The coagulation system provides physiologic host defense, but it can also be exploited by pathogens for infection. On the HSV1 surface, host-cell–derived tissue factor (TF) and virus-encoded glycoprotein C (gC) can stimulate protease activated receptor 1 (PAR1)–enhanced infection by triggering thrombin production. Using novel engineered HSV1 variants deficient in either TF and/or gC, in the present study, we show that activated coagulation factors X (FXa) or VII (FVIIa) directly affect HSV1 infection of human umbilical vein endothelial cells in a manner that is dependent on viral TF and gC. The combination of FXa and FVIIa maximally enhanced infection for TF+/gC+ HSV1 and receptor desensitization and Ab inhibition demonstrated that both proteases act on PAR2. Inhibitory TF Abs showed that the required TF source was viral. Individually, TF or gC partly enhanced the effect of FXa, but not FVIIa, revealing gC as a novel PAR2 cofactor for FVIIa. In sharp contrast, thrombin enhanced infection via PAR1 independently of viral TF and gC. Thrombin combined with FXa/FVIIa enhanced infection, suggesting that PAR1 and PAR2 are independently involved in virus propagation. These results show that HSV1 surface cofactors promote cellular PAR2-mediated infection, indicating a novel mode by which pathogens exploit the initiation phase of the host hemostatic system.
Introduction
Tissue factor (TF) is an essential cofactor in the cellular initiation of coagulation. Once accessible to the circulating clotting proteins, TF functions as a receptor on the cell surface for the zymogen factor VII (FVII) and its activated form, FVIIa.1 The TF-FVIIa complex proteolytically activates factor X (FX) to FXa. In complex with activated factor V and anionic phospholipid, FXa functions as the only known physiologic mediator of the prothrombin to thrombin conversion. In addition to direct effects on clot formation, thrombin, FXa, and FVIIa trigger numerous cell-stimulatory responses that contribute to hemostasis and inflammation.2,–4 The accessibility of TF is therefore highly regulated to prevent the uncontrolled generation of active coagulation proteases in the blood.
The main pathway of coagulation protease–induced cell signaling is through protease activated receptors (PARs).5,–7 PARs belong to a unique group of G protein–coupled receptors. After extracellular proteolysis of the PAR, a new N-terminus is unmasked that serves as a tethered ligand, which than binds intramolecularly or in receptor cross-activation to stimulate G-protein signaling.8 Once activated, PARs are rapidly uncoupled from signaling and internalized by phosphorylation- and β-arrestin–dependent mechanisms, leading to desensitization or G protein–independent PAR signaling.9
In humans, thrombin predominantly facilitates cell signaling by cleavage of PAR1 and, at approximately 50-fold higher concentrations, through the low-affinity receptor PAR4.10 Like thrombin, FXa may stimulate cells via PAR1, but may also do so through PAR2.4,7,11,12 PAR1 and PAR2 are expressed on the endothelial cell surface. TF, which is also found on the endothelial surface, activates FVIIa allosterically through binary cofactor-protease complex formation, TF-FVIIa, which triggers the thrombin-insensitive PAR2. Further studies have established that the ternary complex TF-FVIIa-FXa also signals through PAR1 and PAR2, reducing the functional concentration of FXa required.4,7,11,12 In contrast to thrombin, which is released from the cell and re-associates in close proximity to the scissile bond to facilitate PAR cleavage, FVIIa and FXa remain cell surface localized and concentrated through cofactor or phospholipid interactions proximal to PARs. The respective signaling capabilities of these upstream coagulation proteases are therefore an important consideration in cell activation.
Our previous work has shown that members of the herpesvirus (HV) family, HSV1 and HSV2 and CMV, can initiate the generation of thrombin directly on their surface envelopes through incorporation of host-cell–derived TF and procoagulant phospholipid.13,14 These viruses also promote FX activation by the contact phase of coagulation.15 A further mechanism for FX activation that involves virus-encoded glycoprotein C (gC) has been identified on HSV1.16,17 Both the TF- and gC-dependent mechanisms require FVII/VIIa to facilitate virus-dependent FXa production and to enable the virus to override the cellular control of coagulation-initiated events, thereby generating potent cell-signaling proteases. Therefore, even before host-cell entry, the virus may trigger activation of the hemostatic system to initiate cell signaling, with potential implications for the early steps in cellular infection. Thrombin can enhance HSV1 infection consistently through a PAR1-mediated mechanism.18
Because thrombin activity in vivo is tightly controlled by vessel wall and plasma anticoagulant mechanisms, in the present study, we investigated whether earlier stages in the initiation of coagulation also play a role in virus entry. The HSV1 surface participates in FX16 and FVII activation14 and expresses TF, a cofactor that directly supports PAR activation by associated proteases. We report that, in contrast to thrombin, FXa and FVIIa enhance HSV1 infection of endothelial cells through a mechanism that involves virus surface TF and gC and PAR2 rather than PAR1. Therefore, activation of coagulation proteases upstream of thrombin is a proximal and thrombin-independent strategy used by the virus to hasten permissive host-cell entry.
Methods
Reagents and proteins
The PAR1-specific activating peptide (PAR1ap) TFLLR-NH2 was from Tocris Bioscience and the PAR2-specific activating peptide (PAR2ap) SLIGKV-NH2 was from Bachem. An additional peptide that triggers PAR1 and PAR2, SFLLRNPNDKYEPF-NH2 (PAR1/2ap), BSA, purified nonimmune mouse IgG, purified sheep IgG, purified rabbit IgG, and heparin were from Sigma-Aldrich. Purified recombinant hirudin was from Calbiochem. Purified human thrombin, FX, FXa, and FVIIa were from Haematologic Technologies. Anti-HSV1 major capsid protein (Biodesign International), anti-TF (4503 and 4508, American Diagnostica; PQ and TF-10H10, Santa Cruz Biotechnology), anti-gC (1C8),19 HRP-conjugated goat anti–mouse IgG (Jackson ImmunoResearch Laboratories), anti-PAR1 (WEDE15, Beckman and ATAP2, Santa Cruz Biotechnology), and anti-PAR2 (SAM11, Santa Cruz) Abs were obtained commercially. Anti-TF 9C320,21 and 6B420,22 and recombinant nematode anticoagulant protein c2 (NAPc2)23 were prepared and purified as described.
Cell culture and virus production
The TF-deficient human melanoma cell line A7 was grown in Earl minimum essential medium supplemented with newborn calf serum (8%), FBS (2%), glutamine (2mM), HEPES (10mM), and geneticin (500 μg/mL; Invitrogen). These cells were engineered to generate a new cell line, A7/TF, which expresses TF under the control of zeocin (500 μg/mL), as described previously.24
HSV1 NS gC-null (gC deficient, gC−) and restored NS gC-null (gC containing, gC+) viruses (kind gifts of Dr Harvey Friedman, University of Pennsylvania, Pittsburgh, PA) were generated from HSV1 NS, a low-passage clinical isolate.19 These viruses were propagated in African green monkey kidney cells (Vero CCL-81; ATCC) to produce the initial viral inoculum and purified as described previously.16
To generate surface host protein–selected virus strains, the gC+ and gC− viruses were propagated in the TF-deficient or replete cell line. Briefly, a small amount of the initial Vero-produced virus was used to inoculate either A7 or A7/TF cells. The clarified medium from the first round of the A7 and A7/TF infections was used to inoculate subsequent passages of A7 and A7/TF cells. Virus was then collected from the media of these infections, which allowed the generation of TF+/gC+, TF+/gC−, TF−/gC+ or TF−/gC− virus particles. All virus preparations were sucrose gradient purified, evaluated for purity, and quantified to derive virus particle number per milliliter (vp/mL) by negative-staining electron microscopy.14 After purification, prolonged incubation of virus with chromogenic substrate failed to detect the possibility of trace FX in the presence of purified FVIIa (subnanomolar sensitivity; data not shown). Viral TF and gC Ag were characterized by Western blot analysis after separation by SDS-PAGE and transfer to PVDF membranes using mAb toward TF (7nM; 4503), gC (70nM), or HSV1 major capsid protein (10nM), and then visualized using HRP-conjugated goat anti–mouse IgG (0.7nM) and enhanced chemiluminescence (GE Healthcare).
Human umbilical vein endothelial cells (HUVECs) were obtained from Cambrex (CC-2519) and maintained in complete medium (IMDM supplemented with 20% FBS, 14μM glutamine, 2 μg/mL of gentamycin, 2.5 μg/mL of Fungizone [Invitrogen], 20 U/mL of heparin, and 20 μg/mL of endothelial cell growth supplement (BD Biosciences). Cells were split at a ratio of 1:4 every 3 days into 0.2% gelatin-coated flasks or 24-well plates. Experiments were conducted on HUVECs up to passage 10.
Plaque assays
The infectivity of each virus produced was determined by plaque assays on HUVECs grown in 24-well plates. Before inoculation, the cells were washed once with PBS and serum-free medium supplemented with 1 mg/mL of BSA (SFM/BSA). The inoculation of cells by virus was conducted in SFM/BSA (200 μL/well) for 90 minutes at 37°C. After removing the inoculum, the cells were washed and replaced with reduced-serum (2%) medium and endothelial cell growth supplement (5 μg/mL) with heparin (RSM) to enable continued cell culture. Twenty-four hours after infection, the cells were stained to derive the number of productive infectious events (plaques). All viruses used in the study had similar numbers of vp/mL and PFUs/mL in SFM/BSA and comparable amounts of virus particles were added in each experiment. To maintain the integrity of the cell monolayer and to have a reproducible number of plaques prior to and subsequent to the various cell treatments that were anticipated to be enhancing, the initial number of plaques for each virus was relatively low (2-5).
Enzyme-dependent plaque assays
After washing, cells were inoculated (5-10 PFUs/mL) in the presence or absence of thrombin, FXa, or FVIIa in SFM/BSA. After inoculation, the cells were washed and replaced with RSM. Additional experiments were conducted in which enzyme and inhibitors were added simultaneously. The data were corrected for the number of plaques detected in the absence of added enzyme, taking into account any enzyme-independent infection.
To determine the effect of enzyme combinations in the system, cells were inoculated with a constant PFUs/mL of virus, and FVIIa and FXa were titrated. Alternatively, the amount of virus was held constant while the amount of FXa, FVIIa, and thrombin was titrated in SFM/BSA. To evaluate only the protease-dependent plaque formation, the data were corrected for plaque formation due to added virus alone.
To demonstrate the role of FX activation on infection, HUVECs were inoculated with a constant amount of virus at various concentrations of FX. Identical experiments were also conducted in the presence of a constant amount of FVIIa. The data were corrected for the number of plaques detected in the absence of added FX or FX and FVIIa.
Cell activation/desensitization
To determine the effect of receptor activation on HSV1 infection, HUVECs were treated with PARaps of differing receptor selectivity in the presence of a constant amount of virus. To confirm the involvement of specific PARs in infection, virus and anti-PAR1 or anti-PAR2 Abs were added simultaneously to cells with concentrations of thrombin, FXa, FVIIa, or FXa/FVIIa shown to enhance infection. Similar experiments were conducted in the presence of anti-TF Abs or the protease-targeted anticoagulants hirudin or NAPc2. Desensitization experiments were performed to confirm the PAR pathway for each enzyme. Cells were pretreated with PAR1ap, PAR2ap, or PAR1/PAR2ap for either 10 minutes or 2 hours. After incubation, cells were washed and the virus was added with either purified thrombin or FXa. All data were corrected for the amount of infection due to virus alone.
Data analysis
The data are presented as the means ± SEM of at least 3 separate experiments conducted on different days and cell passage numbers. To account for day-to-day variations in total plaque number, the data were converted to the percentage of enhancement or inhibition. Each experi-ment consisted of 8 replicates. Titration data were arbitrarily fit to the simplest dose-response model (a rectangular hyperbola) using GraphPad Prism Version 4.0 software. Significance probability (P) was determined using the Student t test.
Results
Coagulation initiators on the virus surface
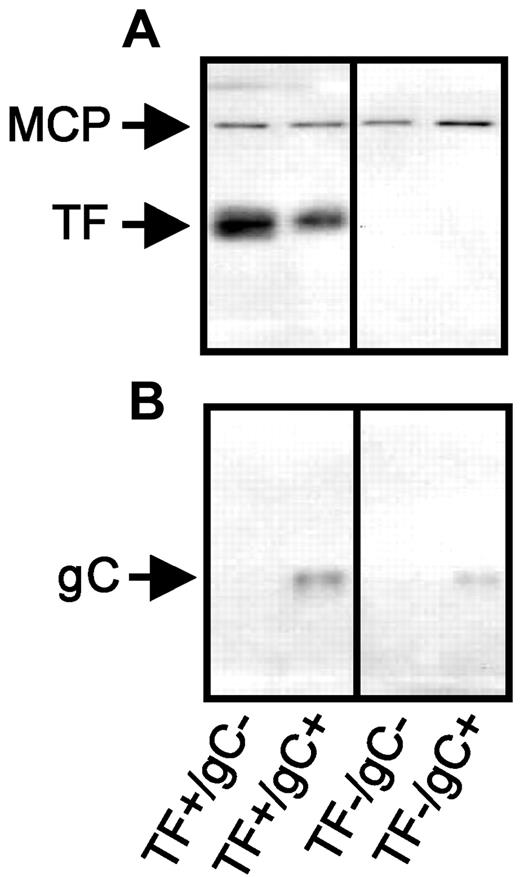
Our previous work14 demonstrated that FVIIa-dependent activation of FX on the surface of HSV1 is through host-cell–derived TF and virus-encoded gC.14,16,17 To independently evaluate the role of these receptors in protease-mediated virus infection, a novel panel of HSV1 was generated. HSV1 gC+ and gC− viruses were propagated in a human melanoma cell line (A7) that does not express TF, for the first time allowing the production of TF−/gC+ and TF−/gC− virus. Additional virus was propagated in A7 cells engineered to express TF (A7/TF)24 to generate TF+/gC+ and TF+/gC− virus. Antigenic characterization of the virus particles by Western blot confirmed the presence or absence of TF (Figure 1A) and gC (Figure 1B) on each purified virus as predicted.
TF- and gC-restricted HSV1 panel. An equal number of purified virus particles (7.5 × 1010) were separated electrophoretically through a 10% polyacrylamide gel, followed by Western blot analysis. The presence of TF (A) and HSV1 gC (B) were detected by Western blot. HSV1 major capsid protein (MCP) was used as a virus particle loading control (co-blotted in panel A). Protein Ag identification was consistent with molecular weight markers (not shown).
TF- and gC-restricted HSV1 panel. An equal number of purified virus particles (7.5 × 1010) were separated electrophoretically through a 10% polyacrylamide gel, followed by Western blot analysis. The presence of TF (A) and HSV1 gC (B) were detected by Western blot. HSV1 major capsid protein (MCP) was used as a virus particle loading control (co-blotted in panel A). Protein Ag identification was consistent with molecular weight markers (not shown).
Thrombin enhances infection independently of viral TF and gC
Because endothelial cells are well characterized with respect to cell-signaling events in vascular biology4,25 and because HSV1 is endothelial cell trophic,26 the effect of proteases on the infection of HUVECs by HSV1 was evaluated. To investigate the involvement of thrombin on infection by the new HSV1 panel and to give potential insight into respective roles for TF and gC, the serum-free viral plaque assay described previously was used. In agreement with previous studies,18 the addition of purified thrombin during the inoculation period resulted in increased HSV1 infection (Figure 2A). Thrombin enhanced infection by approximately 500% regardless of whether the virus had TF and/or gC on its surface. This effect was specific for thrombin, because the addition of hirudin prevented the thrombin-dependent increase in infection for each virus (data not shown). However, hirudin only partially blocked infection in the presence of serum (data not shown), indicating that enzymes other than thrombin may be involved in signal-induced HSV1 infection, which is consistent with our previous study.18
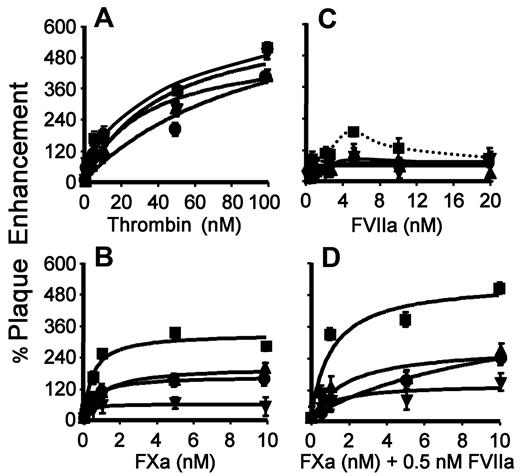
Combined TF and gC on HSV1 optimally enhance FXa/FVIIa-mediated infection. HUVECs in SFM were inoculated with HSV1 (4.5 × 105 vp/mL: ■, TF+/gC+; ▴, TF+/gC−; ●, TF−/gC+; ▾, TF−/gC−) at the indicated concentration of thrombin (A), FXa (B), FVIIa (C), and FXa at a constant concentration of FVIIa (0.5nM; D). The cells were stained 24 hours after infection and the amount of infection was determined. The data were corrected for the number of plaques detected in the absence of added protease. For panel A, n = 4; B, n = 4; C, n = 6; and D, n = 6. Data are ± SEM.
Combined TF and gC on HSV1 optimally enhance FXa/FVIIa-mediated infection. HUVECs in SFM were inoculated with HSV1 (4.5 × 105 vp/mL: ■, TF+/gC+; ▴, TF+/gC−; ●, TF−/gC+; ▾, TF−/gC−) at the indicated concentration of thrombin (A), FXa (B), FVIIa (C), and FXa at a constant concentration of FVIIa (0.5nM; D). The cells were stained 24 hours after infection and the amount of infection was determined. The data were corrected for the number of plaques detected in the absence of added protease. For panel A, n = 4; B, n = 4; C, n = 6; and D, n = 6. Data are ± SEM.
FXa and FVIIa enhance infection with differential effects of TF and gC
We evaluated the role of FXa and FVIIa as potential upstream coagulation proteases in signaling-dependent enhancement of HSV1 infection. When purified FXa was added with the virus during inoculation, increased infection was observed. A maximal enhancement of approximately 400% was noted when both TF and gC were present on the virus particle (Figure 2B). When either TF or gC was omitted from the virus surface, there was approximately half the amount of infection compared with both receptors being present. Increased infection in the presence of FXa was dependent on proteolytic activity, because the addition of active site–blocked FXa or zymogen FX had no effect on HSV1 infection (data not shown). When TF and gC were both absent from the virus, the FXa effect on infection over the same concentration range was further reduced. These data indicate that both TF and gC expressed on the virus act as independent coreceptors for FXa in supporting HSV1 infection, which is consistent with previous data demonstrating direct binding of FXa with gC17 and TF.27
Because FVIIa activates PAR2 when bound to TF,28,29 we assessed the ability of FVIIa to increase HSV1 infection. Indeed, the addition of purified FVIIa increased HSV1 infection (Figure 2C) in the presence of TF and gC. Maximal enhancement by FVIIa (185% ± 18%) was comparable only to the increase observed by FXa (Figure 2B) with virions expressing either TF (148% ± 22%) or gC (148% ± 24%). Enhanced infection by the TF+/gC+ virus in the presence of FVIIa required proteolytic activity, because the addition of ρ-aminobenzamide inhibited the respective increase in infection (data not shown). However, FVIIa enhanced HSV1 infection with a peak at 5nM (Figure 2C dotted line, arbitrary fit) and blocked infection at higher concentrations. Whereas we have not resolved this dose-response behavior, high concentrations of FVIIa are known to induce the association of TF with integrins independently of PAR signaling,30 and this interaction may adversely influence viral entry. In addition, incorporation of TF or gC alone into HSV1 virus was not sufficient to increase FVIIa-enhanced infectivity. Therefore, FVIIa is involved in complex interactions that depend on both TF and gC on the virus.
Ternary coagulation protease complex formation enhances infection
During the initial stages of coagulation, the TF/FVIIa complex activates FX to FXa. Once generated, FXa can remain transiently associated with TF/FVIIa, forming a ternary complex that mediates FXa-dependent cell signaling through PAR1 or PAR2.1,7,30,31 To determine whether such a complex can be formed on the virus surface, purified FXa was added with a relatively low concentration of FVIIa (0.5nM) during inoculation, which alone had a negligible effect on HSV1 infection (Figure 2C). In the presence of both proteases, infection by the TF+/gC+ virus was enhanced relative to FXa alone (compare Figure 2B,D), and infection was comparable to the approximately 500% increase seen with the addition of high concentrations of thrombin. When either TF or gC or both were missing from the virus, there was reduced enhancement of infection in the presence of both proteases and the infectivity was not enhanced compared with the respective TF−/gC+ or TF+/gC− virus in presence of FXa alone. Increasing concentrations of FVIIa had no further effect on infectivity at a fixed concentration of FXa, suggesting that saturation of procoagulant TF with subnanomolar FVIIa is sufficient for the enhancement of infectivity (data not shown). These results indicate that TF and gC cooperate in the assembly of the TF-FVIIa-FXa ternary complex on the virus to enhance infection.
In situ FXa generation enhances infection
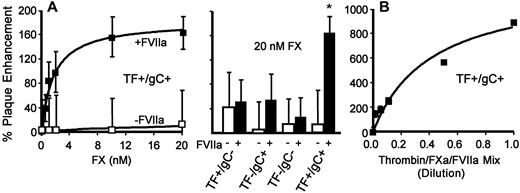
To demonstrate the role of in situ FX activation in infection directly, viruses were incubated with FX in the presence or absence of FVIIa in the serum-free cell-inoculation system. Adding increasing concentrations of FX alone did not significantly enhance infection regardless of viral surface constituents (Figure 3A open symbols). In contrast, FX added with a low concentration of FVIIa (0.5nM) enhanced infection (Figure 3A closed symbols) when both TF and gC were present on the virus. When neither was present, enhancement was comparable to that observed in the absence of FVIIa (Figure 3A bar graph). This is consistent with our previous finding that gC is a cofactor for FVIIa-dependent FX activation.18 Maximal enhancement of the TF+/gC+ virus infection was less than that for the addition of purified FXa (Figure 2B), which may reflect a limited amount of FX activation under the conditions used.
In situ FX zymogen activation or combining thrombin/FXa/FVIIa enhances HSV1 infection. HUVECs in SFM were inoculated with the indicated type of HSV1 (4.5 × 105 vp/mL) in the absence (open symbols) or presence (closed symbols) of FVIIa (0.5nM) and varied FX or 20nM FX (A; n = 3; data are ± SEM) or a mixture of thrombin (100nM)/FXa (10nM)/FVIIa (5nM) at various dilutions (B; n = 4; data are ± SEM). The number of plaques was determined 24 hours after infection. The data were corrected for the number of plaques detected in the presence of FVIIa alone (0.5nM) or added protease mix, respectively.
In situ FX zymogen activation or combining thrombin/FXa/FVIIa enhances HSV1 infection. HUVECs in SFM were inoculated with the indicated type of HSV1 (4.5 × 105 vp/mL) in the absence (open symbols) or presence (closed symbols) of FVIIa (0.5nM) and varied FX or 20nM FX (A; n = 3; data are ± SEM) or a mixture of thrombin (100nM)/FXa (10nM)/FVIIa (5nM) at various dilutions (B; n = 4; data are ± SEM). The number of plaques was determined 24 hours after infection. The data were corrected for the number of plaques detected in the presence of FVIIa alone (0.5nM) or added protease mix, respectively.
Upstream and downstream coagulation proteases have a cumulative effect on infection
Because HSV1 simultaneously activates coagulation proteases in plasma, the combined effect of multiple purified enzymes was investigated. When thrombin, FXa, and FVIIa were added together during the inoculation period at a fixed molar ratio, infection increased by approximately 1000% (Figure 3B). The maximal enhancement in these experiments corresponded to the sum of the increase in infection seen when the enzymes were evaluated individually at 100nM for thrombin, 10nM for FXa, and 5nM for FVIIa (Figure 2 TF+/gC+). In the most simplistic interpretation, these data suggest that under these conditions, the protease pathways triggered by thrombin and FXa with FVIIa act cumulatively by independent mechanisms to increase infection.
PAR1 and PAR2 are involved in infection
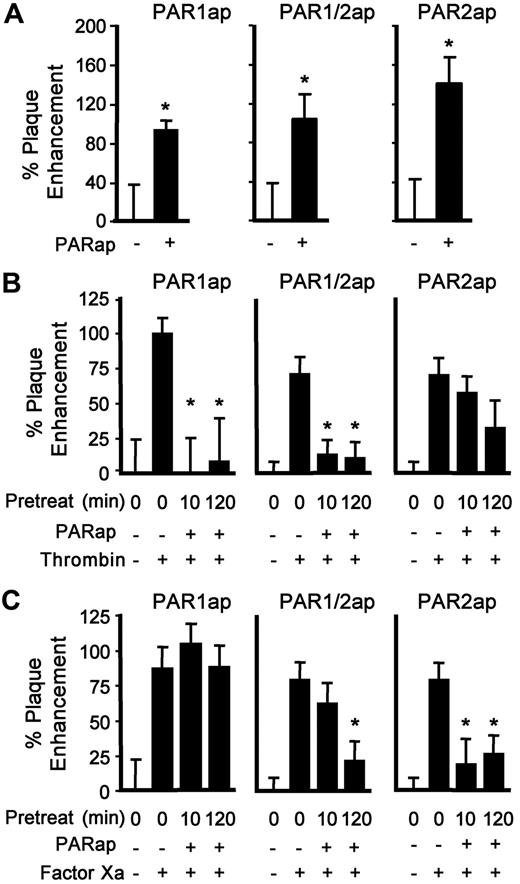
The main pathway of coagulation protease–dependent signaling is through PAR activation.6,32 To eliminate the proteolytic cleavage events and to evaluate PAR involvement in infection of the HSV1 variants prepared here directly, synthetic agonist peptides analogous to the tethered ligand of a cleaved PAR were used. As we have shown previously for other strains of HSV1, infection by the newly developed TF+/gC+ virus was enhanced by PAR1ap and PAR1/2ap treatment of HUVECs (Figure 4A). In addition, PAR2ap increased infection, demonstrating a new role for PAR2-mediated signaling in HSV1 entry.
PAR1ap or PAR2ap enhance infection and receptor desensitization affects coagulation protease-mediated infection. (A) HUVECs were inoculated with HSV1 (TF+/gC+, 4.5 × 105 vp/mL) in the presence of PAR1ap (10μM), PAR1/2ap (10μM), or PAR2ap (200μM) in SFM. The data were corrected for the amount of infection in the absence of added peptide (n = 4; data are ± SEM). *P ≤ .05 compared with no PARap. As in panel A except afterward, thrombin (10nM; B) or FXa (1nM; C) was added for 90 minutes. The data were corrected for the amount of infection without added protease or PARap (n = 6; data are ± SEM). *P ≤ .05 compared with the addition of protease.
PAR1ap or PAR2ap enhance infection and receptor desensitization affects coagulation protease-mediated infection. (A) HUVECs were inoculated with HSV1 (TF+/gC+, 4.5 × 105 vp/mL) in the presence of PAR1ap (10μM), PAR1/2ap (10μM), or PAR2ap (200μM) in SFM. The data were corrected for the amount of infection in the absence of added peptide (n = 4; data are ± SEM). *P ≤ .05 compared with no PARap. As in panel A except afterward, thrombin (10nM; B) or FXa (1nM; C) was added for 90 minutes. The data were corrected for the amount of infection without added protease or PARap (n = 6; data are ± SEM). *P ≤ .05 compared with the addition of protease.
To evaluate the role of individual PARs in protease-supported entry, desensitization with PAR agonist peptides was performed for either 10 minutes or 2 hours. Preincubation with the PAR1ap or PAR1/2ap at 10μM (Figure 4B) inhibited the increase in infection induced by thrombin, but had little effect on the FXa-mediated increase in infection (Figure 4C). After a 2-hour preincubation, thrombin effects remained desensitized. However, the nonselective PAR1/2ap, but not the selective PAR1ap, also attenuated the FXa-mediated increase in infection (Figure 4C), indicating that longer preincubation times with the PAR1/2ap were needed to desensitize PAR2 and implicating PAR2 in the infection-enhancing effects of FXa. When HUVECs were pretreated with the specific PAR2ap for either 10 minutes or 2 hours, followed by the addition of thrombin and virus (Figure 4B), there was insignificant effect on the thrombin-mediated increase in HSV1 infection. In contrast, the PAR2ap significantly decreased the FXa-dependent increase in infection at both preincubation times (Figure 4C). These data showed that the signaling pathway for FXa in virus infection was predominately through PAR2.
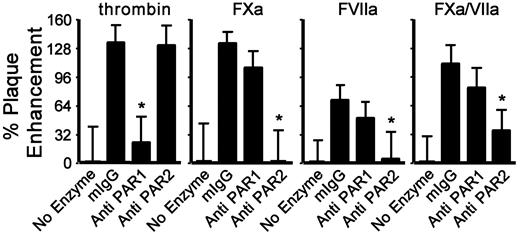
The role of PARs in the thrombin, FXa, and FVIIa effects on the TF+/gC+ virus infection was further evaluated with cleavage-blocking Abs. In agreement with previous studies using other virus strains, the anti-PAR1 Abs inhibited the thrombin-dependent increase in infection (Figure 5), but anti-PAR1 had no effect on the enhancement caused by FXa-, FVIIa-, or FXa/FVIIa-dependent infection. In contrast, a blocking Ab to PAR2 inhibited the FXa-, FVIIa-, and FXa/FVIIa-dependent enhancement of virus infection without affecting thrombin-supported infection (Figure 5). This confirmed the desensitization experiments with PARap by showing that HSV1 uses either PAR1- or PAR2-dependent pathways to enhance protease-specific cell entry.
PAR1 and PAR2 differentially affect coagulation protease-mediated infection. HUVECs were incubated with HSV1 (4.5 × 105 vp/mL, TF+/gC+) and thrombin (10nM), FXa (1nM), FVIIa (2.5nM), or FXa/FVIIa (1nM/0.5nM) with control murine IgG (50nM), control IgG plus enzyme, anti-PAR1 (150nM) plus enzyme, or anti-PAR2 (50nM) plus enzyme. The data were corrected for the amount of infection without added protease in the presence of control IgG (n = 4; data are ± SEM). *P ≤ .05 compared with control IgG plus enzyme.
PAR1 and PAR2 differentially affect coagulation protease-mediated infection. HUVECs were incubated with HSV1 (4.5 × 105 vp/mL, TF+/gC+) and thrombin (10nM), FXa (1nM), FVIIa (2.5nM), or FXa/FVIIa (1nM/0.5nM) with control murine IgG (50nM), control IgG plus enzyme, anti-PAR1 (150nM) plus enzyme, or anti-PAR2 (50nM) plus enzyme. The data were corrected for the amount of infection without added protease in the presence of control IgG (n = 4; data are ± SEM). *P ≤ .05 compared with control IgG plus enzyme.
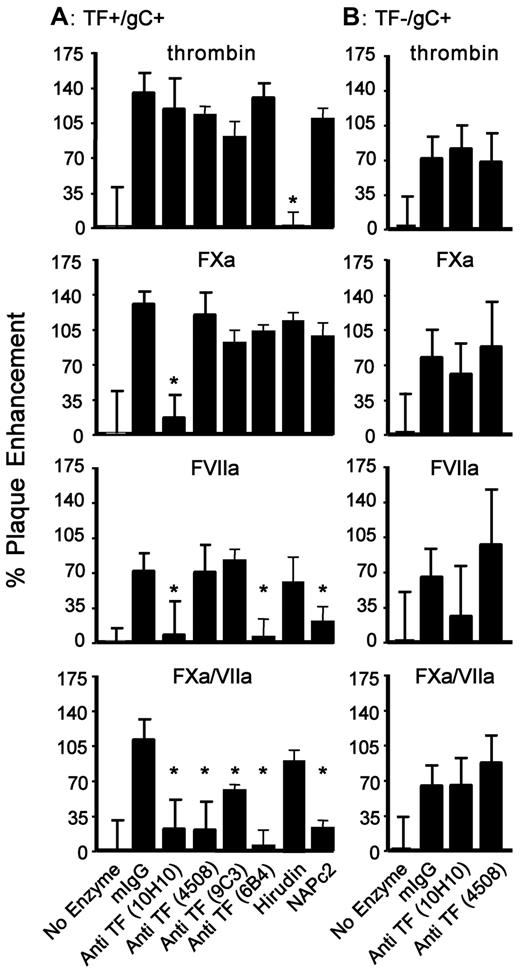
The differential cofactor involvement of TF for the presentation of coagulation proteases to PARs in HSV1 infection is shown in Figure 6A. Anti–TF 10H10 is known to attenuate the PAR2 signaling function of TF without affecting its procoagulant cofactor activity.21 In the present study, anti–TF 10H10 inhibited the enhancement of TF+/gC+ HSV1 infection by FXa alone, FVIIa alone, and FXa/FVIIa. Anti–TF 4508 (called VD8 in the literature) is a known anticoagulant specific for TF residues 1-25.33,34 It had an effect on TF+/gC+ HSV1 infection that was similar to that of anti–TF 9C3, which is competitive for FVIIa binding to TF,35 although nonlinear epitope specificity has precluded localization of its binding site.36 These 2 mAbs inhibited infection only when FXa/FVIIa were both present. With TF association contained within residues 40-83,36 anti–TF 6B4 inhibited the enhancement of TF+/gC+ HSV1 infection due to FVIIa alone or FVIIa/FXa, but not to FXa alone. Therefore, Abs of unique TF epitope specificity blocked HSV1 infection distinguishably, which is consistent with FVIIa and FXa being organized with TF on the virus surface analogous to the ternary coagulation initiation complex. None of the TF Abs altered thrombin-enhanced cell uptake of TF+/gC+ HSV1 significantly. The lack of 10H10 effects on thrombin furthermore indicated a selective role for TF during PAR2-dependent infection mediated by upstream coagulation proteases.
Viral TF is involved in FXa- and FVIIa-mediated HSV1 infection. HUVECs were incubated with a constant amount of TF+/gC+ (A) or TF−/gC+ (B) HSV1 (4.5 × 105 vp/mL) and thrombin (10nM), FXa (1nM), FVIIa (2.5nM), or FXa/FVIIa (1nM/0.5nM) with murine IgG (No Enzyme, 55nM), mIgG plus enzyme (mIgG), or enzyme plus the indicated anti-TF (55nM), NAPc2 (50nM) or hirudin (10 U/mL). The data were corrected for the amount of infection detected without added protease (n = 4; data are ± SEM). *P ≤ .05 compared with control IgG plus enzyme.
Viral TF is involved in FXa- and FVIIa-mediated HSV1 infection. HUVECs were incubated with a constant amount of TF+/gC+ (A) or TF−/gC+ (B) HSV1 (4.5 × 105 vp/mL) and thrombin (10nM), FXa (1nM), FVIIa (2.5nM), or FXa/FVIIa (1nM/0.5nM) with murine IgG (No Enzyme, 55nM), mIgG plus enzyme (mIgG), or enzyme plus the indicated anti-TF (55nM), NAPc2 (50nM) or hirudin (10 U/mL). The data were corrected for the amount of infection detected without added protease (n = 4; data are ± SEM). *P ≤ .05 compared with control IgG plus enzyme.
As expected, an excess of the highly specific thrombin inhibitor hirudin prevented thrombin-enhanced TF+/gC+ HSV1 infection. Hirudin had no effect on the stimulation of infection by FXa alone, FVIIa alone, or FXa/FVIIa (Figure 6A).
NAPc2 is an inhibitor of the ternary TF/FVIIa/FXa complex that binds to a FXa exosite and the FVIIa active site23 and has been shown to inhibit TF-PAR2 signaling pathways in vivo.37 Although NAPc2 inhibits FXa-dependent prothrombin activation,38 it does not block the FXa active site and signaling activity12 and had no effect on FXa alone. Confirming the specificity of this inhibitor, NAPc2 had no effect on thrombin- or FXa-enhanced infection of TF+/gC+ HSV1 (Figure 6A). However, supporting the formation of TF/FXa/FVIIa and TF/FVIIa complexes leading to enhanced virus infection, NAPc2 inhibited FXa/FVIIa- and FVIIa-mediated infection, indicating that in both cases the active site of FVIIa was involved in enhancing infection through PAR2 signaling.
The availability of TF-restricted HSV1 provides a means with which to evaluate the role of viral constituents in PAR-stimulation processes. Like TF+/gC+ HSV1, Figure 6B shows that neither anti–TF 10H10 nor anti–TF 4508 significantly affected thrombin-mediated enhancement of infection for TF−/gC+ HSV1. In sharp contrast, these Abs lost their significant effect on FXa-, FVIIa-, or FXa/FVIIa-mediated enhancement of TF+/gC+ HSV1 infection (Figure 6A) when the TF−/gC+ virus was evaluated (Figure 6B). These data show that TF associated with the virus, and not the target cell, was responsible for the presentation of FXa, FVIIa, and FXa/FVIIa to PAR2.
Discussion
Our earlier work demonstrated that constitutive host cell–derived TF and proPL on the surface of HSV1 and other members of the HV family initiate plasma coagulation.13,14 The thrombin that is generated is used by the virus to enhance infection through PAR1 stimulation of target cells.18 Similar to thrombin, FXa and FVIIa are known to elicit PAR-mediated cell modulation.4,7,39 In the present study, we show that these upstream coagulation proteases contribute to HSV1 cell infection through a distinct mechanism. Indeed, the combination of FXa and FVIIa increased virus infection of HUVECs by activating PAR2, but not PAR1, at the nanomolar concentrations predicted to accumulate physiologically or to drive coagulation in plasma.40,–42
Although TF is integral to virus-initiated coagulation, the HSV1 genome–encoded gC has been shown to bind to FX17 and to enhance FVIIa-dependent FXa generation.16 gC can also facilitate virus uptake by interaction with heparin-sulfated proteoglycans (Figure 7) and complement-mediated immune evasion,43 but our data indicate that a novel evolutionary advantage of gC may be related to the interactions of upstream coagulation proteases. Indeed, gC has pronounced effects on FXa-dependent PAR2 signaling that facilities viral infection and is essential for the additive effects of FVIIa in enhancing FXa's potency of TF-expressing virus particles. Moreover, gC is essential for FVIIa to enhance the infectivity of TF+ HSV1, uncovering an unexpected cofactor for the prototypic TF-FVIIa-PAR2 signaling complex.9 Therefore, a unique mechanism involving cooperative host- and virus-derived receptors in virus infection emerges from these experiments.
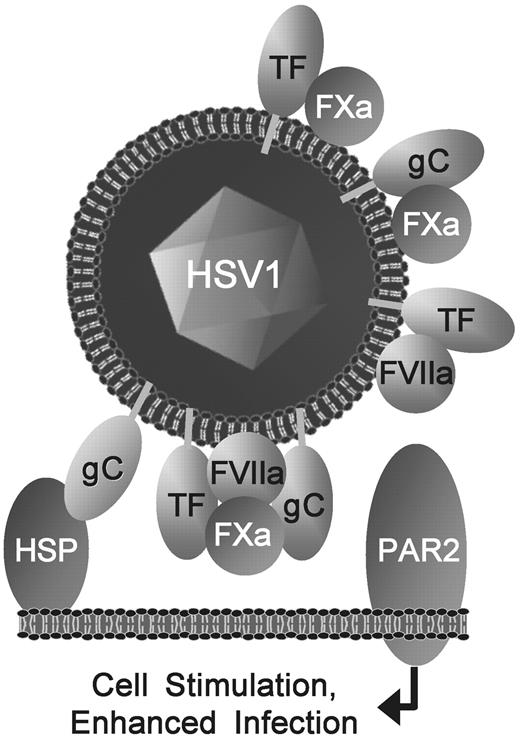
Pleiotropic viral TF and gC enhance FXa- and FVIIa-mediated infection by activating cellular PAR2. TF, gC, and proPL on the HSV1 surface initiate coagulation.14,16,17 Binding of HSV1 to the host cell (eg, via gC-heparan sulfate proteoglycan, HSP), positions FVIIa and FXa proximal to PARs, where viral TF and gC combine with FVIIa and FXa, presumably in a quaternary complex, to enhance infection optimally by stimulating PAR2. A novel gC-FXa binary combination and known TF-FXa and TF-FVIIa also enhance PAR2-mediated infection. In contrast, independently of TF or gC on the virus, thrombin increases HSV1 infection through PAR1 (not shown). Therefore, HSV1 exploits multiple hemostatic functions of TF and gC to augment infection of cells.
Pleiotropic viral TF and gC enhance FXa- and FVIIa-mediated infection by activating cellular PAR2. TF, gC, and proPL on the HSV1 surface initiate coagulation.14,16,17 Binding of HSV1 to the host cell (eg, via gC-heparan sulfate proteoglycan, HSP), positions FVIIa and FXa proximal to PARs, where viral TF and gC combine with FVIIa and FXa, presumably in a quaternary complex, to enhance infection optimally by stimulating PAR2. A novel gC-FXa binary combination and known TF-FXa and TF-FVIIa also enhance PAR2-mediated infection. In contrast, independently of TF or gC on the virus, thrombin increases HSV1 infection through PAR1 (not shown). Therefore, HSV1 exploits multiple hemostatic functions of TF and gC to augment infection of cells.
The results presented here also show that PAR1 and PAR2 mediate distinct and additive effects on viral infectivity. Whereas thrombin promotes infection in a manner dependent on PAR1 but independent of virus-expressed TF or gC, optimal FXa- and FVIIa-enhanced virus infection is remarkably dependent on TF and gC and facilitated by PAR2. In addition, a distinct and independent role for gC is uncovered here with the newly developed TF-deficient virus particles, demonstrating that gC can serve as a cofactor for FXa to mediate PAR2-dependent cellular responses in endothelial cells.
Resting HUVECs express little or no functional TF, but constitutively express PARs. The present study provides evidence that HSV1 TF can substitute for host-cell TF by assembling coagulation protease signaling complexes directly. The virus may then present these signaling enzymes to the cell-surface PARs, promoting infection. Whereas these data implicate TF and its ligands, alternative protease-presenting cofactors may be incorporated in the virus envelope to mediate or enhance cell signaling. For example, FXa-mediated PAR signaling has been shown recently to be facilitated by cell-surface annexin II,44 which we have shown previously to be present on several HVs13,45,46 and to be triggered by thrombin to the HUVEC surface.47 Furthermore, Annexin II has been implicated in HV cell entry and may therefore serve a dual function as a virus receptor and a facilitator of signal modulation leading to enhanced virus entry into the host cell.45,–47 Other cell-derived PAR cofactors, such as endothelial protein C receptor,48 may be recruited to the virus surface during the envelopment process and, therefore, the virus may exploit several protease-cofactor pairs to mediate signal-enhanced infection in vivo.
Once attached to the cell surface, HSV1 assembles a proteolytic complex of TF/FVIIa/FXa/gC in proximity to PAR2 in an environment that protects the enzymes from rapid inactivation by circulating inhibitors. These low levels of virus-bound proteases and localized thrombin production would restrict cell-surface PAR activation to prepare the host cell for infection without acute propagation of coagulation. A previous study supports this model in vivo, showing that NAPc2 significantly reduced Ebola virus titer and enhanced the survival of Rhesus monkeys from the effects of hemorrhagic fever.49 Although we have not yet conducted analogous experiments for HSV1 in vivo, NAPc2 was highly effective in inhibiting FVIIa/FXa-enhanced infection of cultured cells. Therefore, presentation of the upstream coagulation proteases by the virus to cellular PARs is not only an integrated part of the HSV1 entry process, but may constitute a generalized pathway for enveloped virus infection. The demonstration that proteases can be discretely presented by cofactors such as TF on a virus to cells expressing PAR2 suggests that a similar mechanism may be considered in a broader scope for cofactor-bearing cells.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Harvey Friedman for generously providing the parental HSV1 strains.
This work was supported by the Heart and Stroke Foundation of British Columbia and Yukon (to E.P.) and by infrastructure support from the Canadian Foundation for Innovation and the Michael Smith Foundation awarded to the University of British Columbia Center for Blood Research.
Authorship
Contribution: M.R.S. performed the experiments; and M.R.S., W.R., and E.L.G.P. designed the research, analyzed the results, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Edward L. G. Pryzdial, Canadian Blood Services, Centre for Blood Research, University of British Columbia, 2350 Health Sciences Mall, Vancouver, BC, V6T 1Z3 Canada; e-mail: ed.pryzdial@blood.ca.