Abstract
Natural killer (NK) cells are innate lymphocytes that play an important role against viral infections and cancer. This effect is achieved through a complex mosaic of inhibitory and activating receptors expressed by NK cells that ultimately determine the magnitude of the NK-cell response. The T-cell immunoglobulin– and mucin domain–containing (Tim)–3 receptor was initially identified as a T-helper 1–specific type I membrane protein involved in regulating T-cell responses. Human NK cells transcribe the highest amounts of Tim-3 among lymphocytes. Tim-3 protein is expressed on essentially all mature CD56dimCD16+ NK cells and is expressed heterogeneously in the immature CD56brightCD16– NK-cell subset in blood from healthy adults and in cord blood. Tim-3 expression was induced on CD56brightCD16− NK cells after stimulation with IL-15 or IL-12 and IL-18 in vitro, suggesting that Tim-3 is a maturation marker on NK cells. Whereas Tim-3 has been used to identify dysfunctional T cells, NK cells expressing high amounts of Tim-3 are fully responsive with respect to cytokine production and cytotoxicity. However, when Tim-3 was cross-linked with antibodies it suppressed NK cell–mediated cytotoxicity. These findings suggest that NK-cell responses may be negatively regulated when NK cells encounter target cells expressing cognate ligands of Tim-3.
Introduction
Natural killer (NK) cells comprise 5% to 20% of human peripheral blood lymphoid cells and are a critical component of the immune system, providing protection against viral infections and contributing to tumor immune surveillance. NK-cell activity is regulated by an intricate balance of signals transmitted by inhibitory and activating receptors.1,2 Functionally distinct NK-cell subsets can be defined based on the level of CD56 and CD16 coexpression.3 CD56brightCD16− NK cells produce abundant IFN-γ in response to stimulation with interleukin (IL)–12 and proliferate robustly when cultured in IL-2, whereas CD56dimCD16+ NK cells are more cytolytic and produce significant amounts of cytokine when their activating receptors are engaged.4 CD56dimCD16+ NK cells are considered mature NK cells and are differentiated from the immature CD56brightCD16– NK-cell subset. This is further supported by recent data demonstrating the dynamics of expression of the killer immunoglobulin-like receptors (KIR), CD57, CD94, and CD62L expression on the CD56dimCD16+ NK cells as they mature from CD56brightCD16– NK-cell precursors.5-9
T-cell immunoglobulin– and mucin domain–containing (Tim)–3 is a member of Tim family of receptors of which there are 3 in humans (Tim-1, Tim-3, and Tim-4).10 These molecules are involved in diverse metabolic and immunoregulatory processes.11 Tim-3 is a type I transmembrane protein that contains no defined signaling motifs in its cytoplasmic domain, but it has been implicated both in activation and inhibition of immune responses12,13 and in the induction of apoptosis of Tim-3–bearing cells through interactions with galectin-9.14 Tim-3 is expressed on CD4+ T cells, dendritic cells, monocytes,15-17 CD8+ T cells,18,19 and NK cells.20 In a comparison of lymphocyte populations in healthy human subjects, the highest transcription of the gene encoding Tim-3 was observed in NK cells.21 There is evidence that engagement of Tim-3 on mouse T cells with the ligand galectin-9 promotes aggregation, leading to the death of T-helper 1 cells and the selective loss of interferon (IFN)-γ–producing T cells.14 On human T cells, the expression of Tim-3 regulates cell proliferation and IFN-γ secretion.19,21,22 We and others have observed that increased amounts of Tim-3 on T cells during HIV, hepatitis C virus, and other chronic viral infections correlated with T-cell dysfunction, suggesting that Tim-3 is part of a negative regulatory pathway.19,23-25 In this study, we investigated the expression of Tim-3 on human NK cells and its regulation by cytokines, and we provide evidence for the role of Tim-3 in the restraint of NK cell–mediated cytotoxicity in healthy individuals.
Methods
Primary cells and cell lines
Peripheral blood mononuclear cells (PBMCs) of healthy individuals were obtained from the Stanford Blood Bank. Cord blood PMBCs were obtained from AllCells. The University of California, San Francisco and the University of Hawaii institutional review boards approved the research involving human participants reported in this study (approvals H11613-19149 and 11-06-549-01).
NKL cells, a human NK-cell line,26 were stably transduced with pMXneo-FLAG-Tim-3, a retroviral vector expressing human Tim-3 protein with a FLAG tag on its N terminus. A full-length human TIM-3 cDNA was obtained from Open Biosystems. NKL and NKL-FLAG-Tim-3 were cultured in complete media (RPMI-1640; Invitrogen) supplemented with 10% heat-inactivated FCS, 2mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin) with 200 U/mL IL-2 (NCI Biologic Resources Branch). NKL-FLAG-Tim-3 cells were drug selected with 1 mg/mL Geneticin (G-418).
Cell staining and flow cytometric analysis
PBMCs were washed with PB supplemented with 1% bovine serum albumin and 2mM EDTA (FACS buffer). For staining, 5 × 105 cells were incubated with 10 μg/mL human IgG (Sigma-Aldrich) to block nonspecific antibody binding. To identify NK cells, we stained the cells with PE-conjugated or APC-conjugated anti–Tim-3, FITC-conjugated CD94 (R&D Systems), Alexa 700–conjugated anti-CD4, PE-Cy7–conjugated anti-CD56, Pacific blue–conjugated anti-CD16 (all from BD Biosciences), ECD-conjugated anti-CD3, PE-conjugated anti-CD335 (Beckman Coulter), and Qdot605-conjugated anti-KIR3DL1. APC-Cy7–conjugated anti-CD14 and APC-Cy7–conjugated anti-CD19 (BD Biosciences) were used to exclude monocytes and B cells, respectively, and Amine Aqua (Invitrogen) was used to exclude dead cells. In some experiments, cells were fixed in 2% paraformaldehyde in PBS and permeabilized with FACS-perm (BD Biosciences). Permeabilized cells were stained for intracellular IFN-γ and perforin (both from BD Biosciences). Fluorescence minus one samples were prepared for each fluorochrome to facilitate gating. Cells were fixed with 2% paraformaldehyde in PBS and analyzed by flow cytometry using a 4-laser LSR-II or Fortessa instrument (BD Biosciences). Anti–mouse IgG-coated beads (Invitrogen) were reacted with each fluorochrome-conjugated antibody separately and used for software-based compensation. Cells were analyzed with FlowJo Version 9.4.3 software (TreeStar).
Cell sorting experiments
PBMCs were washed with FACS buffer and incubated with 10 μg/mL human IgG before surface staining to block nonspecific antibody binding. After surface staining, the PBMCs were stained with PE-conjugated anti–Tim-3, FITC-conjugated anti-CD94, PE-Cy7–conjugated anti-CD56, Pacific blue-conjugated anti-CD16, ECD-conjugated anti-CD3, APC-Cy7–conjugated anti-CD14, and APC-Cy7–conjugated anti-CD19; the cells were sorted using a modified BD FACSAria (BD Biosciences). To isolate NK cells, we sorted CD19−, CD14−, CD3−, CD56dimCD16+, or CD56brightCD16− either lacking or expressing Tim-3 on their surface.
NK-cell stimulation
Cryopreserved PBMCs from healthy donors were thawed and analyzed for NK-cell frequency and for receptor expression. PBMCs were cultured in complete media and stimulated with targets cells (721.221, an HLA-class I–deficient EBV-transformed human B lymphoblastoid cell line; or K562, a human erythromyeloblastoid leukemia cell line; 1:5 effector:target ratio) or with different cytokines: human recombinant IL-2 (40-500 IU/mL), IL-12 (50 ng/mL; both from PeproTech), IL-18 (50 ng/mL; MBL Lab), IL-15 (100 ng/mL), IFN-γ (10 ng/mL; both from R&D Systems), or IFN-α (104 U/mL; PBL Interferon Source). PBMCs cultured in complete media were taken as a measure of background activity. In brief, thawed cryopreserved PBMCs were cultured at 5 × 106 cells/mL in 96-well round-bottomed plates with the respective stimulants, and then 10 μg/mL FITC-conjugated anti-CD107a mAb (BD Biosciences) was added to the culture for 4 to 6 hours in the presence of 10 μg/mL brefeldin-A (Sigma-Aldrich). The cells were then harvested and prepared for flow cytometry to analyze their CD107a degranulation or their IFN-γ production. Data were analyzed using FlowJo Version 9.4.3 software (TreeStar).
The 4 sorted NK-cell subsets were cultured in IL-15 (100 ng/mL) for 24 hours and then stained with PE-conjugated anti–Tim-3, FITC-conjugated anti-CD94, PE-Cy7–conjugated anti-CD56, and Pacific blue–conjugated anti-CD16 to analyze Tim-3 and CD94 expression during their maturation by flow cytometry. All data were analyzed using FlowJo Version 9.4.3 software (TreeStar).
NK cell–mediated cytotoxicity
NK cell–mediated cytotoxicity was analyzed using a 4-hour 51Chromium (51Cr) release assay. In the antibody-redirected killing assays, human NKL-FLAG-Tim-3 cells, fresh PBMCs, or overnight IL-2 (200 U/mL)–activated PBMCs were used as effectors and were incubated for 4 hours with the 51Cr-labeled mouse FcR+ P815 cells, a mouse mastocytoma cell line (ATCC). P815 cells were coated with 10 μg/mL antibodies against different receptors: anti-CD16 (clone Leu11a; BD Biosciences), anti-2B4 (clone C1.7; a gift from Dr G. Trinchieri, National Cancer Institute), anti-NKG2D (clone 149810; R&D Systems), anti–Tim-3 (clone 344836; R&D Systems), anti–Tim-3 (clone 344801; a generous gift from Dr J. P. Houchins, R&D Systems), anti-CD94 (clone DX22), anti-CD56 (clone My31.13), or against the FLAG epitope tag (clone M2; Sigma-Aldrich). In these P815 cytotoxicity assays, monoclonal antibodies binding to activating human NK receptors induce the killing of the Fc receptor–bearing P815 target cells, whereas monoclonal antibodies against inhibitory NK-cell receptors suppress the killing of P815 target cells. When 2 antibodies (1 antibody against an activating receptor and 1 antibody against an inhibitory receptor) were combined in the same assay, both antibodies were present at the same total concentration. Furthermore, to demonstrate that the antibody against an inhibitory NK-cell receptor was specifically suppressing NK-cell activation, rather than simply competing for Fc receptor occupancy on the P815 target cells, the antibody against the activating NK-cell receptor was mixed with an equal concentration of an isotype-matched control antibody or an antibody binding to NK cells but not affecting their function (eg, anti–human CD56). We also used 721.221 cells transfected with the human CD32 Fc receptor (hCD32)27 as target cells. The assay with 51Cr-labeled 721.221-hCD32 target cells was done similarly to the assay with P815 cells, with the difference that 721.221-hCD32 cells were directly lysed by the effector cells (NKL-FLAG-Tim-3 or fresh PBMCs), so they did not require stimulation with antibodies to an activating NK receptor to initiate cytotoxicity. The radioactivity released by the lysed target cells was measured with a gamma counter. All experiments were performed in triplicate, and data were expressed as the percentage of lysis of target cells, calculated using the formula (sample release − spontaneous release)/(maximal release − spontaneous release) × 100, in which the value of maximal release was the amount of radioactivity released from the target cells with 1% SDS in distilled water. Spontaneous release was the amount of radioactivity released by target cells in medium alone without effector cells.
Statistical analyses
Statistical analyses were performed using SAS System 9 for Windows XP (SAS Institute) or Prism Version 5 statistical software for MAC (GraphPad Software). Nonparametric statistical tests were used. The Mann-Whitney U test was used for comparison tests.
Results
Differential expression of Tim-3 on human NK-cell subsets
Gene expression studies have identified high levels of Tim-3 transcripts in NK cells.21 We measured the surface expression of Tim-3 protein on NK cells by flow cytometry. NK cells were gated as CD14−CD19−CD3−CD56+ cells (Figure 1A). We observed a high surface expression of Tim-3 on most circulating NK cells (median, 90.7%; interquartile range [IQR], 82.1, 94.6) from healthy donors (Figure 1A) compared with CD8+ (median, 18%; IQR, 13.8, 19.3) or CD4+ (median, 8.3%; IQR, 6.4, 15.1) T cells (Figure 1B-C), which is consistent with previous analyses of HAVCR2 mRNA expression.21 Tim-3 expression on NK cells varied among different healthy donors both in terms of frequency (Figure 1A,C) and cell surface density (as determined by mean fluorescent intensity [MFI]; Figure 1A; data not shown).
Tim-3 expression on human NK cells. (A) Plots depict Tim-3 expression on NK cells for 3 representative healthy donors. PBMCs were gated on forward angle light scatter (FSC-height and FSC-area) to eliminate doublets, followed by side light scatter (SSC) and FSC gating to define lymphocytes. Dead cells were excluded using an Amine Aqua reactive dye, and CD14 and CD19 staining was used to exclude monocytes and B cells, respectively. CD56 and CD16 were used to identify NK cells within the CD14−CD19−CD3− population. (B) The plot shows expression of Tim-3 on NK cells in comparison to Tim-3 expression on CD4+ and CD8+ T cells in a representative donor. (C) Graph depicts the frequency (percentage) of Tim-3 on NK cells (solid circles) and CD4+ and CD8+ T cell (open circles) from multiple donors (n = 27). (D) Plot shows NK cells from a healthy donor, gated into CD56brightCD16− and CD56dimCD16+ NK-cell subsets with the expression of Tim-3 on these subsets presented as histograms. Graphs show the frequency (E) and MFI (F) of Tim-3+ CD56brightCD16– (solid circles) and CD56dimCD16+ NK cells (open circles) for 27 healthy individuals. P values were calculated using the Mann-Whitney test. (G) Representative plot shows Tim-3 expression on NK-cell subsets (CD56brightCD16– [gray shade] and CD56dimCD16+ NK cells [black line]) from cord blood obtained at the time of birth. (H) Graph shows Tim-3 expression on NK-cell subsets (CD56brightCD16– [solid circles] and CD56dimCD16+ NK cells [open circles]) from cord blood of 3 healthy newborn infants. (I) Representative plots of Tim-3 coexpression with CD94 on CD56brightCD16– (left) and CD56dimCD16+ (right) NK cells.
Tim-3 expression on human NK cells. (A) Plots depict Tim-3 expression on NK cells for 3 representative healthy donors. PBMCs were gated on forward angle light scatter (FSC-height and FSC-area) to eliminate doublets, followed by side light scatter (SSC) and FSC gating to define lymphocytes. Dead cells were excluded using an Amine Aqua reactive dye, and CD14 and CD19 staining was used to exclude monocytes and B cells, respectively. CD56 and CD16 were used to identify NK cells within the CD14−CD19−CD3− population. (B) The plot shows expression of Tim-3 on NK cells in comparison to Tim-3 expression on CD4+ and CD8+ T cells in a representative donor. (C) Graph depicts the frequency (percentage) of Tim-3 on NK cells (solid circles) and CD4+ and CD8+ T cell (open circles) from multiple donors (n = 27). (D) Plot shows NK cells from a healthy donor, gated into CD56brightCD16− and CD56dimCD16+ NK-cell subsets with the expression of Tim-3 on these subsets presented as histograms. Graphs show the frequency (E) and MFI (F) of Tim-3+ CD56brightCD16– (solid circles) and CD56dimCD16+ NK cells (open circles) for 27 healthy individuals. P values were calculated using the Mann-Whitney test. (G) Representative plot shows Tim-3 expression on NK-cell subsets (CD56brightCD16– [gray shade] and CD56dimCD16+ NK cells [black line]) from cord blood obtained at the time of birth. (H) Graph shows Tim-3 expression on NK-cell subsets (CD56brightCD16– [solid circles] and CD56dimCD16+ NK cells [open circles]) from cord blood of 3 healthy newborn infants. (I) Representative plots of Tim-3 coexpression with CD94 on CD56brightCD16– (left) and CD56dimCD16+ (right) NK cells.
CD56dimCD16+ NK cells are considered mature NK cells and are differentiated from the immature CD56brightCD16– NK-cell subset. We observed that the CD56dimCD16+ NK cells essentially all expressed Tim-3 (median, 95.6%; IQR, 91.1, 98.6), whereas CD56brightCD16– NK cells were heterogeneous (median, 75.3%; IQR, 62.4, 86.5), with clearly separated Tim-3–negative and Tim-3–positive subsets in the healthy donors evaluated (Figure 1D-E). For most donors, the amount of Tim-3 expressed at the surface of CD56dimCD16+ NK cells was slightly higher than on CD56brightCD16– NK cells (Figure 1F). A similar pattern of expression for Tim-3 was observed on NK cells derived from the cord blood of healthy newborns (Figure 1G-H). Interestingly, the percentage of Tim-3+ NK cells was lower in the CD56brightCD16– NK cells from newborns compared with CD56brightCD16– NK cells from healthy adults (median, 32% [Figure 1G] vs median, 75.3% [Figure 1E]).
Because Tim-3 was mostly expressed by mature CD56dimCD16+ NK cells, we analyzed CD94 and Tim-3 coexpression in NK cells to determine whether Tim-3 was acquired during the maturation of CD56brightCD16– NK cells. Indeed, CD94 density marks the transition from CD56brightCD16– and CD56dimCD16+ NK cells.5,28 In the representative donor shown, all CD56brightCD16− NK cells expressed CD94 at a high cell surface density, but 58.6% were also Tim-3+, whereas most of the CD56dimCD16+ NK cells were Tim-3+ (83.4%) and only 28.3% were Tim3+CD94hi (Figure 1I), suggesting that CD94hi NK cells gain Tim-3 expression before down-regulating CD94 surface expression. A similar expression pattern was observed for CD62L and Tim-3 (data not shown). These data suggest that Tim-3 expression is acquired on CD56brightCD16– NK cells during their transition to the more mature CD56dimCD16+ NK cells.
Stimulation of NK cells leads to marked up-regulation of Tim-3 on CD56bright CD16– NK cells
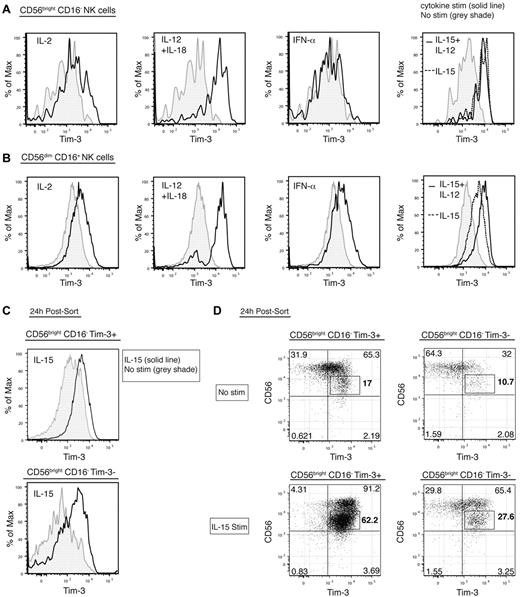
We assessed the expression of Tim-3 on NK cells cultured with cytokines known to stimulate NK-cell proliferation, cytotoxicity, and cytokine production (eg, IL-12, IL-18, IL-12 + IL-18, IL-15, IL-15 + IL-12, IL-2, IFN-γ, or IFN-α). Each of these cytokines induced the up-regulation of Tim-3 expression to varying degrees (Figure 2A), except IFN-γ, which did not up-regulate Tim-3 (data not shown). IL-12 + IL-18 prominently led to a marked up-regulation of Tim-3 expression on both the CD56brightCD16– (unstimulated MFI median, 1212; IQR, 867.8, 1456; median, 76.8%; IQR, 71.6, 82.3; [IL-12 + IL-18 stimulation] MFI median, 2880; IQR, 2534, 3336; median, 87.7%; IQR 78.0, 93.1; MFI; P = .002) and the CD56dimCD16+ NK subsets (unstimulated MFI median, 1354; IQR, 1029, 1579; [IL-12 + IL-18 stimulation] MFI median, 2932; IQR, 2434, 3734; MFI; P = .002; Figure 2A). Previously, we reported that stimulation of CD8+ T cells with IL-15 lead to an increase in Tim-3 expression.18 Similarly, Tim-3 expression was up-regulated on the 2 NK subsets by IL-15 and was further increased on the CD56dimCD16+ NK-cell subset by IL-12 and IL-15 (Figure 2A-B). IL-2 induced modest Tim-3 up-regulation on the CD56brightCD16– NK cells, whereas IFN-α did not change Tim-3 expression on this NK-cell subset (Figure 2A). Conversely, Tim-3 expression on CD56dimCD16+ NK cells was up-regulated by either IL-2 or IFN-α stimulation but to a lesser extent compared with IL-12 and IL-18, IL-15, or IL-12 and IL-15 (Figure 2B).
Tim-3 expression on NK cells is increased by maturation and cytokine stimulation. (A-B) Plots from a representative donor show the surface expression of Tim-3, on CD56brightCD16– (A) and CD56dimCD16+ (B) NK-cell subsets after culture overnight without stimulation (gray shade) or with IL-2, IL-12 and IL-18, IFN-α, IL-15 and IL-12 (black line for all these stimulations), and IL-15 (dotted line). (C-D) CD19−CD14−CD3−CD56brightCD16− NK cells either lacking or expressing Tim-3 were sorted and incubated for 24 hours with either no stimulation or IL-15. (C) Histograms show Tim-3 expression of sorted cells from a representative donor after culture without (gray shade) or with IL-15 stimulation (black line). (D) Plots depict CD56 and Tim-3 coexpression on sorted CD56brightCD16– Tim-3+ (left) and Tim-3− (right) NK cells without (top) or with (bottom) IL-15 stimulation. These experiments were repeated 3 times with different unrelated healthy donors.
Tim-3 expression on NK cells is increased by maturation and cytokine stimulation. (A-B) Plots from a representative donor show the surface expression of Tim-3, on CD56brightCD16– (A) and CD56dimCD16+ (B) NK-cell subsets after culture overnight without stimulation (gray shade) or with IL-2, IL-12 and IL-18, IFN-α, IL-15 and IL-12 (black line for all these stimulations), and IL-15 (dotted line). (C-D) CD19−CD14−CD3−CD56brightCD16− NK cells either lacking or expressing Tim-3 were sorted and incubated for 24 hours with either no stimulation or IL-15. (C) Histograms show Tim-3 expression of sorted cells from a representative donor after culture without (gray shade) or with IL-15 stimulation (black line). (D) Plots depict CD56 and Tim-3 coexpression on sorted CD56brightCD16– Tim-3+ (left) and Tim-3− (right) NK cells without (top) or with (bottom) IL-15 stimulation. These experiments were repeated 3 times with different unrelated healthy donors.
Tim-3 expression is up-regulated by various cytokines and is higher on mature CD56dimCD16+ NK cells; thus, we determined whether Tim-3 was up-regulated during NK-cell maturation. CD56brightCD16– and CD56dimCD16+ NK cells expressing or not Tim-3 at their surface were sorted to high purity (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) and were cultured with or without IL-15. Tim-3 expression spontaneously increased in 24-hour culture even without stimulation (supplemental Figure 1B compared with Figure 2C-D). However, 24 hours after IL-15 stimulation, Tim-3 expression was highly increased on both Tim-3+ and Tim-3− CD56brightCD16– NK cells compared with cells cultured without stimulation (Figure 2C). To correlate Tim-3 induction by IL-15 on CD56brightCD16– NK cells with maturation of this subset, we analyzed CD94 and CD56 expression on these cells. IL-15 stimulation did not change CD94 expression (data not shown). Interestingly, after culture CD56brightCD16– NK cells not only acquired Tim-3 expression but also down-regulated CD56 expression, and strikingly the cells that became CD56dim were Tim-3+ (Figure 2D). IL-15 stimulation highly induced the maturation of NK cells and increased this population of CD56dim Tim-3+ cells (Figure 2D). Indeed, the percentage of CD56dim Tim-3+ NK cells after 24-hour culture without stimulation was 17% and 10.7% for sorted CD56brightCD16− Tim-3+ and Tim-3− cells, respectively, and became 62.2% and 27.6% with IL-15 stimulation. These data show that Tim-3 expression is acquired during activation of NK cells, principally by IL-12 and IL-18 or maturation by IL-15. This suggests that Tim-3 is an activation marker, differentiation marker, or both on NK cells.
Expression of Tim-3 and NK-cell activity
We have shown previously that increased amounts of Tim-3 on T cells renders them dysfunctional.19 Therefore, we examined whether the amount of Tim-3 on the cell surface of NK cells correlated with their functional responses. CD56brightCD16– NK cells are the predominant NK-cell subset secreting IFN-γ in response to IL-12 + IL-18.29 Indeed, a higher percentage of the CD56bright NK-cell subset produced IFN-γ compared with the CD56dim NK cells in response to activation with these cytokines, although a significant proportion of CD56dim NK cells also produced IFN-γ in response to IL-12 + IL-18 (Figure 3A-B). Most of the CD56bright NK cells that produced IFN-γ in response to IL-12 + IL-18 stimulation were Tim-3+ cells (Figure 2A-B) and displayed a high cell surface density of Tim-3 (Figure 3C). The CD56dim NK cells that produced IFN-γ in response to IL-12 + IL-18 stimulation also expressed the highest cell surface density of Tim-3 (Figure 3A-C). The MFI of Tim-3 staining was higher on CD56dim NK cells producing IFN-γ (MFI median, 3110; IQR, 2903, 3693) compared with the IFN-γ–negative NK cells (MFI median, 2240; IQR, 1404, 2532; P = .004; Figure 3C).
NK cells with the highest surface density of Tim-3 secrete the most IFN-γafter IL-12 and IL-18 stimulation. (A) Plots depict coexpression of Tim-3 and IFN-γ production in unstimulated (top) or stimulated by IL-12 and IL-18 (bottom) NK-cell subsets (CD56brightCD16– [left plots] and CD56dimCD16+ [right plots]) in a representative healthy donor. (B) Graph shows frequency of IFN-γ+ NK cells based on Tim-3 expression on NK-cell subsets (CD56brightCD16– [solid circles] and CD56dimCD16– [open circles]) after IL-12 and IL-18 stimulation in 6 unrelated healthy donors. (C) Histograms show Tim-3 surface expression on IFN-γ− (gray shade) and IFNγ+ (black line) cells gated from IL-12– and IL-18–stimulated NK-cell subsets (CD56brightCD16– [left] and CD56dimCD16+ [right]) from a representative donor (n = 6).
NK cells with the highest surface density of Tim-3 secrete the most IFN-γafter IL-12 and IL-18 stimulation. (A) Plots depict coexpression of Tim-3 and IFN-γ production in unstimulated (top) or stimulated by IL-12 and IL-18 (bottom) NK-cell subsets (CD56brightCD16– [left plots] and CD56dimCD16+ [right plots]) in a representative healthy donor. (B) Graph shows frequency of IFN-γ+ NK cells based on Tim-3 expression on NK-cell subsets (CD56brightCD16– [solid circles] and CD56dimCD16– [open circles]) after IL-12 and IL-18 stimulation in 6 unrelated healthy donors. (C) Histograms show Tim-3 surface expression on IFN-γ− (gray shade) and IFNγ+ (black line) cells gated from IL-12– and IL-18–stimulated NK-cell subsets (CD56brightCD16– [left] and CD56dimCD16+ [right]) from a representative donor (n = 6).
On stimulation with 721.221 (Figure 4) or K562 target cells (data not shown), the NK cells that degranulated, as marked by cell surface CD107a staining, were predominantly cells expressing Tim-3 (Tim-3+ NK cells; Figure 4A-B). Interestingly, the CD56brightCD16– NK cells degranulating expressed the highest surface density of Tim-3, suggesting that the more mature CD56brightCD16– Tim-3+ NK cells were the cells degranulating when cocultured with target cells (Figure 4C). Tim-3 cell surface expression was similar between CD56dimCD16+ NK cells degranulating or not (Figure 4C). Overall, Tim-3 preferentially marked the most responsive subset of CD56bright and CD56dim NK cells with respect to both cytokine production and degranulation. This suggests that Tim-3+ NK cells are not dysfunctional or exhausted as observed previously for Tim-3+ T cells but that Tim-3 marks highly functional NK cells.
NK cells with the highest surface density of Tim-3 degranulate the most when stimulated with target cells. (A) Representative plots depict expression of Tim-3 and surface CD107a in CD56brightCD16– (left) and CD56dimCD16+ (right) NK-cell subsets unstimulated (top) or stimulated (bottom) with the human HLA class I–deficient 721.221 target cells. (B) Graphs shows the frequency of CD107a+ NK cells based on Tim-3 expression on CD56brightCD16– (solid circles) and CD56dimCD16– (open circles) NK-cell subsets, after stimulation with 721.221 cells. Experiment performed with cells from 6 unrelated healthy donors. (C) Histograms depict Tim-3 surface expression on CD107a− (gray shade) and CD107a+ (black line) cells gated from IL-12– and IL-18–stimulated NK-cell subsets (CD56brightCD16– [left] and CD56dimCD16+ [right]) from a representative donor (n = 6).
NK cells with the highest surface density of Tim-3 degranulate the most when stimulated with target cells. (A) Representative plots depict expression of Tim-3 and surface CD107a in CD56brightCD16– (left) and CD56dimCD16+ (right) NK-cell subsets unstimulated (top) or stimulated (bottom) with the human HLA class I–deficient 721.221 target cells. (B) Graphs shows the frequency of CD107a+ NK cells based on Tim-3 expression on CD56brightCD16– (solid circles) and CD56dimCD16– (open circles) NK-cell subsets, after stimulation with 721.221 cells. Experiment performed with cells from 6 unrelated healthy donors. (C) Histograms depict Tim-3 surface expression on CD107a− (gray shade) and CD107a+ (black line) cells gated from IL-12– and IL-18–stimulated NK-cell subsets (CD56brightCD16– [left] and CD56dimCD16+ [right]) from a representative donor (n = 6).
Tim-3 cross-linking inhibits NK cell–mediated cytotoxicity
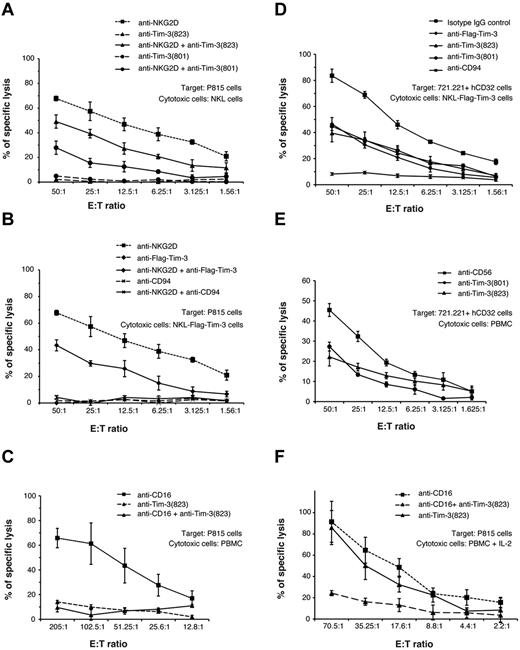
To directly assess the involvement of Tim-3 in NK-cell functional activity, we examined the effects of cross-linking the Tim-3 receptor on NKL, a human NK-cell line, or primary human peripheral blood NK cells from PBMCs. In these experiments, effector cells were cultured with the relatively NK-resistant mouse Fc receptor–bearing P815 target cells in the presence or absence of agonist antibodies against well-defined activating or inhibitory NK receptors. We have previously used this “antibody-redirected” cytotoxicity assay to define the relationship between the activating and inhibitory receptors on human NK cells.30 As shown in Figure 5A, human NKL cells killed P815 cells in the presence of antibodies against the activating NKG2D receptor, and this response was significantly inhibited in the presence of 2 different antibodies against Tim-3. To further confirm that Tim-3 was able to inhibit NK-cell killing, we transduced NKL cells with a Tim-3 construct containing a FLAG epitope tag on the N terminus. Similar to results obtained with the 2 anti–Tim-3 antibodies, an anti-FLAG antibody inhibited NKG2D-induced cytolysis of NKL-FLAG-Tim-3 cells to a similar extent (Figure 5B). These results with the anti-FLAG antibody exclude that the inhibitory effects of the anti–Tim-3 antibodies are unique to special epitopes on the Tim-3 receptor. Control antibodies of the same isotype as the anti–Tim-3 and anti-FLAG antibodies and included in the assays at the same concentration had no effect on the anti-NKG2D–induced cytotoxicity (data not shown). Anti-CD94, recognizing the inhibitory CD94-NKG2A receptor on NKL cells,30 also potently blocked NKG2D-induced cytotoxicity, in general more robustly than anti–Tim-3 or anti-FLAG antibodies (Figure 5B). When NKL cells were incubated with P815 target cells with the anti–Tim-3 or anti-FLAG antibodies alone (ie, in the absence of an antibody to an activating NK receptor), there was no evidence of induction of cytotoxicity (Figure 5A-B) or cytokine production (data not shown). Primary human peripheral blood NK cells potently killed P815 target cells in the presence of anti-CD16 antibodies, and this was suppressed in the presence of anti–Tim-3 antibodies (Figure 5C) but not control antibodies (data not shown). Again, anti–Tim-3 antibodies alone did not induce killing mediated by primary NK cells.
Tim-3 cross-linking inhibits killing by NK cells. (A-C) Each graph depicts the percentage of specific lysis of mouse P815 target cells mediated by the human NK-cell line NKL (A); NKL cells transduced with human Tim-3 containing a FLAG epitope tag on the N terminus, NKL-FLAG-Tim-3 (B); or freshly isolated PBMCs from a representative donor in the absence or presence of the indicated single or combined mAbs: anti–Tim-3 (clone 344801), anti–Tim-3 (clone 344823), anti-NKG2D, anti-CD94, anti-CD16, or anti-FLAG (C). (D-E) Graphs represent lysis of human 721.221 cells transduced with human CD32 Fc receptors by NKL-FLAG-Tim-3 (D) or freshly isolated PBMCs from a representative donor in the presence of the indicated mAbs: anti–Tim-3 (clone 344801), anti–Tim-3 (clone 344823), or anti-CD56 (E). Anti-CD56 was used as a negative control because it does not affect NK-cell cytotoxicity. (F) Graph represents lysis of mouse P815 target cells mediated by PBMCs stimulated overnight with IL-2 from a representative donor in the absence or presence of the indicated single or combined mAbs: anti–Tim-3 (clone 344801), anti–Tim-3 (clone 344823), and anti-CD16. Results are representative of 3 independent experiments performed in triplicate.
Tim-3 cross-linking inhibits killing by NK cells. (A-C) Each graph depicts the percentage of specific lysis of mouse P815 target cells mediated by the human NK-cell line NKL (A); NKL cells transduced with human Tim-3 containing a FLAG epitope tag on the N terminus, NKL-FLAG-Tim-3 (B); or freshly isolated PBMCs from a representative donor in the absence or presence of the indicated single or combined mAbs: anti–Tim-3 (clone 344801), anti–Tim-3 (clone 344823), anti-NKG2D, anti-CD94, anti-CD16, or anti-FLAG (C). (D-E) Graphs represent lysis of human 721.221 cells transduced with human CD32 Fc receptors by NKL-FLAG-Tim-3 (D) or freshly isolated PBMCs from a representative donor in the presence of the indicated mAbs: anti–Tim-3 (clone 344801), anti–Tim-3 (clone 344823), or anti-CD56 (E). Anti-CD56 was used as a negative control because it does not affect NK-cell cytotoxicity. (F) Graph represents lysis of mouse P815 target cells mediated by PBMCs stimulated overnight with IL-2 from a representative donor in the absence or presence of the indicated single or combined mAbs: anti–Tim-3 (clone 344801), anti–Tim-3 (clone 344823), and anti-CD16. Results are representative of 3 independent experiments performed in triplicate.
These findings demonstrate that anti–Tim-3 antibodies suppress NK-cell killing induced by the activating NKG2D and CD16 receptors. To address whether cross-linking Tim-3 also can inhibit the spontaneous killing against human NK-sensitive target cells, we transduced the HLA class I–deficient 721.221 cell line with the human CD32 Fc receptor to permit cross-linking of antibodies bound to the effector NK cells. NKL cells transduced with FLAG epitope–tagged Tim-3 (Figure 5D) and primary human peripheral blood NK cells (Figure 5E) efficiently killed the CD32 + 721.221 targets, and killing was suppressed in the presence of 2 distinct anti–Tim-3 antibodies or anti-FLAG antibody but not control anti-CD56 antibody or a nonbinding isotype-matched control antibody. We did not observed augmentation of NK-cell killing of CD32 + 721.221 cells in the presence of anti–Tim-3 antibodies, suggesting that Tim-3 functions as an inhibitor of cell-mediated cytotoxicity in NK cells. Interestingly, Tim-3 cross-linking of human peripheral blood NK cells activated by overnight culture in IL-2 did not inhibit killing of P815 target cells induced by CD16 (Figure 5F) or NKG2D (data not shown), suggesting that Tim-3 is a weak inhibitory receptor that can be overcome by cytokine stimulation of the effector cells.
Discussion
In this study, we have demonstrated that Tim-3 serves as a marker for NK-cell activation or maturation and when cross-linked can suppress NK cell–mediated cytotoxicity. A host of informative markers have been used to define the maturation of immature CD56brightCD16– NK cells into the mature CD56dimCD16+ NK-cell subset.9,31 Tim-3 is highly expressed on all mature CD56dimCD16+ NK cells but is heterogeneously expressed on CD56brightCD16– NK cells. Both CD56dimCD16+ and CD56brightCD16− NK cells increased Tim-3 expression after stimulation with certain cytokines, in particular with IL-12 + IL-18 and IL-15 + IL-12. The increase of Tim-3 expression on CD56brightCD16− NK cells stimulated with IL-15 was correlated with CD56 down-regulation in the cells that acquired Tim-3. These data suggest that Tim-3 may be a marker of NK-cell activation or differentiation acquired during the maturation of CD56bright into CD56dim cells at the same time that down-regulation of CD56, CD94, CD62L, and NKp46 and the acquisition of KIR is occurring.
In the T-cell lineage, the expression of Tim-3 has been correlated with functional exhaustion of effector T cells.19,32 In addition, Tim-3 has been shown to function as a receptor for galectin-9, which can induce apoptosis in T cells.14,33-35 By contrast, when stimulated with IL-12 + IL-18 to induce IFN-γ production or cultured with NK-sensitive target cells to induce degranulation, the NK cells with the highest amounts of cell surface Tim-3 were the most responsive subset. This clearly demonstrates that in the NK-cell lineage, Tim-3 marks the most functionally responsive NK cells within the population, rather than marking dysfunctional or anergic NK cells. It should be noted that certain NK receptors can be expressed on T cells in an aging immune system, and after chronic activation, which reflects the accumulation of senescent effector T cells.36 Thus, the same receptor can mark senescent T cells as well as fully functional NK cells. We propose that Tim-3 marks fully functional mature NK cells but that Tim-3 can restrain full NK-cell cytotoxic potential, similar to other NK-cell inhibitory receptors like the inhibitory KIR, when Tim-3+ NK cells encounter target cells expressing cognate ligand(s) of Tim-3. In addition to galectin-9, Tim-3 has been reported to bind phosphatidylserine16,37 as well as other undefined ligands.38
Although our functional assays clearly demonstrate that Tim-3 can function as an inhibitory receptor its cytoplasmic domain does not possess any canonical inhibitory motifs that can explain this activity. Moreover, a recent study by Kane et al showed that when transfected into Jurkat cells, Tim-3 can act as a costimulatory receptor with the CD3–TcR complex, interacting with Fyn and the p85 subunit of PI3 kinase, augmenting NF-κB and NFAT activation, and increasing IL-2 production.13 However, when primary mouse T-helper 1 T cells were simultaneously cross-linked with antibodies against CD3 and Tim-3, anti–Tim-3 suppressed anti-CD3–induced production of IFN-γ. Ju et al reported a modest increase in cytotoxicity and cytokine production when the human NK92 cell line was stimulated with the HepG2.2.15 tumor cell line in the presence of an anti–Tim-3 antibody20 ; however, in this case it is unclear whether the anti–Tim-3 is blocking an inhibitory signal or functioning as an agonist. Our study clearly demonstrates that the simultaneous cross-linking of antibodies against Tim-3 with agonist antibodies against the activating NKG2D and CD16 receptors suppresses NK cell–mediated cytotoxicity, as measured by both killing of the target cells and degranulation of the responding NK cell. The mechanisms by which cross-linking suppressed NK cell–mediated killing is unknown, but cross-linking of Tim-3 on NKL cells or primary NK cells did not affect the viability of the NK cells; there was no evidence that cross-linking Tim-3 with antibodies induced apoptosis in NK cells. In addition although galectin-9, a ligand of Tim-3, can induce apoptosis in NK cells, our preliminary studies suggest that in human NK cells Tim-3 is not required for galectin-9–induced death, implying that other receptors for galectin-9 are responsible (L.C.N., unpublished data, 2012).
Collectively, these finding indicate that the function of Tim-3 is context dependent and may differ in different cell types. Other receptors on NK cells have been reported previously to have different functional outcomes depending on the context. For example, the 2B4 (CD244) receptor has been reported to inhibit or activate NK cell–mediated cytotoxicity and cytokine production, which seems to depend on the availability or concentration of intracellular adapter proteins that interact with the cytoplasmic domain of 2B4.39,40 The human KIR2DL4 protein contains a canonical immunoreceptor tyrosine-based inhibitor motif in its cytoplasmic domain, yet associates with the immunoreceptor tyrosine-based activation motif (ITAM)–bearing FcϵRIγ adapter protein. Depending on the context, KIR2DL4 has been suggested to have activating or inhibitory properties.41,42 Similarly, the ITAM-bearing DAP12 and FcϵRIγ adapter proteins can either augment or suppress immune responses depending on the receptor with which they associate and the cell type in which they are expressed.43-45 Therefore, Tim-3 may regulate NK cells both positively and negatively in different circumstances, possibly depending on the nature of the Tim-3 ligand presented by the potential target cell.
The online version of the article contains a data supplement.
Presented in oral form at the 14th International Congress of Immunology, Kansai, Japan, August 23, 2010.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Trevor Burt (University of California, San Francisco [UCSF]) for specimens and Alexandra Gurary (University of Hawaii) for assistance with cell sorting.
This work was supported by National Institute of Allergy and Infectious Diseases grant AI083112 (L.C.N.). The project also was supported by grants from the San Francisco–Gladstone Institute of Virology & Immunology Center for AIDS Research (P30 AI027763), the UCSF AIDS Biology Program of the AIDS Research Institute, and National Institutes of Health grants AI068129, AI60379, AI68498, AI64520, AI066917, AI080129, and AI076014. L.L.L. is an American Cancer Society Professor, and S.L.V. was a Cancer Research Institute postdoctoral fellow.
National Institutes of Health
Authorship
Contribution: L.C.N., S.L.-V., and J.D.B. designed the research, performed the experiments, prepared the figures, and wrote the paper; R.B.J. and B.R.L. contributed to the analysis of data and figure design; A.R.J., E.C.S., and T.F. performed experiments and analyzed results; and D.F.N. and L.L.L. contributed to the study design, analyzed results, and edited the manuscript.
Conflict-of-interest disclosure: L.C.N., R.B.J., and D.F.N. are inventors on a patent application relating to Tim-3 and viral infections. The remaining authors declare no competing financial interests.
Correspondence: Lishomwa C. Ndhlovu, MD, PhD, Hawaii Center for AIDS, Department of Tropical Medicine, University of Hawaii, John A. Burns School of Medicine, 651 Ilalo St, Rm 325C, Honolulu, HI 96813; e-mail: lndhlovu@hawaii.edu.
References
Author notes
L.C.N. and S.L.-V. are co–first authors.
D.F.N. and L.L.L. contributed equally to this study.

![Figure 1. Tim-3 expression on human NK cells. (A) Plots depict Tim-3 expression on NK cells for 3 representative healthy donors. PBMCs were gated on forward angle light scatter (FSC-height and FSC-area) to eliminate doublets, followed by side light scatter (SSC) and FSC gating to define lymphocytes. Dead cells were excluded using an Amine Aqua reactive dye, and CD14 and CD19 staining was used to exclude monocytes and B cells, respectively. CD56 and CD16 were used to identify NK cells within the CD14−CD19−CD3− population. (B) The plot shows expression of Tim-3 on NK cells in comparison to Tim-3 expression on CD4+ and CD8+ T cells in a representative donor. (C) Graph depicts the frequency (percentage) of Tim-3 on NK cells (solid circles) and CD4+ and CD8+ T cell (open circles) from multiple donors (n = 27). (D) Plot shows NK cells from a healthy donor, gated into CD56brightCD16− and CD56dimCD16+ NK-cell subsets with the expression of Tim-3 on these subsets presented as histograms. Graphs show the frequency (E) and MFI (F) of Tim-3+ CD56brightCD16– (solid circles) and CD56dimCD16+ NK cells (open circles) for 27 healthy individuals. P values were calculated using the Mann-Whitney test. (G) Representative plot shows Tim-3 expression on NK-cell subsets (CD56brightCD16– [gray shade] and CD56dimCD16+ NK cells [black line]) from cord blood obtained at the time of birth. (H) Graph shows Tim-3 expression on NK-cell subsets (CD56brightCD16– [solid circles] and CD56dimCD16+ NK cells [open circles]) from cord blood of 3 healthy newborn infants. (I) Representative plots of Tim-3 coexpression with CD94 on CD56brightCD16– (left) and CD56dimCD16+ (right) NK cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/119/16/10.1182_blood-2011-11-392951/5/m_zh89991289630001.jpeg?Expires=1769672120&Signature=nqsOfTatQbtbGrra2q3tBmtOsackjK5ahYR7lckxj2lOCBQxWctwraDyPHwHQb2bf7vrDxiCX6TgwASdpdtqF~E3EElUPNA2deziBom1flgIOs3ZzOx67O6TDFrD6uTAbZKYynOG-5fl2BO7jy77RfjbAAhsiUWATEwVuNcwt0s2Moi1BWv6CdlChQU5P3oRJxAf1x4YllZpSpN4D5AdaqklVXi0QcPXYV7wl5NFKXaY20a~5wK5l9gtX1~CxuovCxXdwHc6NdK8RYCfiz8akCfPlVZR2OztGobfvisj3ZD3L8Q3VY~qNNEakSWykAP62fBaysefDy3iSerkFiKRwA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. NK cells with the highest surface density of Tim-3 secrete the most IFN-γ after IL-12 and IL-18 stimulation. (A) Plots depict coexpression of Tim-3 and IFN-γ production in unstimulated (top) or stimulated by IL-12 and IL-18 (bottom) NK-cell subsets (CD56brightCD16– [left plots] and CD56dimCD16+ [right plots]) in a representative healthy donor. (B) Graph shows frequency of IFN-γ+ NK cells based on Tim-3 expression on NK-cell subsets (CD56brightCD16– [solid circles] and CD56dimCD16– [open circles]) after IL-12 and IL-18 stimulation in 6 unrelated healthy donors. (C) Histograms show Tim-3 surface expression on IFN-γ− (gray shade) and IFNγ+ (black line) cells gated from IL-12– and IL-18–stimulated NK-cell subsets (CD56brightCD16– [left] and CD56dimCD16+ [right]) from a representative donor (n = 6).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/119/16/10.1182_blood-2011-11-392951/5/m_zh89991289630003.jpeg?Expires=1769672120&Signature=v~U6aGjmXG8eP38thcVhnJ29SZv0pM15B5i9QfULLvOQNPSySnObEuDPcx~GEnSub89HZG0TMfa52cmqDDidVlDEYFPhDQN3jmKZyB-HzuBdg18Ydpom80FYt8SHcmxcKUHy0hxB8MlR880766IqH8f~Y-uod9NHmWkk8kWdbOHOlFcLM9urIuW2eNvwlXuzezT~35AyBgZ~mbcKPNSPja-uKFPRuu2MVTgty0ABWslaDg~YBIgrZDUwVR6awdizuxMbuvxZEMP02wvLBYY1Rd2S28zbOsQQKDJhvwquhQoz5h4IlAMUhQ~mFtW3NgfzcXJbEnvJFn-tbjT~S5msKQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. NK cells with the highest surface density of Tim-3 degranulate the most when stimulated with target cells. (A) Representative plots depict expression of Tim-3 and surface CD107a in CD56brightCD16– (left) and CD56dimCD16+ (right) NK-cell subsets unstimulated (top) or stimulated (bottom) with the human HLA class I–deficient 721.221 target cells. (B) Graphs shows the frequency of CD107a+ NK cells based on Tim-3 expression on CD56brightCD16– (solid circles) and CD56dimCD16– (open circles) NK-cell subsets, after stimulation with 721.221 cells. Experiment performed with cells from 6 unrelated healthy donors. (C) Histograms depict Tim-3 surface expression on CD107a− (gray shade) and CD107a+ (black line) cells gated from IL-12– and IL-18–stimulated NK-cell subsets (CD56brightCD16– [left] and CD56dimCD16+ [right]) from a representative donor (n = 6).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/119/16/10.1182_blood-2011-11-392951/5/m_zh89991289630004.jpeg?Expires=1769672120&Signature=emYpvlf8qv4qFtvqqhu85MgO-L~CBsjNQmJ65YrbDZNisgs43HVqCORCg5ko9fwDjNSQvjU9zq0i8NyjBvYjZFzOzwSvbBBhNHgHfiOFrCMQ8rXiVY4Dqqo05O3YGWH8BpukeD7bhzwVt3W-UBU-5FpW2B~9VGjx8HxzARIs4mL9tTkOrMkE9HyuoBmvGFEj5-HSVqAE-Vq6q8CNyjoKKWDrZT3-R1zHn7wff-Kpge69pw3ljtSuAjCwup1BTLxI8ukTXsQQsZ5kS9PTdbLRmU6L4PP5Fio6veWQtlhZ1ftuRJAxaVRtaMhlJdAoEIuKcc2GTcXejN4Zh6YMjExA0A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)