean + SEM from 3 separate experiments. (E) Respiratory deficient Raji subclone C2 was treated with mAb as before and the degree of HA visualized after 4 hours by light microscopy. Original magnification ×20.
To definitely determine whether mitochondria are the source of ROS induced by mAbs we used 2 respiration-deficient cell subclones of Raji cells (C2 and C8), which are unable to generate ROS through the mitochondrial respiration chain.28 MAb-induced ROS production and cell death in the respiration-deficient Raji subclones (Figure 5D), as well as HA (Figure 5E) was equivalent to that induced in WT Raji cells, which indicats that mitochondrial respiration is not required for mAb-induced ROS production, and that the source of ROS is probably extra-mitochondrial.
mAb-induced ROS production and cell death is mediated by NADPH oxidase
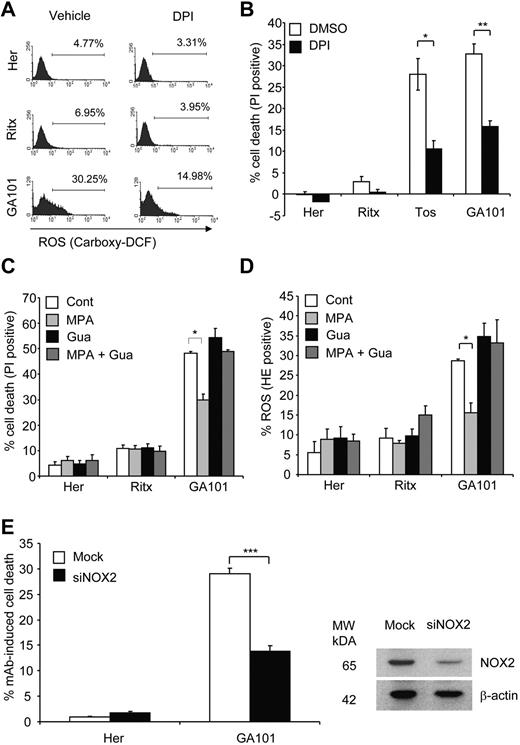
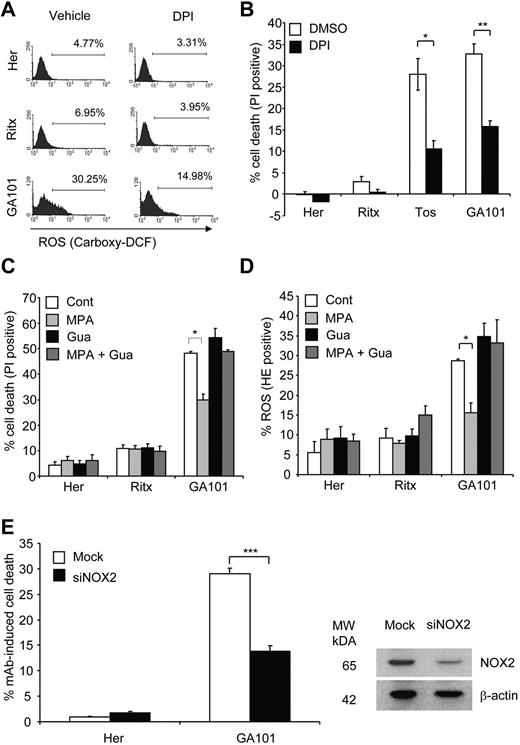
The NOX family of NADPH oxidases are an important source of nonmitochondrial ROS. Although initially known for their ROS-producing role in the respiratory burst-dependent antimicrobial activity of phagocytes, it is now well established that NADPH oxidases conduct various physiologic functions through the regulated production of ROS, including the induction of cell death.36 Furthermore, NADPH oxidases have been shown to produce ROS in B-cells.37,38 Thus, we hypothesized that mAb-induced ROS production and consequent cell death were mediated by NADPH oxidases. Pretreatment of Raji cells with the NADPH oxidase inhibitor DPI significantly attenuated ROS production (as detected by carboxy-H2DCFDA staining) and cell death evoked by tositumomab and GA101 (Figure 6A-B) indicating that NADPH oxidase activity is the source of mAb-induced ROS and is required for cell death. We next confirmed the role of NADPH oxidases in this process using an alternative inhibitor, MPA, previously shown to potently inhibit NADPH oxidase activity.39 Pretreatment of Raji cells with MPA significantly inhibited GA101-induced ROS production and cell death (Figure 6C-D). Because MPA blocks NADPH oxidase activity by depleting cellular GTP and consequently inhibiting Rac,39 an essential component of the NADPH oxidase complex,36 repletion of cellular GTP content by directly adding guanosine abolished the inhibitory effect of MPA, and restored levels of ROS production and cell death to those induced by GA101 alone (Figure 6C-D). Given the role of Rac GTPases in regulating the actin cytoskeleton40 and thus their potential effect on mAb-induced HA and actin relocalization, we demonstrated that DPI and MPA had no effect on mAb-induced HA and actin relocalization (supplemental Figure A-B) thereby confirming that ROS inhibition in this case was not simply because of the inhibition of the actin reorganization, but rather a direct effect on the NADPH oxidase. Furthermore, neither MPA nor DPI inhibited mAb-induced LMP as detected by AO staining (not shown) and the release of cathepsin B from lysosomes into the cytosol (supplemental Figure 3C). These findings are in keeping with the notion that ROS production is downstream of HA and LMP elicited by mAb. The inhibitory effect of MPA and DPI on mAb-induced cell death was further validated using 51Cr-release killing assays, in which both inhibitors significantly attenuated GA101-induced cell death (data not shown).
mAb-induced ROS and cell death is mediated by an NADPH oxidase. (A) Pharmacologic inhibition of NADPH oxidase blocks mAb-induced ROS production. Raji cells were pretreated with the NADPH oxidase inhibitor, diphenylene iodonium (DPI; 50μM) 1 hour before the addition of mAb (10 μg/mL). Generation of ROS was assessed 4 hours later using carboxy-H2DCFDA staining (25μM, 30 minutes, 37°C), and analyzed by flow cytometry. Representative histograms from 3 independent experiments are shown. (B) The effect of NADPH oxidase inhibition on cell death. Raji cells were treated with DPI and mAb as in (A) and cell death quantified 4 hours post-mAb treatment using PI staining analyzed by flow cytometry. Bars represent mean + SEM from 3 separate experiments. (C) The effects of NADPH oxidase inhibition on mAb-induced cell death and (D) ROS were confirmed using a second pharmacologic inhibitor. Raji cells were pretreated with mycophenolic acid (MPA; 10μM) for 16 hours in the presence or absence of guanosine (20μM) followed by addition of anti-CD20 mAb (10 μg/mL) for 4 hours. Cell death (C) and ROS (D) were then assessed by flow cytometry using PI and HE, respectively. Bars represent mean + SEM from 3 separate experiments. (E) Raji cells were nucleofected with siRNA against NOX2 and incubated for 48 hours. Cells were then treated with mAbs (10 μg/mL) and assessed for cell death after 4 hours using PI staining (left panel). Knockdown of NOX2 was confirmed by Western blotting (right panel). Bars represent mean + SEM from 3 separate experiments.
mAb-induced ROS and cell death is mediated by an NADPH oxidase. (A) Pharmacologic inhibition of NADPH oxidase blocks mAb-induced ROS production. Raji cells were pretreated with the NADPH oxidase inhibitor, diphenylene iodonium (DPI; 50μM) 1 hour before the addition of mAb (10 μg/mL). Generation of ROS was assessed 4 hours later using carboxy-H2DCFDA staining (25μM, 30 minutes, 37°C), and analyzed by flow cytometry. Representative histograms from 3 independent experiments are shown. (B) The effect of NADPH oxidase inhibition on cell death. Raji cells were treated with DPI and mAb as in (A) and cell death quantified 4 hours post-mAb treatment using PI staining analyzed by flow cytometry. Bars represent mean + SEM from 3 separate experiments. (C) The effects of NADPH oxidase inhibition on mAb-induced cell death and (D) ROS were confirmed using a second pharmacologic inhibitor. Raji cells were pretreated with mycophenolic acid (MPA; 10μM) for 16 hours in the presence or absence of guanosine (20μM) followed by addition of anti-CD20 mAb (10 μg/mL) for 4 hours. Cell death (C) and ROS (D) were then assessed by flow cytometry using PI and HE, respectively. Bars represent mean + SEM from 3 separate experiments. (E) Raji cells were nucleofected with siRNA against NOX2 and incubated for 48 hours. Cells were then treated with mAbs (10 μg/mL) and assessed for cell death after 4 hours using PI staining (left panel). Knockdown of NOX2 was confirmed by Western blotting (right panel). Bars represent mean + SEM from 3 separate experiments.
The NOX isoform most commonly associated with B cells is the prototypical phagocyte NADPH oxidase NOX2 (gp91phox/cytochrome b558).37 We confirmed that NOX2 is expressed in Raji cells by Western blotting, and that expression levels of NOX2 remained unchanged after mAb treatment (data not shown). Importantly, the down-regulation of NOX2 expression using siRNA significantly inhibited GA101-induced cell death (Figure 6E). As neither inhibition of mAb-induced cell death nor NOX2 knockdown was complete in our model system, it is currently unclear as to whether NOX2 is the only mediator of cell death or that other NOX isoforms also contribute. Nevertheless, these data support a direct role for NOX2 in mAb-induced cell death. Altogether, these data show that mAb-induced ROS production and cell death is dependent on NADPH oxidase activity.
Discussion
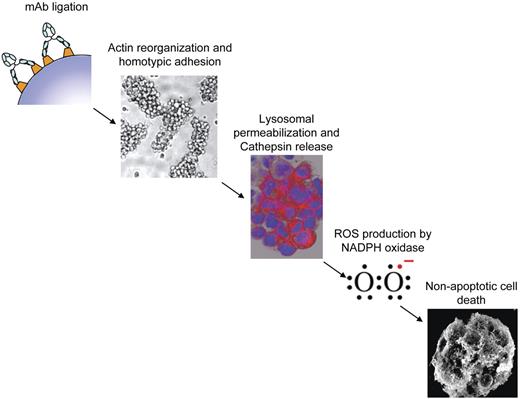
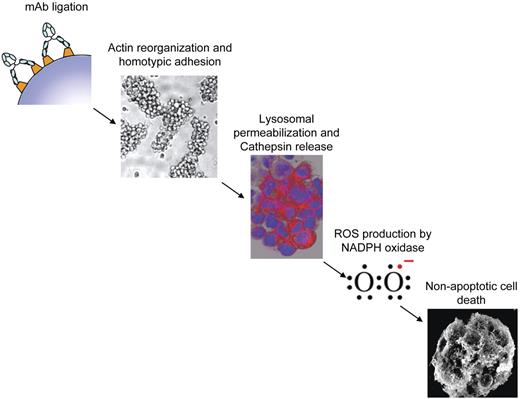
Increasing evidence suggests that ROS may play a central role in mediating various forms of nonapoptotic, caspase-independent PCD either through the activation of pro-death signal transduction programs or direct damage to cellular structure. Here we investigated the involvement of ROS in the direct nonapoptotic PCD evoked by a range of mAbs and identified several key findings. First, the ability of mAbs to elicit PCD directly correlated with their ability to generate ROS. Second, ROS production was required for cell death. Third, ROS production occurred downstream of mAb-mediated HA, actin reorganization, and LMP. Fourth, although mAbs that induced PCD also promoted the loss of ΔΨm, the mitochondrial respiration chain was not essential for mAb-induced ROS production. Finally, ROS production was found to be mediated by NADPH oxidase, which was required for cell death. Taken together, our understanding of the mAb-induced cell death pathway characterized in B-cell malignancies thus far is summarized in Figure 7.
Proposed mechanism of mAb-induced PCD. Schematic diagram illustrating the proposed sequence of events in the proposed cell death pathway evoked by type II anti-CD20 and anti-HLA DR mAb. mAb ligation results in HA and actin reorganization followed by lysosomal membrane permeabilization, release of cathepsins, and generation of ROS via NAPDH oxidase which ultimately culminates in nonapoptotic cell death.
Proposed mechanism of mAb-induced PCD. Schematic diagram illustrating the proposed sequence of events in the proposed cell death pathway evoked by type II anti-CD20 and anti-HLA DR mAb. mAb ligation results in HA and actin reorganization followed by lysosomal membrane permeabilization, release of cathepsins, and generation of ROS via NAPDH oxidase which ultimately culminates in nonapoptotic cell death.
The ability of mAbs to elicit ROS and PCD appears to depend not only on the target antigen, but also on the specific interaction between mAb and target, as demonstrated with the superior ability of type II anti-CD20 mAbs (tositumomab, GA101) to evoke ROS and cell death compared with type I anti-CD20 mAbs (rituximab, ofatumumab, and AME-133V). Although the mechanism underlying this difference is still not fully understood, and could not be explained by the epitope binding given that epitopes bound by type I and II anti-CD20 mAbs are largely overlapping, Niederfellner et al recently proposed that the difference lies in the orientation of the binding and compartmentalization of CD20 within the plasma membrane on mAb ligation.41 Such factors may alter the strength of intracellular signal transduction mediated by cell surface receptors. Interestingly, although the PCD signal evoked by type I anti-CD20 mAbs is limited, a recent study has shown that B-CLL cells can be further sensitized to rituximab-induced PCD by an increase in basal ROS levels, mediated through CD40 stimulation.42 Similarly, combining rituximab with anti-CD74 mAb (milatuzumab) increases HA and ROS production, enhancing cell death.12 Clearly, certain stimuli can enhance the limited cell death signal evoked by type I anti-CD20 mAbs, so it will be interesting to examine whether such stimuli further augment the high levels of cell death and ROS evoked by type II anti-CD20 mAbs and anti-HLA DR mAbs.
ROS generation has been reported to serve as both an inducer and a consequence of LMP.43 In our model system, ROS production appears to occur downstream of the mAb-induced lysosomal derangement, because inhibition of ROS had no effect on LMP and cytosolic cathepsin release, whereas inhibition of vacuolar ATPase and cathepsin activity blocked ROS production. Lysosomes can produce ROS directly, or LMP can act as a trigger for MMP and subsequent hyper-production of ROS from mitochondria.33,44 We demonstrated here that mAb-induced PCD correlates with the induction of MMP as detected by loss of ΔΨm, consistent with earlier studies.5,11 However, BCL-2 overexpression did not protect mitochondria from loss of ΔΨm evoked by mAb, in contrast to loss of ΔΨm induced by apoptotic stimuli, and in keeping with the BCL-2 independent nature of mAb-induced cell death.5,7 Indeed, although BCL-2 is a potent suppressor of MMP, mitochondrial damage that is insensitive to BCL-2 has been previously reported, including MMP triggered by granzyme A.18 Intriguingly, although GA101 potently induced MMP, the ability of respiration-deficient Raji subclones to efficiently produce ROS and undergo cell death on mAb treatment was unaffected. This observation taken together with our earlier finding in which cytoplasts devoid of mitochondria were efficiently killed by tositumomab,5 strongly suggests that mitochondria are not essential for mAb-induced ROS production and cell death. The accompanying MMP is therefore probably an epi-phenomenon that is not critical for the mechanism of cell death, and is perhaps secondary to LMP. In support of this hypothesis, we noted that inhibition of vacuolar ATPases and cathepsins prevented the mAb-induced loss of ΔΨm (J.H. and M.A., unpublished observations, October 2010).
In contrast to the mitochondrial electron transport chain, which generates ROS as a byproduct of respiration, NADPH oxidases produce ROS as their primary function.36 We demonstrated here that NADPH oxidases are a major source of ROS in mAb-induced cell death, and that their activity is required for the execution of cell death. NADPH oxidases are essential in various models of ROS-dependent cell death, including the NOX1-dependent PCD induced by TNFs.45 Interestingly, TNF-induced PCD appears to be regulated by both the actin cytoskeleton,46 and lysosomal cathepsins,47 and is associated with LMP.48 Thus it appears that TNF-induced PCD shares striking similarities with mAb-induced PCD and therefore mechanistic relationship between these 2 cell death pathways warrants further investigation.
The molecular basis underlying the loss of plasma membrane integrity after ROS production in mAb-induced cell death remains to be elucidated. Our data suggests that ROS, given their close proximity to the plasma membrane at areas of cell-cell contact as detected by our live cell imaging analysis, may damage plasma membrane lipids through peroxidation. In support of this hypothesis, NADPH oxidase activation has been previously reported to induce the peroxidation of all 3 major classes of membrane phospholipids: phosphatidylcholine, phosphatidylethanolamine, and phosphatidylserine in neutrophils.49 Furthermore, because NADPH oxidases are membrane-bound and can localize to both the plasma and lysosomal membranes it is still undetermined whether the NADPH oxidase in mAb-induced cell death generates ROS within the lysosomal lumen (with ROS subsequently released near areas of cell-cell contact on LMP), or directly at the plasma membrane. The specific site of ROS production and its subsequent impact on plasma membrane integrity forms the subject of our ongoing research.
The importance of this ROS-mediated mAb-induced cell death pathway to the clearance of B-cell tumors in the clinic remains unproven. However, preclinical evidence is compelling that some antibody specificities such as anti–HLA-DR are able to induce PCD, with type II anti-CD20 mAb demonstrating greater potency than their type I counterparts. However, further preclinical in vivo studies are required to determine the relative contribution of PCD to the therapeutic efficacy of mAb compared with other mAb effector mechanisms and these will probably vary depending on the antibody, the targeted antigen and the host micro-environment of the malignant B cells. Whether the PCD associated with GA101 leads to improved clinical responses remains unknown. A recent randomized phase 2 trial comparing rituximab to GA101 in patients with relapsed follicular lymphoma demonstrated modest increases in response rate of GA101 over Rituximab, yet this failed to translate into improvements in progression free surivival.50 Larger phase 3 studies are required to definitively establish whether GA101 will lead to improved clinical outcomes over rituximab, some of which are already underway. However, even if GA101 demonstrates superior efficacy, it will be difficult to determine whether this is attributed to improved PCD, enhanced recruitment of FcγR-expressing immune effector cells, or reduced tendency to evoke antigenic modulation,51 emphasizing the importance of in vivo studies in preclinical mouse models to address these questions.
In defining the mechanisms underlying this mAb-induced PCD pathway, rational drug combinations can be further investigated to provide additive or synergistic tumor cell death. For example, having established a role for ROS in mAb-induced PCD the ability to further enhance cell death by combining mAbs with clinical reagents that promote ROS accumulation through interfering with ROS scavenging systems could be further investigated. Such ROS-generating reagents include platinum-based chemotherapy,52 or the novel radiosensitizer compound motexafin gadolinium, which is currently undergoing phase 3 clinical trials.16
In conclusion, this study provides further mechanistic insights into mAb-induced cell death and reveals a previously unrecognized role for NADPH oxidase-derived ROS in mediating this nonapoptotic, lysosomal cell death pathway. Further investigation of this newly characterized cell death pathway may lead to novel therapeutic approaches that may further improve the clinical efficacy of mAbs.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful to all collaborators for providing the various reagents used in this study. GA101 was provided by C. Klein, Roche GlycartAG. They also thank the Manchester Cancer Research Center Biobank, Mrs Deepti Wilks and Dr Adrian Bloor (Christie Hospital NHS F.T) for facilitating the provision and preparation of patient samples.
This work was supported by fellowships and grants from Cancer Research UK and the Ministry of Higher Education of Kuwait.
Authorship
Contribution: T.M.I., J.H. and W.A. initiated the studies and designed the research; J.H., W.A., M.A., E.J.C., H.P., and A.I. performed experiments; all authors analyzed the data; M.S.C. provided novel reagents; W.A. wrote the paper; and J.H., E.C., M.S.C., P.H. and T.M.I. edited the paper.
Conflict-of-interest disclosure: T.M.I. received honorarium for consultancy work with Hoffman La Roche. The remaining authors declare no competing financial interests.
Correspondence: Tim M. Illidge, School of Cancer and Enabling Sciences, Manchester Academic Health Science Centre, University of Manchester, Manchester, M20 4BX, United Kingdom; e-mail: tmi@manchester.ac.uk.
References
Author notes
J.H. and W.A. contributed equally to this work