In this issue of Blood, Laidlaw and colleagues show that in subjects with aspirin exacerbated respiratory disease (AERD), dysregulated platelet-leukocyte cross-talk may be partly responsible for the respiratory tissue inflammation and overproduction of cysteinyl leukotrienes, providing new clues for the treatment of the disease.1
In some individuals the ingestion of aspirin and other nonsteroidal anti-inflammatory drugs (NSAIDs) induces AERD.2 NSAIDs are a chemically heterogeneous class of drugs that act by reducing the biosynthesis of prostanoids for their inhibitory effect on cyclooxygenase (COX)–1 and COX-2 activity.3 The mechanism of NSAID sensitivity is not immunologically mediated but seems related to their ability to interfere with arachidonic acid (AA) metabolism. Because the COX product prostaglanding (PG)E2 has beneficial anti-inflammatory effects in the lung,4 numerous studies have been performed to assess whether this prostanoid was reduced in AERD. However, no in vivo study has found diminished levels of PGE2 at baseline in aspirin-sensitive patients. Thus, it is difficult to draw any firm conclusion on this hypothesis.
In contrast, numerous studies consistently showed that AERD is associated with an excessive production of cysteinyl leukotrienes (cys-LTs).2 The cys-LTs are active compounds with smooth muscle–stimulating and edema-inducing properties that are thought to contribute to several of the characteristic features of AERD.5 They comprise LTC4, D4, and E4. LTC4 is formed from AA through the activity of 5-lipoxygenase (5-LO) that catalyzes a 2-step reaction leading to the generation of the unstable intermediate LTA4 that is further converted, by LTC4 synthase (LTC4S), to the glutathione conjugate LTC4. The other cys-LTs are formed by hydrolytic removal of γ-Glu and Gly from LTC4 (yielding LTD4 and LTE4) by the activity of enzymes present in plasma (see figure). Leukocytes express the complete enzymatic machinery necessary to generate cys-LTs.5 However, in some circumstances cells that do not express the complete enzymatic repertoire of eicosanoid biosynthesis can use an intermediate product generated and released from another cell type to complete the conversion into the biologically active mediator; this phenomenon is called trans-cellular biosynthesis.6 However, the detailed cellular and molecular determinants controlling eicosanoid transcellular generation in vivo in health and disease have not been fully elucidated. Laidlaw et al have found that the adhesion of platelets to leukocytes contributes to the higher level of cys-LTs generated in vitro by activated granulocytes isolated from subjects with AERD compared with aspirin-tolerant controls.1 Because platelets express LTC4S but not 5-LO, it is plausible that enhanced LTC4 biosynthesis occurred by transcellular metabolism of AA through platelet-leukocyte cross-talk.7 In addition to this mechanism, activated platelets can induce LTC4 biosynthesis in leukocytes through the release of soluble products, such as free AA and granulocyte-macrophage colony stimulating factor (GM-CSF), which can activate 5-LO enzyme.8
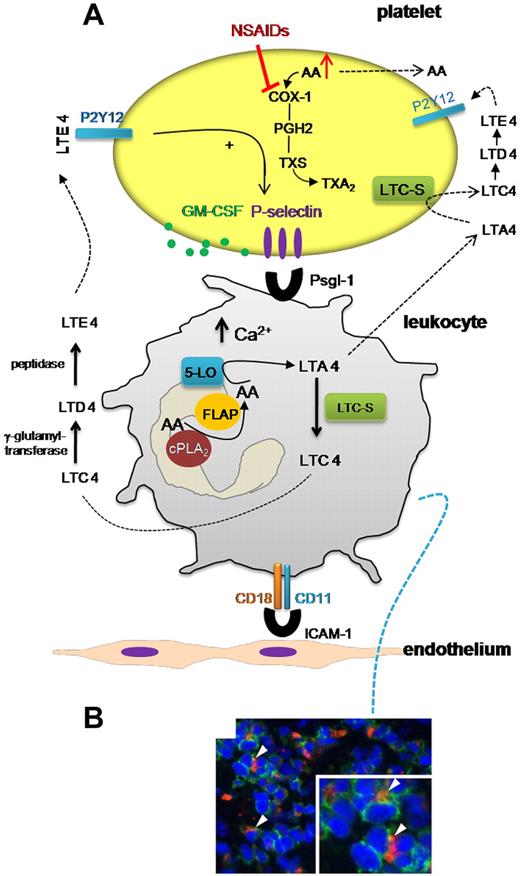
Inside platelet-leukocyte cross-talk in aspirin exacerbated respiratory disease. (A) In AERD, enhanced propensity of platelets to adhere to leukocytes translates into increased biosynthesis and release of LTC4 that is metabolized to LTE4 by the activity of enzymes present in plasma. LTE4 may interact with platelet P2Y12 receptor, thus inducing platelet P-selectin expression and facilitating the formation of platelet-leukocyte aggregates. Other platelet products, such as GM-CSF, can be released and may activate leukocyte 5-LO and induce a pro-adhesion phenotype and prolong the survival of eosinophils. As shown in panel B (from Figure 1 in the article by Laidlaw et al beginning on page 37901 ), nasal polyps from subjects with AERD contained many extravascular platelets that co-localized with leukocytes. In this scenario, NSAID treatment may increase the biosynthesis of cys-LTs. NSAIDs inhibit platelet COX-1 that can cause the accumulation and release of free AA that can be taken up by leukocytes thus inducing 5-LO translocation and enhancing cys-LT generation. PGH2 indicates prostaglandin-H2; and TXS, thromboxane synthase.
Inside platelet-leukocyte cross-talk in aspirin exacerbated respiratory disease. (A) In AERD, enhanced propensity of platelets to adhere to leukocytes translates into increased biosynthesis and release of LTC4 that is metabolized to LTE4 by the activity of enzymes present in plasma. LTE4 may interact with platelet P2Y12 receptor, thus inducing platelet P-selectin expression and facilitating the formation of platelet-leukocyte aggregates. Other platelet products, such as GM-CSF, can be released and may activate leukocyte 5-LO and induce a pro-adhesion phenotype and prolong the survival of eosinophils. As shown in panel B (from Figure 1 in the article by Laidlaw et al beginning on page 37901 ), nasal polyps from subjects with AERD contained many extravascular platelets that co-localized with leukocytes. In this scenario, NSAID treatment may increase the biosynthesis of cys-LTs. NSAIDs inhibit platelet COX-1 that can cause the accumulation and release of free AA that can be taken up by leukocytes thus inducing 5-LO translocation and enhancing cys-LT generation. PGH2 indicates prostaglandin-H2; and TXS, thromboxane synthase.
In AERD, the systemic biosynthesis of LTC4 in vivo, as assessed by measuring urinary levels of LTE4, was also increased and correlated strongly with percentages of circulating platelet-adherent granulocytes.1 Interestingly, it was found that platelet-adherent subsets of leukocytes had higher expression of several adhesion markers, such as CD11b/CD18 (MAC-1), than did platelet nonadherent subsets.1 Altogether, these findings suggest that AERD is associated with enhanced adhesion of circulating platelets to leukocytes and this event may trigger enhanced cys-LT biosynthesis and induce a pro-adhesive phenotype in leukocytes thus facilitating respiratory tissue inflammation (see figure).
Enhanced circulating LTE4 levels can mediate pulmonary inflammation (mucosal eosinophilia and airway hyperresponsiveness),2 but this effect is not dependent on the activation of the type 1 and 2 receptors for cys-LTs (CysLT1R and CysLT2R). In contrast, the activation of the purinergic (P2Y12) receptor (the target of the thienopyridine antiplatelet drugs) by LTE4 seems to play a role.9 The finding of Laidlaw et a1 that steady-state urinary excretion of LTE4 correlates with the frequencies of platelet-adherent leukocytes in the peripheral blood may suggest a possible contribution of LTE4 in the formation of platelet-leukocyte aggregates through the activation of platelet P2Y12 and P-selectin expression.1 This hypothesis might be addressed by testing the effects of thienopyridines on the formation of circulating platelet-leukocyte aggregates and correlating it with the clinical outcomes in this setting. Further studies are required to identify the mechanisms involved in enhanced propensity of platelets of AERD to adhere to leukocytes compared with those of aspirin-tolerant individuals.
The administration of NSAIDs may cause the accumulation of free AA, in platelets, for their inhibitory action on COX-1 activity. The presence of dysregulated platelet-leukocyte cross-talk may facilitate the transfer of platelet AA to leukocytes thus inducing 5-LO translocation and increasing cys-LT biosynthesis8 (see figure panel A). Increased circulating levels of LTE4 can then contribute to enhanced formation of platelet-leukocyte aggregates possibly through the activation of platelet P2Y12. Activated platelets may cause eosinophil accumulation in respiratory tissue by inducing a pro-adhesive phenotype of eosinophils and prolonging their survival, possibly through the release of GM-CSF10 (see figure panel B).
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■