Abstract
Follicular lymphoma (FL) is a B-cell tumor arising in germinal centers and retaining features of its normal B-cell counterpart. Lymphomagenesis appears stepwise from the t(14;18) translocation, through FL-like cells, to FL in situ, then to overt FL. Surface Ig is mandatory and carries a striking V-region modification because of introduction of glycan addition sites during somatic mutation. These are positively selected and acquire unusual high mannoses, which interact with lectins. The Ig-associated mannoses appear essential for FL, providing a disease- specific target for antibody attack. Antibody therapy is currently focused on anti-CD20 (rituximab), which appears to rely predominantly on the Fcγ module recruiting suitably activated macrophages. Immunogloblulin and, to some extent, CD20, can each escape antibody attack in vitro by modulation, but this is difficult to demonstrate clinically. Instead, studies of anti-CD20 therapy of FL suggest that effector modulation, similar to that seen in the suppression of autoimmune inflammation by infusions of normal human IgG, may be important. Both antigenic and effector modulations might be minimized by repeated small doses of more potent antibodies. Clearly, mechanisms of attack vary with the malignancy, the target molecule, and the antibody design, offering opportunities for optimizing this promising strategy.
Introduction
Antibody is now a major contributor to the treatment of B-cell malignancies. However, there remains a pressing need to understand better how to encourage the destruction of antibody-coated neoplastic cells. We direct our attention to human follicular lymphoma (FL), to its pathogenesis and a therapeutic opportunity this reveals. We focus on 2 of its cell-surface antigens: first, the key receptor of B cells, surface immunoglobulin (Ig), which has a renewed target potential; and second, an established target, CD20. Antibodies against each molecule are clinically effective but may differ in the mechanism of killing. They differ in susceptibility to endocytosis/modulation after engagement by antibody and illustrate the need to understand the behavior of the target molecule to optimize strategies for attack.
Development of FL
FL occupies the immune system, surviving and proliferating mainly in the germinal centers (GCs) of lymph nodes. The architecture of normal GCs tends to be retained, with lymphoma cells located in a network of follicular dendritic cells, follicular CD4+ T cells, and stromal elements. For normal B cells, GCs are the sites where Darwinian evolution occurs with binding to antigen being the positive selector and death awaiting nonselected B cells. To persist in this hostile environment, FL cells need to escape the default death pathways. One protective factor evident in the majority of cases is the up-regulated expression of the antiapoptotic BCL2 protein via the t(14;18)(q32;q21) translocation. The translocation appears to occur principally as an error during Ig VDJ recombination in the bone marrow, which places the BCL2 proto-oncogene under the control of the IgH enhancers on the nonexpressed IgH allele. Perhaps not surprisingly, translocational errors are relatively common in normal B cells undergoing potentially dangerous genetic recombination and somatic mutational processes1 and reveal the price to be paid for the production of high-affinity antibody. Fortunately, they represent only one step in lymphomagenesis; and, in the case of t(14;18), the “seed” cells can proceed through maturation and exit the GC without necessarily generating FL.2
Subsequent steps to FL are less clear, although pathologists do occasionally detect BCL2-positive FL, termed FL in situ (FLIS), as an incidental finding in reactive lymph nodes.3 Because FLIS shows a very low rate of progression, it may represent a stage en route to fully developed lymphoma. FL is therefore a spectrum of disease stages, ranging from FLIS to “overt” FL, the latter divided into clinical stages based on the relative proportion of centrocyte- and centroblast-like cells. FL appears to evolve along complex progression pathways,4 with diffuse areas of growth coexisting with follicular structures. In some cases, transformation to diffuse large B-cell lymphoma occurs.5-7 Genome sequencing is revealing mutational events in a proportion of cases of FL, with several located in histone-modifying genes.8 It will be of interest to determine whether these are present in early disease and might therefore contribute to lymphomagenesis.
Importance of surface Ig
One feature of normal B cells that is maintained by FL cells is the expression of surface Ig (sIg). This is despite the loss of one IgH allele by translocation and potential damage to the functional allele by somatic mutation. It indicates an essential function for the B-cell receptor, expected for normal B cells9 but apparently also required for FL cells. Targeting this molecule is attractive and might explain the efficacy of anti-idiotypic (Id) antibody as a treatment for FL.10 The logistical challenges of making individual anti-Id antibodies has so far inhibited this promising approach. However, our recent finding that there is a lymphoma-specific modification of sIg in FL may reopen this strategy. It appears that lymphomas of the GC acquire sequence motifs in the Ig variable regions for glycan addition.11 The motifs are introduced during somatic mutation and the glycans added are unusual.12 Not only does this raise intriguing questions about the role of the glycans in the lifestyle of FL, but it exposes a possible Achilles heel. Targeting the lymphoma-specific glycans with a single antibody could offer an intriguing and feasible way of attacking sIg without having to make individual anti-Id antibodies.
Approaches to antibody therapy
FL is clearly vulnerable to antibody therapy. Active vaccination against Id has long been attractive13 and has clinical effects14-16 but faces the problem of inducing variable immunity in a damaged setting. Passive antibody can be manufactured and injected under controlled conditions and is clearly capable of penetrating FL sites.17 Anti-CD20 has shown great success, especially in concert with chemotherapy.18
In general, antibody therapy operates by one or more of 3 mechanisms: direct cytotoxicity caused by engagement of the target-cell surface, recruitment of antibody-dependent cellular cytotoxicity (ADCC), and activation of complement. In contrast to attacks on surface Ig of FL,19 there is inconsistent evidence for direct cytotoxicity in anti-CD20 therapy, although it possibly has a role under certain circumstances20 and with some antibodies.21 Strong evidence from mouse models, supported by clinical observations, suggests that the major mechanism killing anti-CD20–coated FL cells is ADCC effected by macrophages. We discuss this later. An activated complement cascade, unlikely by itself to cause significant lysis of target cells,22 could be important in promoting macrophage activity.23
Antibodies could be improved to exploit current knowledge, but we are impeded by the complexities of antigenic and effector modulation. We think that these problems should be approached with knowledge gained from animal models tempered by invaluable clinical experience.10,24
This perspective aims to bring together new concepts relating to the pathogenesis of FL, refocusing on the critical Ig receptor. Targeting sIg with new antibodies would be attractive, and increased understanding antibody-mediated mechanisms of elimination should allow refinement of the present therapeutic strategies.
Surface Ig in FL
The IgV genes of the majority of FL cases have undergone somatic hypermutation (SHM), which apparently continues after transformation, generating intraclonal variants with some differences in mutational patterns. Although SHM can introduce potentially dangerous aberrant mutations in non-Ig genes, such as SOCS-1,25 this phenomenon appears to be relatively uncommon in FL. Even without further detectable genetic abnormalities, the GC stage of FL appears to have acquired lymphomagenic properties, with no evidence for a pre-GC stem cell.26 However, the route to FL is not completely fixed because occasional codevelopment of other clonally related lymphomas, such as Hodgkin disease, can occur.27
The majority of FL cases express sIgM(D), with an estimated 20% to 30% having undergone isotype switch to IgG or IgA. In the absence of a large study, this figure remains uncertain. The level of SHM, which is high overall, tends to be relatively higher in isotype-switched cases, reflecting the pattern in normal post-GC B cells.28 Despite the intraclonal variation in SHM evident within FL clones, and, in some cases, the presence of activation-induced cytidine deaminase, there is no apparent increase in mutational load over time but rather an expansion or contraction of subclones.28 The picture of FL is of a GC B cell with up-regulated BCL2 largely arrested in a site where normal B cells, or early FL-like B cells with the same translocation, do not linger. Although FL cells have some ability to circulate and spread to other lymphoid sites,2 the question is how the majority are retained in the GC, and how these sIg-expressing cells are able to subvert microenvironmental influences for their own advantage.
Modification of sIg variable region sequences in GC-derived lymphomas
The distinguishing structural feature of the Ig of FL cells is the presence of sequence motifs in the IgV regions, which act as acceptor sites for N-addition of glycan chains.11 The motifs, introduced by SHM and found in virtually all cases, are Asn-X-Ser/Thr (N-X-S/T), where X can be almost any amino acid except proline. As shown in supplemental Figure 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article), 1 or more can be detected in IGHV sequences of FL, often in CDR2, with others found in IGLV.11 Motifs are also found in other GC-derived lymphomas, such as endemic Burkitt lymphoma, and in a proportion of diffuse large B-cell lymphoma.11,29 Because they are rare in normal B cells, in myeloma, and in the mutated subset of chronic lymphocytic leukemia, this modification appears to characteristic of GC-located tumors. Presumably, SHM generates similar sites in all V genes, regardless of the cell of origin; therefore, their presence in GC-derived lymphomas probably reflects positive selection. Negative selection in normal B cells is an alternative possibility, but this argument would have to apply to sIg-negative myeloma and to nonfunctional V genes, making it less likely. In contrast, the importance of the motifs for FL cells is underlined by their conservation during ongoing somatic mutation.30
Unusual nature and function of the IgV-region glycan
As expected, glycans are added to the motifs in vitro, confirming functionality12 and, importantly, are present on the sIg of FL cells.31 An unexpected finding was that the added glycans differ from those typical of cell surface glycoproteins, in not acquiring branched sugars but being limited to high mannose.12 Perhaps because of steric hindrance in the V region, further sugars are not added, leaving mannosylated glycan exposed in the FL antigen-binding sites. We speculated that this might allow sIg of FL to interact with lectins in the GC.
C-type (Ca+2-dependent) lectins are pattern recognition receptors, which bind to glycans expressed by pathogens. Lectin-glycan binding is also important in endogenous cell-cell interactions, including those within the immune system involved in cell adhesion, trafficking, and in antibody-mediated functions.32 During B-cell development, an N-linked glycosylation site in the first constant region of the μ-chain is an absolute requirement for a functional pre-B cell receptor.33 Glycan modification of B- and T-cell receptors occurs in the Golgi apparatus by transferase-mediated linkage of terminal sugars. Interestingly, blockage of this step in T cells has been shown to increase clustering of the T-cell receptor at the cell surface, apparently because of release from a galectin-3 lattice.32 The consequent lowering of the threshold of activation is one example of control exerted by lectins on lymphocyte function. Modulation of B-cell receptor activity is already known to involve lectin properties of CD22, an inhibitor of B-cell activation, whose function is controlled by binding to specific sialic acid-terminating glycans.34
We approached the question of interaction between sIg-expressed mannoses and lectins by showing that the candidate lectins, mannose-binding lectin and DC-SIGN, could bind to FL-derived mannosylated Ig in vitro. More significantly, the lectins also bound to primary FL cells and signaled via sIg leading to a rise in intracellular Ca2+.31 No signal was mediated in normal B cells, although both normal B and FL cells could signal equally well with anti-IgM. In terms of potential lectin-bearing cells in the GC, we demonstrated that monocyte-derived dendritic cells could bind to mannosylated FL Ig via DC-SIGN (dendritic cell-specific ICAM-3–grabbing nonintegrin), CD209.31 Thus, a hypothetical bridge could be constructed between a lectin-expressing stromal cell and an FL cell that would signal via the mannosylated sIg.
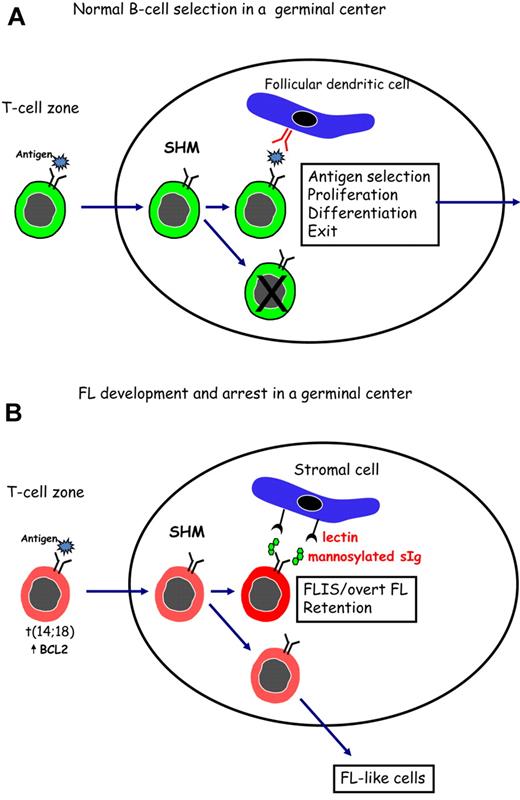
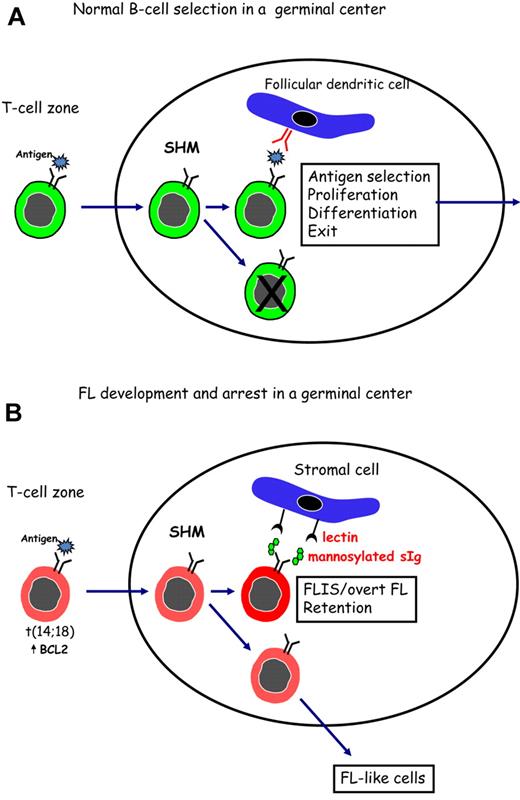
Figure 1 indicates a possible way in which FL cells might subvert the process of antigen selection, which occurs during normal B-cell development in the GC. In Figure 1A, normal B cells are shown undergoing SHM in the dark zone of the GC. Antigen, held as immune complexes on follicular dendritic cells, then selects B cells with higher affinity for interaction with CD4+ T cells, followed by proliferation, differentiation, and exit. Although it is likely that a conventional antigen could stimulate B cells with t(14;18), this would not permit long-term residence in a hostile site. To rescue FL cells and allow persistence in the GC, an alternative driver able to stimulate the B-cell receptor of all FL cases, is proposed. This could involve a lectin-mannosylated sIg interaction (Figure 1B) and may be the reason for retention of signal-competent sIg by FL cells.
Surface Ig-mediated events occurring in normal B cells or in FL B cells after entry into the GC. (A) Normal B cells encounter antigen in the T-cell zone and then locate in a GC where SHM, antigen selection, and isotype switching occur. Antigen, held as immune complexes on follicular dendritic cells, selects B cells of appropriate affinity. After interaction with specific T cells, selected B cells proliferate and differentiate before leaving the site to develop into memory B cells or plasma cells. (B) B cells that have undergone the t(14;18) translocation can respond to antigen and undergo SHM in a GC. Introduced N-glycosylation motifs in the Ig variable regions are positively selected by FL cells. Interaction of the added mannoses with lectins can occur and might provide a foundation for development of FLIS or overt FL in the GC site. B cells that do not acquire N-glycosylation motifs can exit, and possibly revisit, the GC.
Surface Ig-mediated events occurring in normal B cells or in FL B cells after entry into the GC. (A) Normal B cells encounter antigen in the T-cell zone and then locate in a GC where SHM, antigen selection, and isotype switching occur. Antigen, held as immune complexes on follicular dendritic cells, selects B cells of appropriate affinity. After interaction with specific T cells, selected B cells proliferate and differentiate before leaving the site to develop into memory B cells or plasma cells. (B) B cells that have undergone the t(14;18) translocation can respond to antigen and undergo SHM in a GC. Introduced N-glycosylation motifs in the Ig variable regions are positively selected by FL cells. Interaction of the added mannoses with lectins can occur and might provide a foundation for development of FLIS or overt FL in the GC site. B cells that do not acquire N-glycosylation motifs can exit, and possibly revisit, the GC.
Signaling via sIg is clearly a critical influence on the behavior of FL cells.35 Analysis of sIg-mediated signaling in vitro has revealed that most FL cells have functional pathways. However, a subset of the FL clone exists in some patients with apparently down-regulated membrane proximal signaling pathways, presumably after events in vivo. Down-regulation was linked to a reduced expression of CD20 and was reversible after inhibition of negative regulators. Interestingly, the proportion of FL cells with these features was associated with a poorer prognosis, suggesting that the number of cells that have undergone B-cell receptor signaling in vivo may be an indicator of lymphoma progression and of resistance to therapy.35 One possibility is that the signal in vivo results from the lectin-mannosylated sIg bridge.
Candidate lectin-expressing stromal cells
If sIg signaling is mediated via a lectin in the GC, we speculate as to which cells express relevant lectins. Candidates include macrophages, dendritic cells, and follicular dendritic cells. Although, as for other tumors,36 stromal cells probably contribute multiple factors to support FL cells, direct interaction with sIg may be a novel lymphoma-specific pathway. An interesting insight from solid tumors is that mannose receptors are up-regulated on tumor-associated macrophages, and that binding to partner glycans leads to an M2 profile with an increase in IL-10 and a decrease in the Th1-attracting chemokine CCL3.36 This situation may be mirrored in FL where increased levels of IL-4 would indicate a predominance of Th2 cells.37
For FL, initial gene expression profiling implicated genes associated with macrophages and dendritic cells as a bad prognostic factor.38 Because these are lectin-expressing cells, this could be advantageous for FL cells. However, further profiling data added complexity to the links between macrophages and prognosis.39 Similar complexity surrounds the role of T-cell infiltration, initially considered to be a good prognostic factor.38 Surprisingly, this appears to be the case for FOXP3+ Treg,40 but the impact of other T-cell subsets remains controversial.39 Perhaps this is inevitable given that prognosis includes both pathogenesis and response to treatment, and the factors influencing each might be operating in different directions. More immunohistochemical data on the nature and maturational status of the candidate stromal cells, and on T-cell subsets, in early untreated FL are clearly required.39
Antibody attack on sIg
The ability of anti-Id antibodies to suppress FL is well documented.10 Indeed, the only way a patient's FL clone could “hide” from injected mAb was by outgrowth of an Id-negative sIg-positive variant,41 demonstrating both efficacy of the single-mouse mAb in FL and the need for retention of sIg. Because of technical challenges in raising anti-Id antibodies, the approach of active vaccination was developed. It soon became clear both from models and from clinical data in FL that, although T-cell responses are induced and can act as effectors,42 the main mediator of protection induced by Id vaccination is antibody.43,44 Direct apoptosis mediated via sIg signal transduction45 may be one component of this.19
For normal GC B cells, survival is dictated by the strength of interaction with antigen located on follicular dendritic cells, with the subsequent processes of terminal differentiation or recycling ability dependent on CD4+ T cells.46 Our studies have shown that, in the absence of T-cell help, memory B cells are highly susceptible to antigen-induced death in vivo with escape only possible for low-affinity B cells.47 We do not yet know how FL cells might gain sustenance, and not death, via interaction of sIg with local lectin-bearing cells, but this again could reflect a low affinity. If this is the case for FL, engaging sIg with high affinity anti-Id or antimannosylated sIg could provide effective therapy. An alternative approach would be to develop a blocking nonagonistic antibody, which would interrupt the sustaining interaction and possibly detach the FL cells from their protective niche.
Antibodies against the common mannosylated sites expressed by sIg of FL may therefore mediate direct killing supplemented by indirect attack similar to that mediated by anti-CD20. Fortunately, antibodies against mannoses are being actively raised in a different context by investigators in the field of HIV. This virus expresses a shield of host-derived oligomannose glycans, which protect the envelope protein gp120 from antibody recognition.48 Raising antimannose neutralizing antibodies that penetrate this shield is the goal, and such antibodies could have relevance for FL.
Effectors killing antibody-coated tumor cells
After the importance of ADCC in killing antibody-coated tumor cells had been long suspected,49 decisive papers22,50,51 appeared confirming this and identifying macrophages as the probable major effectors. In a variety of tumor models in genetically manipulated mice, antibody therapy was found to require activating FcγR and was enhanced by absence of the inhibitory FcγRIIB.51
Among candidate cells mediating ADCC are macrophages, granulocytes, dendritic cells, NK cells, and some γδ T cells.52 All express activating FcγR, but only macrophages, granulocytes, and dendritic cells express inhibitory FcγRIIB. Three pieces of evidence favor macrophages as the predominant effectors: the heightened therapeutic effect of antitumor antibodies in FcγRIIB−/− mice,51 the role of monocytes/macrophages in depleting normal B lymphocytes in mice injected with mouse anti-CD20 antibody,53-55 and the ubiquity of macrophages, including their predominance among cells infiltrating human tumors.56 Mechanisms used by the macrophages remain undetermined. Synapses between macrophages and tumor targets in mice were reported to be multifocal, involving accumulated actin, FcγR, and phospho-tyrosines. No phagocytosis was reported.57
The role of ADCC in the response of FL to rituximab was investigated by determining in a group of patients the allotype of FcγRIIIA, the FcγRIII species located on monocytes/macrophages and NK cells.58 The 158V allotype has a higher affinity for IgG1 and mediates ADCC better than the alternative 158F. Superior response rates were seen in V/V compared with V/F or F/F subjects, a finding consistent with a major role for macrophage-mediated ADCC. The finding was soon confirmed.59
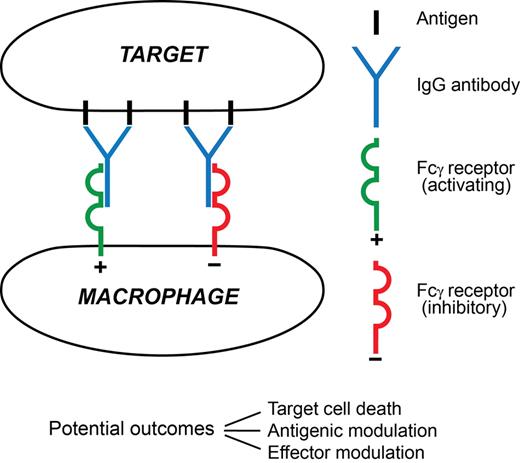
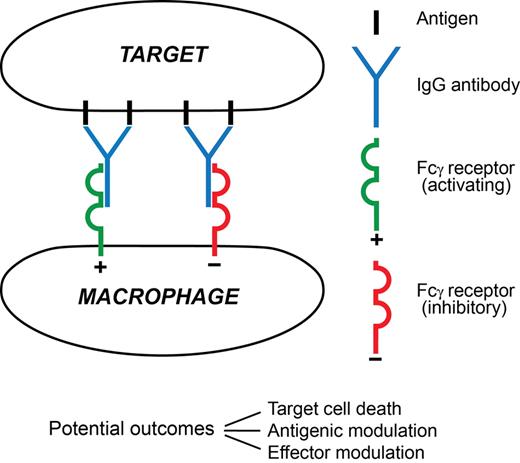
A macrophage attack on an antibody-coated tumor cell and the major sequelae visualized in this paper are summarized in Figure 2.
A synapse between an antibody-coated target cell and a macrophage effector. In this minimal depiction 4 surface antigen molecules are engaged by 2 IgG antibody molecules. One has docked with an activating FcγR (typically FcγRIIIA), the other with an inhibitory FcγR (FcγRIIB), on the surface of the effector. Three possible major outcomes between the cells are listed. Among major determinants of outcome are the extents of signaling from each FcγR and the prevailing mediator environment. The 3 outcomes are not mutually exclusive, for example, the target cell might die despite extensive antigenic modulation, and the encounter will influence effector modulation by altering the local mediator environment. Much remains to be learned about this type of synapse.
A synapse between an antibody-coated target cell and a macrophage effector. In this minimal depiction 4 surface antigen molecules are engaged by 2 IgG antibody molecules. One has docked with an activating FcγR (typically FcγRIIIA), the other with an inhibitory FcγR (FcγRIIB), on the surface of the effector. Three possible major outcomes between the cells are listed. Among major determinants of outcome are the extents of signaling from each FcγR and the prevailing mediator environment. The 3 outcomes are not mutually exclusive, for example, the target cell might die despite extensive antigenic modulation, and the encounter will influence effector modulation by altering the local mediator environment. Much remains to be learned about this type of synapse.
To investigate the role of complement in rituximab-treated FL, a correlation was sought60 between tumor responses and expression by target cells of the complement inhibitors CD46, CD55, and CD59. No correlation was found. However, without directly causing lysis, an activated complement cascade could usefully promote macrophage ADCC, as shown by the anaphylatoxin C5a increasing the ratio of activating to inhibitory FcγR on macrophages.23 ADCC mediated by NK cells can actually be impeded by complement-derived C3b on the target-cell surface.61 It is doubtful that this would apply to ADCC mediated by macrophages, which, unlike NK cells, possess surface receptors for C3b.
Antigenic modulation
Antigenic modulation is the disappearance from a cell surface of an antigen under immunologic attack. It was first reported62 in 1963 for mouse thymus leukemia (TL) antigens belonging to the MHC class Ib family. Leukemia cells bearing TL were seen to survive passaging in hosts displaying anti-TL antibody from a previous immunization. Recovered cells were apparently TL-negative as judged by resistance to lysis by anti-TL antibody and complement but regained positivity when repassaged in the absence of antibody. It was later shown that the initial exposure to an antibody led to rapid movement of surface antibody-antigen complexes to form patches and caps, from both of which endocytosis occurred.63 Sparseness of modulated surface Ig was seen to be prolonged by a diminished delivery of newly synthesized molecules to the surface.64
Recently, it has been recognized that much of the removal of immune complexes in antigenic modulation might in some cases be accomplished by trogocytosis,65 implying the nibbling off of immune complexes by phagocytes attached to them via their Fc receptors.66 This could be followed by the phagocyte endocytosing the immune complexes plus Fc receptors, leading to appreciable loss of receptors from the phagocytic surface. Unfortunately, trogocytosis is difficult to observe in vivo, but there is good evidence for its occurrence.67,68
Modulation of surface Ig
Some variables involved in antigenic modulation are illustrated by surface IgM on B lymphocytes. Polyclonal anti-Ig in vitro led to most of the surface Ig on normal mouse B lymphocytes being endocytosed after 10 minutes at 37°C, and this was even faster for guinea-pig leukemic B lymphocytes.69,70 Subsequent experiments with monoclonal anti-Id IgG antibodies yielded slower modulation than with polyclonal anti-Id. But in vivo, modulation by monoclonal anti-Id became comparable in speed with that given by polyclonal preparations (with some uncertainty because of heavy sequestration of cells from the blood). This increase was apparently the result of interactions involving the antibody Fcγ, as it was not seen in the slow modulation induced by antibody F(ab′γ)2.71 Whether the faster modulation in vivo is the result of interaction with Fcγ receptors providing a signal to the antibody-coated cell, or to a large degree of trogocytosis, is difficult to determine and could vary.
Indirect evidence of antigenic modulation impeding anti-Id therapy was obtained on comparing bivalent (IgG) and univalent (Fab/c) polyclonal anti-Id reagents in treating a guinea-pig leukemia.72 The univalent reagent will not induce modulation by crosslinking but is expected to do so, at a rate slower than the bivalent, on interaction with Fcγ receptors.71 A much better therapy achieved by the univalent reagent suggested an important role for antigenic modulation in impeding anti-Id killing of cells in this model.
The Stanford experience of treating FL with monoclonal anti-Id antibodies has been described.10,73,74 Successes were encouraging (45 persons, 66% overall responses, 18% complete remissions of durations much greater than achieved with anti-CD20), but the logistics proved unsustainable. In some cases, there were significant levels of extracellular idiotypic antigen. The series provided 5 excellent examples of dormancy of subclinical tumor in patients who had enjoyed up to 10 years of clinical complete remission. Unfortunately, observations on antigenic modulation were not included. But there is an obvious lesson in the presumed antigenic modulation not proving a complete bar to good results. It will be of interest to investigate whether antimannose antibodies also lead to endocytosis, and if any secreted Ig, which could act as a potential barrier to antibody, carries the mannosylated glycoform.
Modulation of CD20
Before 2003, most authors reported zero or very slow75 modulation induced by monoclonal anti-CD20 in vitro, nor was it noted in vivo experimentally or clinically in the treatment of FL. In contrast, reports then appeared of modulation of CD20 on chronic lymphocytic leukemia (CLL) cells in response to rituximab therapy.76,77 After infusion of 30 mg, C3 fragments were demonstrable on the leukemic cells. Cell numbers then decreased considerably. However, they increased again toward the end of the approximately 600-mg, approximately 5-hour infusion, but with surface CD20 reduced by more than or equal to 90%.78 The timing, rapidity, and extent of this antigen depletion suggested to the authors a removal of complexes by trogocytosis after cytotoxic mechanisms had been exhausted by an initial killing of antibody-coated cells.
Antigenic modulation in rituximab therapy of FL, where neoplastic cells are absent from blood or present only in small numbers, presents a different picture. The beginning of the first infusion is possibly the most critical part of an antibody course. It is the stage most prone to yield toxicity and might initiate prolonged effector modulation (next section). Any antigen-positive cells in the blood are hazarded by the slowly rising antibody concentration but have an opportunity to respond by undergoing antigenic modulation. Normal B lymphocytes evidently fail to modulate sufficiently to escape. In the Stanford trials of therapy of FL,24,79 B lymphocytes had largely disappeared from the circulation after treatment by 4 antibody infusions at 375 mg/m2 at 1-week intervals. They remained nearly undetectable (as judged by CD19 positivity) for approximately 6 months after treatment, and then increased slowly in number. Apparently cytotoxic mechanisms killing antibody-coated B lymphocytes were not exhausted as they were during infusions in CLL (where B-lymphocyte counts can exceed 100 times normal). The fate of the normal lymphocytes in these FL cases is in complete contrast to TL+ mouse lymphocytes surviving intact in vivo in the face of high titer anti-TL antibody.
Absent or inefficient antigenic modulation was also revealed by neoplastic B lymphocytes in nodal masses. In a phase 1, escalating single-dose (10-500 mg/m2) study of FL,17 these cells, when examined by flow cytometry of biopsies taken 2 weeks after treatment, still exhibited a coating of antibody. We discuss later this prolonged survival of antibody-coated FL cells.
Type I/II dichotomy
Antigenic modulation displayed among a number of anti-CD20 antibodies has been investigated.21 A type I/II dichotomy based on rates of modulation was observed. Rituximab is in the type I group, whereas the type II group contains antibodies modulating even more slowly. Among other differences, the type I antibodies activate complement better, and some of the type II antibodies induce a nonapoptotic form of receptor-initiated cell death. But it is the slower modulation by type II that has claimed the most attention, on the basis that the longer persistence of antibody coating could favor ADCC and phagocytosis. For whatever reason, a type II antibody proved superior to type I in depleting, in vivo, B cells from mice transgenic for human CD20.80 Another observation was that rates of antigenic modulation varied also with the density of FcγRIIB on the target-cell surface.81 It should be noted in passing that, in FL with no recorded antibody treatment, deregulated FcγRIIB production by neoplastic B lymphocytes was correlated with tumor progression.82,83
Effector modulation
A major feature of anti-CD20 therapy in humans, not addressed in mouse experiments, is the remarkable delay in onset of tumor regression in patients exhibiting a significant response. This is seen on comparing the Stanford trials of anti-Id and anti-CD20.10,17 Delays before tumor responses were noted, taking the first day of the first infusion as day 0, were in the anti-Id trial 8 to 16 days and, in the anti-CD20 trial, 28 to 133 days (median, 71 days). Speculation regarding the nature of late responses includes a “vaccinal” immune response to the tumor. A brief report has appeared of variable T-cell responses to the tumor idiotype in 5 rituximab-treated patients,84 and more data on this phenomenon are awaited.
We think that the evidence at present is greatly in favor of the late responses being the result of antibody. In a multicenter trial,85 the median plasma levels of rituximab at 3 months after treatment (105 days after the first of 4 infusions) were as follows: in responders (complete response and partial response, 62 patients), 25.4 μg/mL; in nonresponders (42 patients), 5.9 μg/mL. Not only are the antibody levels among responders good for the time elapsed since treatment, they also predict levels in extravascular fluids86 well within the cytotoxic range of ADCC.
Suppression of autoimmune inflammation: a model for effector modulation in antibody therapy?
We postulate that the delay in tumor responses to rituximab therapy is the result of effector modulation, which may be defined as a failure of immunologic effectors to kill antibody-coated targets at antibody levels predicted to lie within the cytotoxic range. The postulate relies heavily on an apparent analogy with suppressed autoimmune inflammation.
Autoimmune inflammation in humans is in certain cases usefully suppressed by one of 2 measures: infusion of intravenous normal human IgG (IVIG) or infusion of human IgG antibody to the rhesus D antigen (anti-D). IVIG preparations are pooled from plasma of at least 1000 normal donors. Large amounts are infused, for example, up to a total of 2 g/kg (twice the normal body content of IgG) given over 2 to 5 days. This has proved particularly valuable in childhood immune thrombocytopenic purpura (ITP), for which the approach was introduced.87 Although platelet autoantibodies persist, the platelet count rises over approximately 5 days; and in cases of acute ITP, the procedure is often curative. Chronic ITP usually needs repeated infusions, but these might be spaced weeks apart.
Speculation about the mechanisms of action of IVIG has yielded almost a surfeit of possibilities.88 One pathway leading to decreased macrophage activity has been demonstrated in detail by Ravetch et al.89,90 A minor (∼ 2%) population of human IgG possesses Fcγ glycans terminating in α-2,6 sialic acid with affinity for the lectin DC-SIGN. These glycans initiate a pathway with anti-inflammatory activity marked by increased expression of FcγRIIB on macrophages. Another response to IVIG, which blocks inflammatory IFN-γ signaling, has been defined as a suppression of the IFNGR2 subunit of the IFN-γ receptor.91 There is persuasive evidence that dimers or higher aggregates of IgG, formed after mixing monomeric IgG preparations from multiple donors, are required for IVIG to initiate effective anti-inflammatory activity.88,91-93
The large infusions of IVIG described in the preceding paragraph may be replaced by small infusions of anti-D IgG, consisting of more than 90% polyclonal anti-D94 and given in doses up to 75 μg/kg per day. This procedure is applicable to rhesus-D positive subjects (∼ 85% of whites, more than 90% of blacks and Asians) and forms a coating of immune complexes on the recipient's red cells. Results have been broadly comparable to those given by IVIG, but studies of spontaneous ITP in humans95 and of a mouse model of ITP96 have both suggested that IVIG and anti-D achieve ameliorations by different mechanisms. These results attest again to the complexity of the variables involved. The anti-D procedure could be claimed to resemble anti-CD20 therapy more closely than does IVIG administration.
Similarities between the delay in responding to anti-CD20 therapy and the therapeutic suppression of autoimmune inflammation97 can now be summarized. In both maneuvers, there is a large initial exposure of macrophages and other effectors to IgG aggregates or immune complexes (preformed in IVIG, formed on red cells by anti-D, formed on normal and neoplastic B cells by anti-CD20). In both maneuvers, the patient frequently experiences rigors and fever during the first infusion, symptoms typical of the release of cytokines, phospholipids, and other mediators. Such releases are thought to arise mainly from effector cells activated by cross-linkage of their surface FcγR. Again, in both settings, second and later infusions contrast with the first in being generally symptom-free. We attribute this tentatively to the initial excessive mediator release leading to modulation, with changes such as production of IL-4, polarization of T cells to the TH2 state, and polarization of macrophages to a quiescent state associated with high expression of FcγRIIB.98 This modulation appears to last for weeks or months.
These concepts imply that a view of the undesirability of antigenic modulation cannot hold in all situations. In comparing the Stanford anti-Id and anti-CD20 experiences, the prompter occurrence and better durability of remissions with anti-Id might have owed much to early antigenic modulation reducing antibody coating of targets, and so reducing the excessive stimulation of effector cells, which leads to effector modulation.
More effective antibodies?
A number of new anti-CD20 antibodies are presently undergoing clinical trials as alternatives to rituximab in the treatment of FL, CLL, and other lymphoid tumors.99 Final verdicts on these comparisons are not available, but 2 of the antibodies with interesting features, ofatumumab and GA101 (obinutuzumab), are considered in the following paragraphs.
It should be noted here, in assessing performances in vitro of ADCC by novel antibodies, that NK cells from peripheral blood are convenient standard effectors because the cells do not need preliminary activation and need not be separated from other mononuclear cells. However, as a surrogate for macrophage ADCC, results could be deceptive: NK cells do not possess the crucially important FcγRIIB,51 and they possess complex inhibitory systems of their own, which will be variably recruited by nonautologous targets.
Ofatumumab is a fully human IgG1κ anti-CD20 antibody produced in mouse cells by transgenic technology. It (referred to as 2F2) was shown to engage a CD20 epitope distinct from that used by rituximab, to possess a slower off-rate from CD20 than does rituximab, and to perform better in assessments in vitro of ADCC and complement-mediated cytotoxicity.100 Despite these favorable features, data from a phase 2 trial of first-line immunochemotherapy of CLL show it performing less well than rituximab.101 Other trials are still current.
GA101 is a type II IgG1κ anti-CD20 antibody. It was derived by humanization of the murine IgG antibody B-ly1, selection of variants to improve direct cell-killing, and growth in a line of CHO cells, which endow the Fcγ with nonfucosylated glycans. Binding to FcγRIIIa and ADCC performance by NK cells in vitro were enhanced as anticipated by the choice of glycan. Performance exceeded that of rituximab in treating lymphoma xenograft models, and in depleting normal B lymphocytes in both blood and tissue in cynomolgus monkeys.102
Further engineering of the Fcγ module
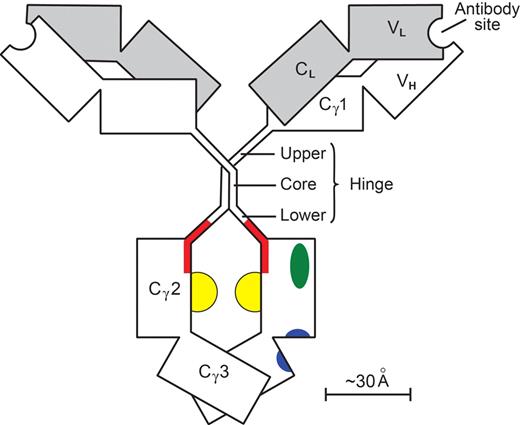
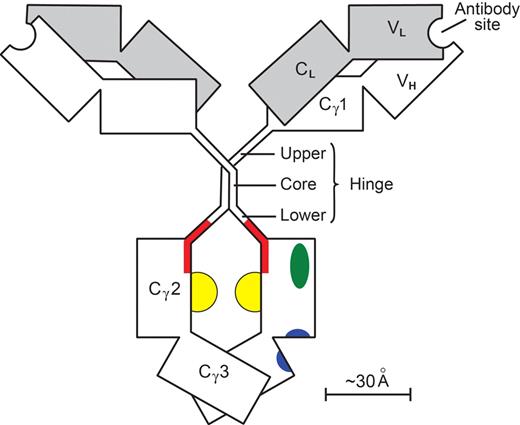
Results achieved with antitumor antibodies in FcγRIIB−/− mice51 stimulated efforts to improve, by mutagenesis of Fcγ, the ratio of (KA for union with FcγRIII) to (KA for union with FcγRIIB). Increases in the ratio of 9- and 10-fold were reported.103,104 An increase of up to 50-fold was reported for human IgG with nonfucosylation of the glycans,105 forming the floor of the docking site for all FcγR (Figure 3).
A human IgG1 molecule. Heavy (γ) chains are clear; light (κ or λ) chains are shaded. Individual domains (V, variable; C, constant) are depicted as rectangles. The paired Cγ2 and Cγ3 domains with their N-linked glycans (yellow) make up the Fcγ module. The remainder of the molecule (including its hinge) is composed of 2 Fab′γ modules. The hinge (γ-chain residues 221-237) has a rigid core with 2 γ-γ interchain disulfide bonds, whereas the upper and lower hinges are flexible. One of the 2 set of docking sites on Fcγ is shown. The site for all Fcγ receptors (red) involves the medial aspects of both Cγ2 domains, extends into the lower hinge, and has a floor formed by the 2 Fcγ glycans.107 The site for FcRn (blue) involves both Cγ2 and Cγ3 domains at their interface.109 The site for complement C1q (green) is on the surface of the Cγ2 domain lateral to the Fcγ receptor site.110 The contralateral set of sites is obscured. In the FabIgG derivative,108 the hinge disulfide bonds are cleaved; and a third Fab′γ module, with antibody specificity for the activating FcγRIIIA, is attached to one of the resulting cysteine residues. The original FcγR site is deliberately disabled so that there is little residual affinity for FcγRIIB. The sites for FcRn and complement C1q are little affected.
A human IgG1 molecule. Heavy (γ) chains are clear; light (κ or λ) chains are shaded. Individual domains (V, variable; C, constant) are depicted as rectangles. The paired Cγ2 and Cγ3 domains with their N-linked glycans (yellow) make up the Fcγ module. The remainder of the molecule (including its hinge) is composed of 2 Fab′γ modules. The hinge (γ-chain residues 221-237) has a rigid core with 2 γ-γ interchain disulfide bonds, whereas the upper and lower hinges are flexible. One of the 2 set of docking sites on Fcγ is shown. The site for all Fcγ receptors (red) involves the medial aspects of both Cγ2 domains, extends into the lower hinge, and has a floor formed by the 2 Fcγ glycans.107 The site for FcRn (blue) involves both Cγ2 and Cγ3 domains at their interface.109 The site for complement C1q (green) is on the surface of the Cγ2 domain lateral to the Fcγ receptor site.110 The contralateral set of sites is obscured. In the FabIgG derivative,108 the hinge disulfide bonds are cleaved; and a third Fab′γ module, with antibody specificity for the activating FcγRIIIA, is attached to one of the resulting cysteine residues. The original FcγR site is deliberately disabled so that there is little residual affinity for FcγRIIB. The sites for FcRn and complement C1q are little affected.
We have designed a bispecific106 FabIgG construct in which a recombinant Fab′γ module, with antibody specificity for FcγRIII, is attached to the rituximab hinge in a manner deliberately disabling the FcγR docking site.107,108 The affinity for FcγRIII is enhanced, affinities for all other FcγR collapse, and the FabIgG acquires a ratio of KA (FcγRIII) to KA (FcγRIIB) increased more than 1000-fold. The other docking sites on Fcγ109,110 (Figure 3) do not involve the hinge and are minimally affected.
It is hoped that antibody constructs similar to those in the preceding paragraphs, used initially at least at repeated low doses, could achieve a given level of cell kill with less induction of both antigenic and effector modulations. The situations are complex and decisions must depend on clinical observations.
In conclusion, infusion of mAb into patients with FL is now an accepted part of treatment protocols. The outcome depends on the target antigen, and differences are illustrated by comparing anti-Id and anti-CD20. Anti-Id may have direct cytotoxic action, but it undergoes rapid antigenic modulation. Nevertheless, clinical results, although limited because of the need for individual antibodies, are impressive. In contrast, very slow modulation of anti-CD20 yields persisting antibody-coated cells, which exceed cytotoxic capability and produce prolonged effector modulation, an apparent defense against “cytokine storm.” Strengthening antibody attack requires knowledge of the mechanism of action. To return to anti-Id, but using a single mAb is attractive, and a new possibility of targeting the unusual mannosylated Ig variable regions is emerging. Effector cells are probably important for both anti-Id and anti-CD20, and modifications of potency and dose of antibody to conserve effector cell function may be required to improve performance.
The online version of this article contains a data supplement.
Acknowledgments
The authors thank Vania Coelho for her invaluable contributions to the study of Ig V-region mannosylation in FL, Terry Hamblin for perceptive discussions of antibody therapy, and Lynsey Block for invaluable help with preparing the manuscript.
This work was supported by Cancer Research UK and Tenovus.
Authorship
Contribution: F.K.S. and G.T.S. wrote the paper, contributed comments, and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Freda K. Stevenson. Cancer Sciences Academic Unit, Southampton University Hospitals Trust, Southampton, SO16 6YD, United Kingdom; e-mail: fs@soton.ac.uk.