Abstract
New treatments are required for rituximab-refractory follicular lymphoma (FL). In the present study, patients with rituximab-refractory FL received 8 weekly infusions of ofatumumab (CD20 mAb; dose 1, 300 mg and doses 2-8, 500 or 1000 mg; N = 116). The median age of these patients was 61 years, 47% had high-risk Follicular Lymphoma International Prognostic Index scores, 65% were chemotherapy-refractory, and the median number of prior therapies was 4. The overall response rate was 13% and 10% for the 500-mg and 1000-mg arms, respectively. Among 27 patients refractory to rituximab monotherapy, the overall response rate was 22%. The median progression-free survival was 5.8 months. Forty-six percent of patients demonstrated tumor reduction 3 months after therapy initiation, and the median progression-free survival for these patients was 9.1 months. The most common adverse events included infections, rash, urticaria, fatigue, and pruritus. Three patients experienced grade 3 infusion-related reactions, none of which were considered serious events. Grade 3-4 neutropenia, leukopenia, anemia, and thrombocytopenia occurred in a subset of patients. Ofatumumab was well tolerated and modestly active in this heavily pretreated, rituximab-refractory population and is therefore now being studied in less refractory FL and in combination with other agents in various B-cell neoplasms. The present study was registered at www.clinicaltrials.gov as NCT00394836.
Introduction
Patients with follicular lymphoma (FL) refractory to rituximab-containing regimens have limited treatment options.1-4 Radioimmunotherapy (yttrium-90 ibritumomab tiuxetan and iodine-131 tositumomab) and bendamustine have demonstrated high overall response rates (ORRs) of 70%-75% in refractory FL,5,6 albeit with a limited duration of response ranging from 6.4-22.4 months.5,7-9 These agents are approved for the treatment of rituximab-refractory FL, but are associated with significant hematologic toxicities.5,10-12 In addition, radioimmunotherapeutic agents pose logistical challenges that limit their clinical use.11-13
Resistance of lymphoma cells to rituximab can be inherent or acquired after previous successful treatment with rituximab. Nearly half of rituximab-naive patients treated previously with chemotherapy did not respond to 4 weekly doses of rituximab monotherapy.4 In contrast, rituximab monotherapy in previously untreated FL patients with low tumor burden14 or rituximab followed by 16 maintenance infusions in previously untreated patients with indolent non-Hodgkin lymphoma (NHL)15 resulted in an ORR of up to 73%. Sixty percent of patients with low-grade or follicular NHL who responded to initial rituximab therapy failed to respond to repeat rituximab therapy, indicating that initially sensitive patients can acquire rituximab resistance.1
Several mechanisms of action have been proposed for rituximab resistance, including low-affinity Fc receptor polymorphism,16,17 overexpression of the complement-inhibitory molecules CD55 and CD59,18-21 low CD20 expression,18,22-24 and high tumor burden.25 In an in vitro study using lymphoma cell lines, repeated exposure to rituximab led to global down-regulation of CD20 gene and protein expression, thereby affecting lipid raft reorganization and downstream signaling and ultimately inhibiting the ability of rituximab to lyse cells.24 The investigators concluded that this mechanism may lead to rituximab resistance, although additional factors such as up-regulation of CD55 and CD59 may contribute.24 With the increasing use of repeated rituximab exposure in clinical practice, acquired resistance has become a growing challenge, highlighting the need for alternative treatment options.
New therapeutic agents for rituximab-refractory FL include mAbs against CD20 and other lymphoma-associated Ags. Ofatumumab is a human anti-CD20 mAb that binds to a unique epitope encompassing both the small and large loops of CD20, distinct from the epitope recognized by rituximab.18 Ofatumumab depletes B cells using mechanisms similar to those used by rituximab, but with more potent complement-dependent cytotoxicity (CDC).18,26-28 In rituximab-resistant cell lines, ofatumumab induced robust CDC in vitro.28 In addition, the efficacy of ofatumumab in vitro was less sensitive to the CD20 level or the CD55 and CD59 complement-inhibitory protein expression levels than rituximab.18,28 In a dose-escalation trial administering 4 weekly infusions of ofatumumab at 300-1000 mg, the ORR in 37 evaluable patients with relapsed/refractory FL was 43%, with an acceptable toxicity profile. Three of 4 patients with rituximab-refractory FL responded to ofatumumab.29 Therefore, we have performed a randomized study to examine 2 doses of ofatumumab as weekly monotherapy for rituximab-refractory FL.
Methods
Patients
Patients (≥ 18 years of age) with grade 1 or 2 CD20+ FL (as defined by World Health Organization guidelines)30 refractory to rituximab were eligible. The diagnosis was confirmed by central review of a lymph node biopsy (Bio-Analytical Research Corporation). Eligible patients had received at least 4 infusions of rituximab given as monotherapy, in combination with chemotherapy, or as maintenance therapy after chemotherapy or rituximab-chemotherapy. Rituximab-refractory disease was defined as: (1) failure to achieve at least a partial response (PR) to rituximab-based treatment, (2) disease progression while on treatment, or (3) disease progression in responders within 6 months of the last dose of rituximab. Patients had to have measurable disease, defined as 2 or more clearly demarcated lesions with a largest diameter of at least 1.5 cm or 1 clearly demarcated lesion with a largest diameter of at least 2.0 cm by computed tomography scan. No fluorodeoxyglucose-positron emission tomography scans were performed for this study.
Patients were excluded if they had an Eastern Cooperative Oncology Group performance status of 3 or 4; clinical suspicion of transformation to aggressive lymphoma (eg, B symptoms, fast-growing tumor, or increasing lactate dehydrogenase level); previous allogeneic stem cell transplantation at any time or previous autologous stem cell transplantation within 6 months of first infusion; more than 1 previous radioimmunotherapy; radioimmunotherapy within 3 months of first infusion; non-mAb anticancer therapy or glucocorticoid (> 10 mg/d prednisolone) within 4 weeks of first infusion; nonrituximab mAb therapy within 3 months of first infusion; known central nervous system involvement; active infectious disease requiring systemic treatment; clinically significant cardiovascular disease; abnormal laboratory values; or life expectancy less than 6 months. Pregnant and breastfeeding women and those of childbearing potential who were not using adequate contraception were excluded.
All patients provided signed informed consent. The protocol, amendments, and consent forms were approved by health authorities and local Independent Ethics Committees or Institutional Review Boards. The study was conducted in accordance with the Guidelines for Good Clinical Practice and the Declaration of Helsinki. The study was registered at clinicaltrials.gov as NCT00394836.
Study design and treatment
This prospective, phase 3, open-label trial was initially designed to randomize (1:1) patients to 2 dose levels: 500 mg versus 1000 mg. Patients received 8 weekly doses of ofatumumab: 300 mg at dose 1 followed by 500 mg or 1000 mg for doses 2 to 8. Because of slow patient recruitment and no increased toxicity with the 1000-mg dose, the protocol was amended to discontinue recruitment to the 500-mg arm. Because the patient population was diverse, with various degrees of tumor burden and prior treatment at study entry, only the 1000-mg arm was continued to reduce the likelihood of undertreatment of patients. All patients received acetaminophen 1000 mg and cetirizine 10 mg (or equivalent) before all infusions and glucocorticoid (prednisolone 100 mg or equivalent) was required before infusions 1 and 2.
Baseline assessments included vital signs, physical examination, computed tomography scans, bone marrow biopsy, hematology, biochemistry, evaluation of constitutional symptoms, Eastern Cooperative Oncology Group performance status, and prognostic factors. Based on previous experience with rituximab, some of the prognostic factors analyzed included the Follicular Lymphoma International Prognostic Index (FLIPI), FcγRIIIA valine/phenylalanine genotypes, FcγRIIa arginine/histidine genotypes, and B-cell lymphoma 2 (BCL2). The prognostic markers tested were not correlated with response to ofatumumab.
Efficacy evaluations
The primary end point was ORR (including complete response [CR], CR unconfirmed [CRu], and PR), as measured over a 6-month period from start of treatment, assessed by an independent end point review committee according to International Working Group guidelines.31
Secondary end points included duration of response (time from initial response to progression or death), progression-free survival (PFS; time from randomization to progression or death), overall survival (OS; time from randomization to death), and reduction in tumor volume. Disease status and response were assessed with radiologic imaging at months 3, 6, 9, 12, 18, and 24. After month 24, patients were monitored at 6-month intervals for survival.
Safety evaluations
Adverse events (AEs) and their potential relationships to the study drug were reported by investigators. The severity of AEs was graded by investigators according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) Version 3.0. Serious AEs were monitored from the time of informed consent until month 60 or initiation of alternative FL therapy.
Blood chemistry and hematology were assessed at screening and at all visits up to month 24, and human anti–human Abs were assessed at screening and at months 6, 9, 12, and 24. Blood chemistry and hematology (Bio-Analytical Research Corporation) and human anti–human Abs (Charles River Laboratories) were analyzed at central laboratories. Peripheral blood samples were analyzed for CD19+ and CD20+ B cells by flow cytometry. Monitoring continued until B-cell counts were within the normal range or reached or exceeded the baseline level, until alternative FL treatment was initiated, or until month 60.
Pharmacokinetic evaluations
Pharmacokinetic samples were collected at doses 1, 4, and 8 and at months 3, 6, and 24 after the last ofatumumab dose. Ofatumumab concentrations were determined as described previously.26 Noncompartmental methods were used to estimate pharmacokinetic parameter values.
Statistical analysis
The present trial was originally designed to estimate the response rate and corresponding 95% confidence interval (CI) for each treatment arm; no formal statistical test between dose groups was planned or applied. After an amendment, the study was to enroll 81 patients to the ofatumumab 1000-mg arm to estimate ORR with 10% precision. Specifically, with this population size, the lower bound of the 95% CI would be no more than 10 percentage points below a response rate of 30% or less using the large sample normal approximation.
Evaluation of all end points was based on the full analysis population composed of all patients exposed to ofatumumab. The response rate, including 95% CI, was reported using descriptive statistics. Duration of response, PFS, and OS were evaluated using Kaplan-Meier estimates. Reduction in tumor size, AEs, clinical safety data, and pharmacokinetic data were summarized using descriptive statistics. Regression analyses were performed to explore possible associations between clinical outcomes and ofatumumab maximum plasma concentration (Cmax), minimum plasma concentration (Cmin), and area under the concentration time curve (AUC) values.
Results
Patient characteristics and treatment delivery
Between September 2006 and September 2008, 116 patients were enrolled at 44 centers in 10 countries; 86 patients were enrolled in the 1000-mg arm, and 30 were randomized to the 500-mg arm before the protocol amendment. The median age of the overall population was 61 (range, 37-82) years; 86% of patients were Ann Arbor stage III or IV, and 47% had high-risk FLIPI scores of 3-5 (Table 1). Twenty-seven patients were refractory to rituximab monotherapy, 45 were refractory to rituximab maintenance therapy, and 44 were refractory to rituximab-chemotherapy combination therapy (Table 2). Baseline characteristics demonstrated a higher proportion of patients refractory to chemotherapy in the rituximab-chemotherapy combination group and a longer median disease duration in the rituximab monotherapy group.
In total, 87% and 91% of patients in the 500-mg and 1000-mg groups, respectively, completed all 8 infusions of ofatumumab. Of the 12 study withdrawals during treatment, the predominant reason was progressive disease (n = 8; 7%). AEs (n = 1; 1%), protocol violation (n = 1; 1%), and patient decision (n = 1; 1%) contributed to other reasons for study withdrawal during treatment. During follow-up, progressive disease was the most frequent reason for withdrawal.
Efficacy
The ORR was 10% in the 1000-mg group (1 CR and 8 PR) and 13% in the 500-mg group (2 CRu and 2 PR); the ORR for the total population was 11%. The primary end point did not differ between dose groups, and the treatment arms were therefore combined for analyses of secondary end points.
Patients with high-risk (n = 55), intermediate-risk (n = 32), and low-risk (n = 26) FLIPI scores achieved ORRs of 11%, 13%, and 11%, respectively. The ORR was 22% for patients refractory to rituximab monotherapy, 9% for patients refractory to rituximab maintenance therapy, and 7% for patients refractory to rituximab-chemotherapy. Three of 7 patients who progressed on rituximab monotherapy responded, whereas 3 of 11 patients on rituximab maintenance for more than 12 months responded. There was no difference in ORR between patients with bulky (lymph nodes > 5 cm) and nonbulky disease (data not shown). At the 3-month time point from start of treatment, 46% of evaluable patients (49 of 106) demonstrated a reduction in tumor volume (Figure 1).
Reduction in tumor size at 3 months. Tumor size was measured by computed tomography scan and calculated as the sum of the product of diameters for the indicator lesions (assessed by an independent radiology reviewer and based on standard review criteria for NHL31,39 ). The change in tumor size from screening to month 3 was assessed in 106 patients with postbaseline tumor measurement at month 3.
Reduction in tumor size at 3 months. Tumor size was measured by computed tomography scan and calculated as the sum of the product of diameters for the indicator lesions (assessed by an independent radiology reviewer and based on standard review criteria for NHL31,39 ). The change in tumor size from screening to month 3 was assessed in 106 patients with postbaseline tumor measurement at month 3.
The median PFS was 5.8 months for the overall population (Figure 2). For the 32 patients who failed to achieve PR with prior rituximab, the PFS was 6.6 months. There were no statistically significant differences in PFS based on radiology review (P = .22 across groups), by qualifying rituximab therapy (P = .82 across groups), or by FLIPI risk score (P = .10). PFS was calculated based on disease progression in the absence of deaths. The median follow-up time for PFS was 5.8 months (95% CI, 4.8-7.0) from randomization for the total population and 5.2 (95% CI, 3.9-9.4) and 6.1 (95% CI, 4.8-7.0) months for the 500-mg and 1000-mg dose groups, respectively. The median PFS was 9.1 months for patients (n = 46) who had reduction of tumor volume at 3 months, compared with 5.7 months for patients (n = 36) with no change or tumor growth at 3 months (P = .017). The median PFS (8.9-9.2 months) was similar for patients who had 1%-24%, 25%-49%, or 50% or greater tumor reduction (P = .79; supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The median duration of response was 6.0 months in both the 500-mg and 1000-mg dose groups. The median OS has not been reached.
PFS among patients with rituximab-refractory FL treated with ofatumumab. PFS, defined as the time from randomization (week 0) to progression (assessed by an independent end point review committee) or death. Results presented are for the combined dose groups (ofatumumab 500 mg and 1000 mg).
PFS among patients with rituximab-refractory FL treated with ofatumumab. PFS, defined as the time from randomization (week 0) to progression (assessed by an independent end point review committee) or death. Results presented are for the combined dose groups (ofatumumab 500 mg and 1000 mg).
Safety
Most patients had low peripheral blood B-cell counts at baseline, likely because of recent exposure to rituximab and other antilymphoma therapies, but ofatumumab rapidly depleted peripheral blood CD19+ and CD20+ cells further (supplemental Figure 2). The median CD19+ lymphocyte count decreased from 14 cells/mm3 at baseline to 0 cells/mm3 at 3 months after the completion of ofatumumab therapy. At 6 and 9 months after therapy, the median CD19+ lymphocyte counts still remained 0 cells/mm3. Because of profound lymphopenia at baseline, these data are difficult to interpret.
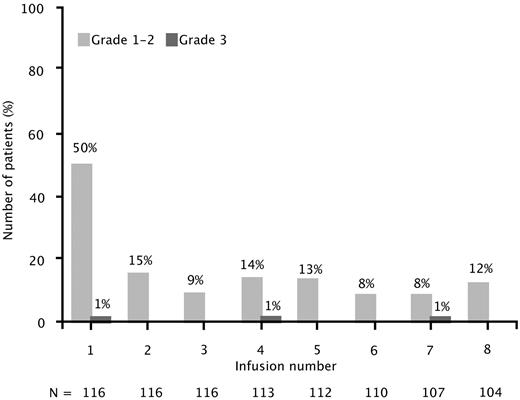
Infusion-related reactions (on the day of or day after infusion) occurred in 51% of patients at infusion 1, which decreased to 8%-15% for the remaining 7 infusions (Figure 3). Nearly all infusion-related reactions were grade 1 or 2. Only 3 patients (3%) experienced grade 3 reactions (ie, cough, hypoxia, or urticaria), which resolved, and they were able to receive subsequent infusions. No grade 4 or 5 infusion reactions were observed.
Infusion-related reactions by infusion number. Infusion-related reactions reported on the day of and day after ofatumumab infusion. Results presented are for the combined dose groups (500 mg and 1000 mg).
Infusion-related reactions by infusion number. Infusion-related reactions reported on the day of and day after ofatumumab infusion. Results presented are for the combined dose groups (500 mg and 1000 mg).
The most common investigator-reported AEs (in more than 10% of total patients) were infections (ie, upper respiratory infections and nasopharyngitis), rash, urticaria, fatigue, pruritus, nausea, cough, neutropenia, and pyrexia. Three patients developed grade 3 infections. Grade 3 or 4 events included neutropenia, infections (all grade 3, including 1 case of febrile neutropenia), cough (all grade 3), and urticaria (all grade 3; Table 3). The incidences of AEs from the start of treatment until 30 days after the last dose (data not shown) were similar to those shown in Table 3. By laboratory assessment, the incidences of grade 3 or higher hematologic toxicities were: neutropenia, 15%; leukopenia, 10%; anemia, 3%; and thrombocytopenia, 3%. Human anti–human Abs were not detected in any available samples (39 patients at month 6, 28 at month 9, and 9 at month 12).
Five patients had fatal AEs. Three patients died of disease progression 7, 37, and 63 days after the last dose, and 2 patients died of nonneutropenic sepsis 37 and 188 days after the last dose. Because of the prolonged B-cell depletion with ofatumumab, a causal relationship in these 2 patients cannot be excluded, although it should be noted that these 2 patients already had significant B-cell depletion (CD19+ and CD20+ cell numbers) at the time of entering this study.
Pharmacokinetics
Ofatumumab Cmax and Cmin values increased with repeated weekly administration. Ofatumumab pharmacokinetics were proportional to dose at infusions 4 and 8 (supplemental Figure 3 and supplemental Table 1). At infusion 8, the geometric mean ofatumumab clearance values were 7.0 and 8.8 mL/h, volume of distribution at steady state values were 4.4 and 5.4 L, and terminal half-life values were 18.5 and 18.4 days in the 500-mg and 1000-mg dose groups, respectively.
Exploratory univariate regression analyses detected a significant relationship between ofatumumab serum exposure and objective response only for AUC at dose 8. Higher Cmax and Cmin values at infusion 4 and higher Cmax, Cmin, and AUC values at infusion 8 were associated with longer PFS values (supplemental Table 2).
Discussion
Ofatumumab monotherapy was well tolerated in this heavily pretreated, rituximab-refractory population with limited treatment options. Although the ORR was modest (11%), it should be noted that two-thirds of patients were refractory to chemotherapy and 47% of patients had high-risk FLIPI scores. The median PFS was 5.8 months for the overall population and 9.1 months for patients who had tumor reduction at 3 months. The favorable safety profile was consistent with results described previously,29 with most AEs being grade 1 or 2. The majority of nonhematologic AEs were grade 1 or 2, with no grade 4 or 5 nonhematologic events among the most common AEs.
The modest ORR in this patient population may be attributable to several factors. It is plausible that CD20 down-regulation occurred after treatment with rituximab,24 and such down-regulation could potentially limit the ability of ofatumumab to effectively kill lymphoma cells in this rituximab-refractory population. Because a repeat tumor biopsy was not required, tumor CD20 expression was not assessed at study entry. Although ofatumumab demonstrates potent cell lysis even in rituximab-resistant cells with low CD20 expression, complete loss of CD20 would abrogate the activity of ofatumumab.18,28 In addition, patients may have had depleted complement levels, as was observed with rituximab therapy in chronic lymphocytic leukemia.32 CDC is thought to be the primary effector mechanism for in vitro cell death with ofatumumab.18,28 Therefore, depleted complement levels may reduce the antitumor efficacy of ofatumumab, even though ofatumumab promotes CDC in vitro at low concentrations of complement components.27 Therefore, ofatumumab-induced CDC may not be sufficient to reverse rituximab-refractory disease, especially in patients with disease concurrently resistant to chemotherapy (Table 1). Finally, heavily pretreated patients may have severely damaged cellular effector mechanisms, which may have contributed to the lack of efficacy via Ab-dependent cellular cytotoxicity by ofatumumab. Another theoretical mechanism that may have affected this heavily pretreated group of patients is the lack of potential T-cell response after exposure to anti-CD20 mAb, which has been described in patients with FL after treatment with rituximab therapy.33
Despite the modest ORR, the results of the present study suggest that ofatumumab therapy may have a role in FL. The higher ORR in patients refractory to rituximab monotherapy (22%) compared with patients refractory to maintenance or combination therapy with chemotherapy (9% and 7%, respectively) suggests that ofatumumab monotherapy may have greater clinical activity in patients with FL that is not refractory to chemotherapy. The low ORR in the other patient subgroups may reflect extreme treatment refractoriness, because most patients had FL also refractory to cytotoxic chemotherapy. Recent in vitro studies suggest that ofatumumab may be more active than rituximab against rituximab-sensitive tumor cells; ofatumumab was more effective than rituximab at killing primary tumor cells derived from patients with either de novo or relapsed B-cell lymphoma.34 Furthermore, a nonrandomized clinical study of ofatumumab combined with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) in previously untreated FL demonstrated high CR/CRu rates across all FLIPI subgroups.35
Similar to the findings of an initial phase 1 study in FL,27 ofatumumab serum exposure variables were largely not associated with objective response. Higher Cmax, Cmin, and AUC values at doses 4 or 8 were associated with longer PFS, suggesting that investigation of higher ofatumumab monotherapy doses may be warranted in highly refractory patients (supplemental Table 2). However, these associations should be interpreted with caution. Higher ofatumumab concentrations or AUC values may result in greater tumor reduction and therefore a longer period of time before tumor progression is observed. Alternatively, patients with lower initial tumor burden or greater tumor cell depletion in response to ofatumumab may subsequently have reduced clearance and higher ofatumumab concentrations with further dosing. Ongoing studies in FL continue to investigate the potential role of ofatumumab pharmacokinetics in predicting clinical outcome.
Despite the low ORR for single-agent ofatumumab, approximately half of evaluable patients in the present study showed a reduction in tumor burden after therapy, and the median PFS was 5.8 months. Furthermore, patients with 1%-49% tumor reduction had PFS that was similar to that of patients who achieved PR, and the median PFS for all patients with tumor reduction was superior to that of patients with tumor growth. Therefore, more patients achieved a biologic response to ofatumumab than is reflected in the 11% ORR. Experience with rituximab in diffuse large B-cell lymphoma and chronic lymphocytic leukemia indicates that mAb therapy can have a significant effect when given in combination with chemotherapy, even when mAb monotherapy elicits a limited clinical ORR.36-38 Furthermore, the combination of ofatumumab and CHOP appeared highly active in previously untreated FL.35 Therefore, ofatumumab in combination with chemotherapy regimens such as CHOP or bendamustine may be effective in rituximab-refractory FL and warrants study.
In summary, in the present study, ofatumumab monotherapy was well tolerated in heavily pretreated patients and achieved an ORR of 11%. Approximately half of patients demonstrated tumor reduction at 3 months and the median PFS was 5.8 months, demonstrating that ofatumumab is biologically active in refractory FL. Further studies should examine ofatumumab monotherapy in less heavily pretreated FL patients who are still sensitive to rituximab and ofatumumab in combination with chemotherapy in patients with relapsed or refractory FL and other NHL histologies.
The online version of this article contains a data supplement.
Presented in part at the 51st American Society of Hematology Annual Meeting, December 8, 2009, New Orleans, LA.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the following investigators in the Hx-CD20-405 study for their participation: in the Czech Republic: D. Belada, T. Kozak, and J. Mayer; in France: M. Bernard, P. Brice, D. Bordessoule, R. Bouabdallah, B. Coiffier, A. Delmer, P. Feugier, R. Gressin, C. Haioun, S. Le Gouill, and N. Milpied; in Germany: U. Dührsen, M. Pfreundschuh, N. Schmitz, and A. Viardot; in Italy: A. Santoro, and P. Zinzani; in Poland: M. Komarnicki, T. Robak, and J. A. Walewski; in Spain: F. J. Capote, and C. Ferrá; in Sweden: M. Jerkeman and A. Själander; in the United Kingdom: P. Johnson, D. Linch, R. Pettengrell, and A. Pettitt; and in the United States: H. Myint and G. Okada. The authors also thank the Independent Radiology Committee (S. Salomon, D. Shepro, and S. Soignet), and Kamilla Begtrup for statistical analyses.
This study was supported by research funding from Genmab A/S and GlaxoSmithKline. Editorial support for this publication was provided by Emily Bauer, PhD, Medicus International New York, and was funded by GlaxoSmithKline.
Authorship
Contribution: M.S.C. contributed to the concept and design of the manuscript, provided study materials and/or patients, collected, assembled, analyzed, and interpreted the data, and wrote the manuscript; L.F., I.G., and C.A.R. collected, assembled, analyzed, and interpreted the data, and wrote the manuscript; V.D., K.K., B.K.L., A. Hellmann, and E.G.-E. collected and assembled the data and wrote the manuscript; G.C., E.J., and L.P.-B. provided study materials and/or patients, collected, assembled, analyzed, and interpreted the data, and wrote the manuscript; J.R. provided study materials and/or patients and collected and assembled the data; C.G.D. contributed to the concept and design of the manuscript, collected, assembled, analyzed, and interpreted the data, wrote the manuscript, and provided administrative support and study materials and/or patients; N.G., R.C.J., T.S.L., and S.L. analyzed and interpreted the data and wrote the manuscript; M.S. contributed to the concept and design of the manuscript, collected, assembled, analyzed, and interpreted the data, and wrote the manuscript; A. Hagenbeek contributed to the concept and design of the manuscript, provided study materials and/or patients, analyzed and interpreted the data, and wrote the manuscript; and all authors approved the final manuscript.
Conflict-of-interest disclosure: M.S.C. acted as a consultant/advisor for GSK and Genmab and received research funding from Genmab. L.F. received research funding from GSK. G.C. acted as a consultant/advisor for Roche and GSK and received honoraria from GlaxoSmithKline and Roche. B.K.L. acted as a consultant/advisor for Genentech/Roche and GlaxoSmithKline and received research funding from Genentech/Roche, Lilly, and GlaxoSmithKline. J.R. acted as a consultant/advisor for GlaxoSmithKline and owns stock in GlaxoSmithKline. A. Hellmann received research funding from Genmab and GSK. C.G.D. is an employee of GSK. N.G. is an employee of and owns stock in GlaxoSmithKline. I.G. is an employee of and owns stock in GSK. R.C.J. is an employee of and owns stock in GSK. T.S.L. is an employee of GSK. S.L. is an employee of Genmab. M.S. is an employee of and owns stock in Genmab. C.A.R. was an employee of Genmab when the study took place, is currently employed at Boehringer Ingelheim, and owns stock in Genmab. A. Hagenbeek acted as a consultant/advisor for Spectrum Pharmaceuticals. The remaining authors declare no competing financial interests.
A complete list of the members of the 405 Study Investigators appears in the online supplemental Appendix.
Correspondence: Myron S. Czuczman, MD, Department of Medicine, Roswell Park Cancer Institute, Elm & Carlton Streets, Buffalo, NY 14263; e-mail: myron.czuczman@roswellpark.org.