Abstract
Natural cytotoxicity receptors (NCRs) were originally identified as specific natural killer cell activating receptors that, on binding to their endogenous ligands, trigger the killing of tumor cell targets. We recently described the differentiation of a novel subset of NCR+ Vδ1 T cells characterized by a remarkably high cytolytic potential against cancer cells. Here we demonstrate that the engagement of NKp30, one of the NCRs expressed de novo on Vδ1 T cells after stimulation, triggers the production of high levels of CCL3/MIP-1α, CCL4/ MIP-1β, and CCL5/RANTES but not of CXCL12/SDF-1. In turn, this NKp30-induced secretion of cc-chemokines is able to significantly suppress the replication of a CCR5 tropic strain of HIV-1 in CD4+/CCR5+ infected PM1 cell lines. This experimental evidence disclosing an unanticipated antiviral function of NCR+ Vδ1 T cells opens new avenues for understanding the pathogenic role and for manipulating the function of γδ T cells in HIV-1 infection.
Introduction
Although natural cytotoxicity receptors (NCRs) have been first identified as surface molecules specifically expressed on NK cells,1 we recently characterized a novel subset of human NCR+ Vδ1 T cells differentiated in vitro on activation with mitogenic stimuli (either PHA or anti-CD3 monoclonal antibody) and cytokines (either IL-2 or IL-15). Among the 3 known NCRs, Vδ1 T cells preferentially expressed NKp30 and showed a significantly higher cytolytic potential against cancer cells compared with that of conventional Vγ9Vδ2 T cells,2 the dominant subset of circulating γδ T lymphocytes.3 Besides being potent effectors against tumors, γδ T cells are also strongly involved in the immune responses against several viral infections.4 In particular, during the course of HIV-1 infection, there is both a significant expansion of Vδ1 T lymphocytes and a loss of Vγ9Vδ2 T cells. This leads to an inversion of Vδ2/Vδ1 ratio in the blood and intestinal mucosa of HIV-1–infected persons.5-8 Although the mechanisms underlying the loss of circulating Vγ9Vδ2 T cells and their clinical correlates in patients have been extensively investigated,8-12 the role of the expanded Vδ1 T cells in the pathogenesis of HIV-1 infection remains largely unknown.
Methods
Expansion of NKp30+ Vδ1 γδ T cells, cross-linking of surface receptor, and detection of chemokines
Human PBMCs were obtained from buffy coats of healthy volunteers and HIV-1–infected patients, all of whom signed consent forms in accordance with the Declaration of Helsinki and with clinical protocols approved by the Institutional Review Board of Desio Hospital, Milan, Italy. Ten HIV-1–infected patients with a prolonged high HIV-1 viremia (≥ 24 months) and history of opportunistic diseases, either naive for ART or and whose therapeutic regimen had been discontinued, were enrolled for this study. PBMCs were obtained in accordance with the clinical protocol approved by the Institutional Review Board of the National Institute of Allergy and Infectious Diseases. Each patient signed a consent form that was approved by the same Institutional Review Board.
To expand and characterize NKp30+ Vδ1 γδ T cells, we followed the same protocol recently developed by our group. Briefly, purified γδ T cells were stimulated in vitro with 1 g/μL of PHA (Sigma-Aldrich) and 100 U/mL of rhIL-2 (Roche Diagnostics) in 1640 RPMI medium supplemented with 10% FCS and penicillin/streptomycin/l-glutamine (Invitrogen). After 14 days of incubation, cells were analyzed by flow cytometry, as previously described.2 Before adding expanded NCR+ Vδ1 T cells, 96 flat-bottom well-plates were coated for 16 hours at room temperature with 10 μg/mL of anti-NKp30, anti-NKp46, anti-NKp44 (clones F252, KL 247, and KS38, respectively), anti-NKG2D and anti–TCR-γδ (clones ON72 and B1.1, respectively; Beckmann and Coulter) mAbs and washed twice. Then, 2 × 106/mL of in vitro expanded NCR+/NKG2D+ Vδ1 T cells were cultured for 18 hours at 37°C in coated plates at 200 μL/well. Cell supernatants were then collected and the levels of CCL3/MIP-1α, CCL4/MIP-1β, CCL5/RANTES, and SDF-1/CXCL12 were measured by ELISA (R&D Systems).
Infection of PM1 cell line and detection of HIV-1 replication
CD4+/CCR5+ PM1 cell line13 was infected with an HIV-1BaL R5 strain (National Institutes of Health–AIDS research and reference reagent program).14 The levels of viral replication was detected by p24 ELISA (Perkin Elmer) in time course experiments either in the presence or in absence of a combination of neutralizing mAbs against cc-chemokines (20 mg/mL each of anti-CCL3, CCL4, and CCL5; R&D Systems; supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Results and discussion
Engagement of NKp30 on Vδ1 T cells induces high levels of cc-chemokines
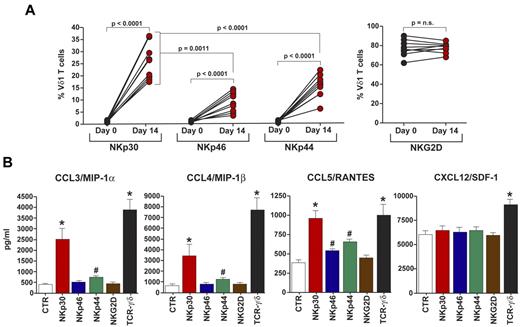
The well-established ability of γδ T cells to produce antiviral factors, such as cytokines and chemokines, has been further confirmed by reports showing that murine Vδ1 T cells and human Vγ9Vδ2 T cells produce high levels of cc-chemokines on TCR-γδ triggering.15-17 In particular, Vγ9Vδ2 T cells have been shown to suppress the replication of R5 and X4-tropic strains of HIV.16 To investigate whether the engagement of de novo expressed NCRs on activated Vδ1 T cells, other than remarkably strengthen their cytolytic potential,2 could also induce the production of cc-chemokines, we expanded NCR+ Vδ1 T cells from PBMCs of 10 healthy donors. In line with our previous results,2 NKp30 was the NCR most highly expressed on activated Vδ1 T cells, followed by NKp44 and NKp46. In contrast, we did not observe any significant modulation of NKG2D expression, another critical NK cell activating receptor that, unlike NCRs, is constitutively present on γδ T cells3 (Figure 1A). We then cross-linked NCRs and NKG2D on Vδ1 T cells and measured chemokine levels in cell supernatants. As positive control, we engaged the function of TCR-γδ that induced the highest production of all chemokines tested. The results showed that the engagement of NKp30 induced a significantly higher production CCL3/MIP-1α, CCL4/ MIP-1β, and CCL5/RANTES compared with that of Vδ1 T cells in the absence of receptor triggering. In contrast, the cross-linking of NKp46, NKp44, and NKG2D did not result in an increment of cc-chemokine secretion similar to that observed for NKp30. Finally, the production of CXCL12/SDF-1 did not change either in the presence or in the absence of NCRs and NKG2D engagement on Vδ1 T cells (Figure 1B).
Phenotypic and functional characterization of NCR+ Vδ1 T cells producing cc-chemokines. (A) Summary graphs of statistical dot plots showing the percentages of freshly purified (day 0) and in vitro activated (day 14) Vδ1 T cells expressing NKp30, NKp46, NKp44, and NKG2D. (B) Levels of CCL3, CCL4, CCL5, and CXCL12 spontaneously secreted by Vδ1 T cells (white bars) compared with those of Vδ1 T cells whose NKp30 (red bars), NKp46 (blue bars), NKp44 (green bars), NKG2D (brown bars), or TCR-γδ (black bars) were previously cross-linked with anti-NKp46, anti-NKp30, anti-NKp44, anti-NKG2D, and anti–TCR-γδ mAbs, respectively. Data are presented as a mean of 10 independent experiments (with P values and SD) performed in duplicates from 10 unrelated healthy donors. *P > .0001. #P < .05. Differences were assessed using the Mann-Whitney test, and all P values are 2-sided and unadjusted. n.s. indicates not significant.
Phenotypic and functional characterization of NCR+ Vδ1 T cells producing cc-chemokines. (A) Summary graphs of statistical dot plots showing the percentages of freshly purified (day 0) and in vitro activated (day 14) Vδ1 T cells expressing NKp30, NKp46, NKp44, and NKG2D. (B) Levels of CCL3, CCL4, CCL5, and CXCL12 spontaneously secreted by Vδ1 T cells (white bars) compared with those of Vδ1 T cells whose NKp30 (red bars), NKp46 (blue bars), NKp44 (green bars), NKG2D (brown bars), or TCR-γδ (black bars) were previously cross-linked with anti-NKp46, anti-NKp30, anti-NKp44, anti-NKG2D, and anti–TCR-γδ mAbs, respectively. Data are presented as a mean of 10 independent experiments (with P values and SD) performed in duplicates from 10 unrelated healthy donors. *P > .0001. #P < .05. Differences were assessed using the Mann-Whitney test, and all P values are 2-sided and unadjusted. n.s. indicates not significant.
NKp30-induced production of cc-chemokines suppresses HIV-1 replication
The protective role of chemokines in controlling HIV-1 replication was disclosed in the mid-1990s, when seminal studies demonstrated that CCL3, CCL4, CCL5, and CXCL12 act as potent inhibitors of HIV-1 entry in the CD4+ T cells. Indeed, these chemokines have been demonstrated to compete with the HIV-1–envelope/CD4 complex for the binding to CCR5 and CXCR4, the 2 coreceptors for cc-chemokines and CXCL12, respectively.18-21 The elucidation of this pathogenic mechanism led to the development of one of the therapeutic regimens currently available to effectively suppress viral replication in HIV-1–infected persons.22
To understand whether in vitro activated NCR+ Vδ1 T cells could naturally suppress HIV-1 replication through the NKp30-induced production of cc-chemokines, we incubated in time-course experiments CD4+/CCR5+ PM1 cell line infected with an R5 tropic strain of HIV-1 either in the presence or in the absence of supernatants from cultures of Vδ1 T cells selectively activated via NCRs and NKG2D. The highest and statistically significant suppression of HIV-1 replication occurred with supernatants from Vδ1 T cells cross-linked with the NKp30 mAb, similar to that observed with the supernatant of Vδ1 T cells activated via TCR-γδ. We did not find any analogous suppression of HIV-1 replication when supernatants of Vδ1 T cells cross-linked with anti-NKp46, -NKp44, and -NKG2D mAbs were added to cultures of HIV-1–infected PM-1 cell line (Figure 2A). To determine whether HIV-1 suppression was indeed associated with the high levels of cc-chemokine production on NKp30 or TCR-γδ engagement on Vδ1 T cells, we blocked the antiviral functions of cc-chemokines by adding neutralizing mAbs for CCL3, CCL4, and CCL5. The results confirmed our hypothesis by showing that the blocking of cc-chemokines restored HIV-1 replication to levels similar to those observed in the cultures of HIV-1–infected PM1 alone (Figure 2B). We then performed blocking experiments individually neutralizing CCL3, CCL4, or CCL5. We found that only the blocking of CCL4 was able to significantly restore HIV-1 replication, although not at the same levels produced by the blocking of all 3 chemokines together (supplemental Figure 2). The fact that CCL4 plays a dominant role in suppressing viral replication is in line with the highest level of production of this chemokine on engagement of NKp30 on Vδ1 T cells (Figure 1B). Overall, these data demonstrate that the highest level of viral suppression is determined by the combined and synergic effect of CCL3, CCL4, and CCL5.
Suppression of HIV-1 replication through cc-chemokines secreted on NKp30 engagement on Vδ1 T cells. (A) Time course experiments showing the levels of viral replication in PM1 cell line infected with HIV-1BaL R5 strain either in the absence (yellow bars) or in the presence of supernatant from cultures of Vδ1 T cells whose NKp30 (red bars), NKp46 (blue bars), NKp44 (green bars), NKG2D (brown bars), and TCR-γδ (black bars) were previously cross-linked with anti-NKp46, anti-NKp30, anti-NKp44, anti-NKG2D, and anti–TCR-γδ mAbs, respectively. *P > .0001. #P < .05. Differences were assessed using the Mann-Whitney test, and all P values are 2-sided and unadjusted. n.s. indicates not significant. (B) The detection of HIV-1 replication in PM1 cells line (yellow bars) was performed either in the absence (red and black bars) or in the presence (pink and gray bars) of neutralizing mAbs to CCL3, CCL4, and CCL5. Data are presented as a mean of 10 independent experiments (with P values and SD) performed in duplicates from 10 unrelated healthy donors.
Suppression of HIV-1 replication through cc-chemokines secreted on NKp30 engagement on Vδ1 T cells. (A) Time course experiments showing the levels of viral replication in PM1 cell line infected with HIV-1BaL R5 strain either in the absence (yellow bars) or in the presence of supernatant from cultures of Vδ1 T cells whose NKp30 (red bars), NKp46 (blue bars), NKp44 (green bars), NKG2D (brown bars), and TCR-γδ (black bars) were previously cross-linked with anti-NKp46, anti-NKp30, anti-NKp44, anti-NKG2D, and anti–TCR-γδ mAbs, respectively. *P > .0001. #P < .05. Differences were assessed using the Mann-Whitney test, and all P values are 2-sided and unadjusted. n.s. indicates not significant. (B) The detection of HIV-1 replication in PM1 cells line (yellow bars) was performed either in the absence (red and black bars) or in the presence (pink and gray bars) of neutralizing mAbs to CCL3, CCL4, and CCL5. Data are presented as a mean of 10 independent experiments (with P values and SD) performed in duplicates from 10 unrelated healthy donors.
We obtained similar results in terms of cc-chemokine production and viral suppression using either supernatants from highly purified FACS-sorted (mean average of NKp30 expression of 99%) or unsorted (mean average of NKp30 expression of 27%) NKp30+ Vδ1 T cells (supplemental Figures 3-4). Moreover, the cross-linking of NKp46 and NKp44 on highly pure and FACS sorted NKp44+ and NKp46+ Vδ1 T cells (data not shown) did not significantly increase the cc-chemokine secretion to levels similar to those observed with NKp30 engagement (supplemental Figure 4B). These data demonstrate the predominant role of NKp30, among NCRs, in promoting the antiviral activity of Vδ1 T cells and also suggest that the production of cc-chemokines is mainly mediated by the specific induction of the NKp30 downstream pathway after its engagement on Vδ1 T cells, rather than depending on the levels of NKp30 cell surface expression. Moreover, we also observed that, among T-cell subsets, this specific antiviral effect is restricted to Vδ1 T cells because in vitro activation of either Vγ9Vδ22 or α/β T (supplemental Figure 5A) cells did not induce the expression of NKp30 and, therefore, did not result in a suppression of viral replication through the NKp30-mediated secretion of cc-chemokines (supplemental Figure 5B-C). Given the lack of induction of CXCL12 on NCRs and NKG2D engagement, we did not analyze the ability of Vδ1 T cells to suppress CXCR4 tropic HIV-1 strains.
To verify whether or not the inducible expression of NKp30 is retained also during the course of HIV-1 infection, we expanded Vδ1 T cells from HIV-1–infected patients. Our data showed very low or undetectable levels of NKp30 on freshly purified γδ T cells from HIV-1–infected patients, whereas the in vitro activation of these cells with PHA plus IL-2 induced in cells from HIV-1–infected donors the de novo expression of NKp30 to levels similar to those observed in healthy donors (Figure 1; supplemental Figure 6A). Furthermore, the cross-linking of NKp30 on expanded Vδ1 T cells from HIV-1–infected persons induced a remarkable and similar production, if compared with that of uninfected individuals, of cc-chemokines capable of significantly suppressing HIV-1 replication in infected CD4+/CCR5+ PM1 cell line (supplemental Figure 6). These results demonstrate that Vδ1 T cells expanded from HIV-1–infected patients, although constantly exposed to the chronic inflammation given by HIV-1 viremia, are not anergic or senescent. Indeed, strong stimulation and NKp30 engagement allow Vδ1 T cells to produce high amounts of cc-chemokines, thus deploying a potent antiviral effect that can potentially limit the spreading of infection even in an autologous system during the course of HIV-1 disease.
Collectively, this study demonstrates that NKp30 engagement on in vitro activated Vδ1 T cells is able to suppress HIV-1 replication through the NKp30-induced production of cc-chemokines. Our novel findings open new important insights for understanding the role and for manipulating the functions of γδ T cells during HIV-1 disease. Indeed, HIV-1 infection is characterized by a great expansion of Vδ1 T cells5-8 and by chronic inflammation.23 In such conditions, activated and expanded Vδ1 T cells producing cc-chemokines might contribute to the control of viral replication. This phenomenon is probably even more relevant in mucosal tissues, given the fact that intestinal and cervical mucosae, where Vδ1 T cells are naturally resident,3 are 2 important gates of HIV-1 entry. In this context, NKp30+ Vδ1 T cell–mediated control of viral replication might play an important role in restraining the establishment of viral reservoirs and in limiting the levels of mucosal and systemic inflammation generated by the pathologic translocation of microflora in the lamina propria and mesenteric lymph nodes after the massive depletion of mucosal CD4+ T cells in acute and highly viremic stages of HIV-1 infection.24
Importantly, and in line with what was recently demonstrated for Vγ9Vδ2 T cells in SIV-infected primates,25 the establishment of novel protocols either inducing NKp30 expression on expanded Vδ1 T cells in vivo or adoptively transferring in vitro differentiated NKp30+ Vδ1 T cells may be of great therapeutic value in the cure of HIV/AIDS.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Prof Alessandro Moretta and Dr Emanuela Marcenaro for providing mAbs anti-NCRs, Ana E. Sousa for critical reading the manuscript, and Dr Anthony S. Fauci for his support and for providing samples from HIV-1–infected patients.
This work was supported by the Italian Ministry of Health (grants RF-ICH-2009-1304134 and RF-ICH-2009-1299677) and the Italian Association for Cancer Research (Proposal IG 9104). B.S.-S. is supported by the European Research Council (StG-260352) and European Molecular Organization Young Investigator Program.
Authorship
Contribution: K.H., M.F., D.V.C., J.M., and A.R. performed research and analyzed data; S.D.B., B.S.-S., and D.M. analyzed data; K.H., B.S.-S., and D.M. wrote the manuscript; and D.M. conceived and planned the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Domenico Mavilio, Laboratory of Clinical and Experimental Immunology, IRCCS, Istituto Clinico Humanitas, Via Alessandro Manzoni, 113 Rozzano, Milano, Italy; e-mail: domenico.mavilio@humanitas.it.
References
Author notes
B.S.-S. and D.M. contributed equally to this study.