Abstract
The effects of eltrombopag, a thrombopoietin-receptor agonist, on platelet function in immune thrombocytopenia (ITP) are not fully characterized. This study used whole blood flow cytometry to examine platelet function in 20 patients receiving eltrombopag treatment at days 0, 7, and 28. Platelet surface expression of activated GPIIb/IIIa, P-selectin, and GPIb was measured with and without low and high adenosine diphosphate (ADP) and thrombin receptor activating peptide (TRAP) concentrations. Before eltrombopag treatment with no ex vivo agonist, platelet activation was higher in ITP patients than controls. Platelet GPIb and activated GPIIb/IIIa expression without added agonist was unchanged following eltrombopag treatment, whereas a slight increase in P-selectin was observed. Expression of P-selectin and activated GPIIb/IIIa in response to high-dose ADP was lower during eltrombopag treatment than at baseline. Eltrombopag led to a slight increase in platelet reactivity to TRAP only in responders to eltrombopag but not to levels above those in controls; whole blood experiments demonstrated that this increase was probably because of higher platelet counts rather than higher platelet reactivity. In conclusion, although thrombocytopenic ITP patients have higher baseline platelet activation than controls, eltrombopag did not cause platelet activation or hyper-reactivity, irrespective of whether the platelet count increased.
Introduction
Immune thrombocytopenia (ITP) is an autoimmune condition characterized by antibody-mediated accelerated platelet destruction and suboptimal platelet production, which leads to various degrees of thrombocytopenia and bleeding symptoms.1,2 Thrombopoietin receptor (TPO-R) agonists have recently been developed for the treatment of patients with ITP, and can dramatically increase platelet counts and reduce bleeding symptoms in the majority of patients, including some with severe refractory thrombocytopenia.3-5 Eltrombopag, is an oral, small-molecule TPO-R agonist that interacts with the transmembrane domain of the receptor in a noncompetitive manner with endogenous TPO, thereby activating intracellular signal transduction pathways and leading to enhanced megakaryocyte differentiation, proliferation, and platelet production.6
Patients with ITP often have few bleeding symptoms despite very low platelet counts, suggesting that ITP platelets are highly functional.7-9 The TPO-R is expressed on circulating platelets,10 and although TPO stimulation in vitro does not directly activate platelets, it potentiates platelet reactivity to several agonists, including adenosine diphosphate (ADP), thrombin, and collagen.11-13 Administration of eltrombopag to healthy volunteers does not increase platelet aggregation or platelet surface expression of P-selectin or activated GPIIb/IIIa.14 However, the effect of eltrombopag on platelet function in vivo in thrombocytopenic patients is unknown and requires study because of the potential for thrombosis with higher (eg, normal) platelet counts. Platelet activation can be hypothesized to occur either by direct stimulation of platelet TPO receptors rendering them more susceptible to lower concentrations of agonists or indirectly because of an influx of young, large, potentially more reactive platelets. In a study of patients with thrombocytopenia because of chronic liver disease who were undergoing a surgical procedure and received eltrombopag for 2 weeks, portal vein thrombosis occurred in 6 patients (4%) treated with eltrombopag, 5 of whom had platelet counts more than 200 × 109/L, compared with 1 patient (1%) receiving a placebo.15
Therefore, this study examined the effects of eltrombopag on the activation state of circulating platelets (no agonist added ex vivo) and on platelet activation in response to ex vivo agonist stimulation in patients with severe thrombocytopenia because of chronic ITP. Whole blood flow cytometry was used to minimize ex vivo platelet activation, and because unlike all other platelet function assays, this method enables platelet function studies at very low platelet counts (< 20-50 × 109/L).16 The end points of platelet activation used in this study were platelet surface P-selection, activated GPIIb-IIIa, and GPIb.17 Platelet surface P-selectin expression is a sensitive marker of platelet degranulation. Activated GPIIb/IIIa was measured using the PAC-1 reporter monoclonal antibody, which only binds to GPIIb/IIIa receptors that have undergone the conformational change that enables fibrinogen to bind and platelet aggregation to occur.17 GPIb is a receptor for von Willebrand factor and its expression is strongly related to platelet surface area, and therefore to platelet size. Whereas platelet surface expression of activated GPIIb/IIIa and P-selectin increases on platelet activation, platelet surface GPIb expression decreases as the receptor is proteolyzed and/or internalized.18-20
Methods
Twenty patients with chronic (> 6 months) ITP who received eltrombopag (Promacta/Revolade; GlaxoSmithKline) treatment as part of 3 phase II/III studies (Repeat Exposure to Eltrombopag Study [REPEAT, TRA108057]21 ; Eltrombopag Extended Dosing Study [EXTEND, TRA105325]22 and Randomised, Placebo-controlled Idiopathic Thrombocytopenic Purpura Study with Eltrombopag [RAISE, TRA102537])23 gave informed consent in accordance with the Declaration of Helsinki and were enrolled in this Weill Medical College IRB-approved study which required only additional blood to be drawn at the scheduled blood draws of the primary treatment studies. Eltrombopag (50 mg) was administered orally once daily and dose adjustment rules for each study were followed.
Patients were studied before starting eltrombopag treatment (day 0), and days 7 and 28 after continuous daily treatment. For the purpose of this analysis, a response to eltrombopag treatment was defined as a platelet count ≥ 50 × 109/L and twice the pretreatment (day 0) platelet count. Two patients had platelet counts > 140 × 109/μL on day 7 of treatment, and their treatment was therefore discontinued before the day 28 time point. A control blood sample from a healthy donor was analyzed together with the patient blood samples on each study day. Twenty controls were studied, of whom all provided written consent and had not taken any antiplatelet medications within 7 days before the study. Samples from the healthy control donors were analyzed in parallel to the ITP patients with no manipulation of platelet counts to avoid artifactual in vitro activation.
Blood was drawn by antecubital venipuncture into 4.5 mL 3.2% trisodium citrate and ethylenediaminetetraacetic acid (EDTA) Vacutainers (BD Biosciences). The EDTA sample was used for measurement of platelet counts and mean platelet volume (MPV) in a Bayer-Advia automated complete blood count (CBC) counter immediately after the blood draw. Immature platelet fraction (IPF%) and the absolute immature platelet fraction (AIPF) were measured in a Sysmex XE-2100 autoanalyzer within 8 hours of blood draw (using the same EDTA sample).24
Platelet activation and reactivity to agonists was assessed by whole blood flow cytometry as this can be used at low platelet counts and correlates well with standard assays of platelet function, such as platelet aggregometry (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Twenty minutes after blood draw, aliquots of whole blood from the citrate sample were incubated with fluorescently labeled monoclonal antibodies and either 0.5μM ADP (low), 20μM ADP (high), 1.5μM thrombin receptor activating peptide (TRAP; low), 20μM TRAP (high), or N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid (HEPES) Tyrode buffer (10mM HEPES, 137mM sodium chloride, 2.8mM potassium chloride, 1mM magnesium chloride, 12 mM sodium hydrogen carbonate, 0.4mM sodium phosphate dibasic, 5.5mM glucose, 0.35% wt/vol bovine serum albumin, and pH 7.4) for exactly 15 minutes. The reaction was stopped with a 15-fold dilution in 1% formaldehyde in HEPES-saline buffer. Samples were maintained at room temperature and not agitated until fixation to prevent handling activation.
The antibodies used were: phycoerythrin (PE)–conjugated anti–P-selectin monoclonal antibody (CD62P, clone 1E3, Santa Cruz Biotech); fluorescein isothiocyanate (FITC)–conjugated monoclonal antibody PAC-1 (BD Biosciences, which only binds to the activated conformation of GPIIb/IIIa25 ; and PE-Cy5–conjugated anti-CD42b (GPIb) monoclonal antibody (clone HIP1, BD Pharmingen). PE-conjugated MIgG2a isotype (Santa Cruz Biotechnology), and FITC–PAC-1 together with 2.5 μg/mL of the GPIIb/IIIa antagonist eptifibatide to block specific binding, served as the negative controls for platelet surface P-selectin and platelet surface activated GPIIb/IIIa, respectively.
To examine the correlation between platelet reactivity as measured by platelet surface activated GPIIb/IIIa expression and platelet aggregation, citrate anticoagulated blood was collected from patients at baseline, 45 minutes, and 24 hours after treatment with the GPIIb/IIIa antagonist tirofiban (supplemental Figure 1). These patients were males and females aged 21 years or older with coronary artery disease who were undergoing percutaneous coronary intervention with a high likelihood of treatment with tirofiban. Platelet aggregation in response to 20μM ADP was performed on platelet-rich plasma after adjustment of platelet count to 250 000/μL. Platelet surface activated GPIIb/IIIa was determined by incubating whole blood with PAC1-FITC/CD61-perCP with or without 20μM ADP for 25 minutes followed by addition of a fixative.
Fixed samples were stored at 4°C and sent by overnight courier to the Center for Platelet Function Studies at the University of Massachusetts Medical School for flow cytometric analysis. This was performed in a Becton Dickinson fluorescence-activated cell sorter FACSCalibur flow cytometer that was calibrated daily to assure proper instrument function and consistent fluorescence measurements over time. A known quantity of RFP-30-5 calibration beads (Spherotech) was added to allow cell counts to be calculated. The platelet count was determined for each sample and the volume was adjusted to achieve a consistent data acquisition rate for flow cytometric analysis. Platelet surface P-selectin, activated GPIIb/IIIa, and GPIb expression were measured relative to the isotype control as mean fluorescence intensity (MFI).
To establish the effect of different platelet counts on agonist responsiveness, control experiments were performed where the platelet count of blood from healthy volunteers was manipulated. Briefly, platelet rich plasma (PRP) and platelet-poor plasma (PPP) were prepared from citrate anticoagulated whole blood from healthy volunteers (n = 5) by centrifugation for 12 minutes at 150g and 2000g for 10 minutes, respectively. The plasma was added back to the original volume of blood centrifuged for PRP. This blood was mixed and recentrifuged for PRP. This second PRP preparation step allowed a reconstituted whole platelet count of approximately 50 000/μL. The remaining packed red blood cell (RBC)/white blood cell (WBC) volume from the second PRP step was mixed and aliquoted (500 μL) into siliconized microcentrifuge tubes. A graduated combination of PRP and PPP was then added back to 6 aliquots ranging from 500 μL PRP to 500 μL PPP to create a range of platelet counts while maintaining a constant RBC/WBC count. Complete blood counts were determined on the 6 aliquots using a Sysmex XE-2100 hematology analyzer. These platelet count-manipulated whole blood samples were then processed for flow cytometry exactly as the samples from patient and control subjects.
Statistical analysis
Results are stated as mean ± SEM. A mixed model repeated measures ANOVA was used to examine the effects of eltrombopag on the platelet surface markers of activation with and without agonist across 3 time points. On finding a significant interaction between agonist condition and time, Bonferroni-adjusted pairwise comparisons for time at each treatment were performed (ie, no agonist: baseline vs week 1, baseline vs week 4, and week 1 vs week 4). Results were declared significant if P was less than .05.
Results
Patient demographics and clinical characteristics
The mean age of the ITP patients was 59 years (range 31-83), 58% were female and 53% were splenectomized. Three patients were taking prednisolone and one patient was taking mycophenolate mofetil and danazol at stable doses before enrollment in the study, and the doses were not altered during the study period. The mean age of the controls was 27 years (range 19-35) and 60% were female; none were taking any medications at the time of study.
Bleeding symptoms were significantly reduced in the patients who responded to eltrombopag treatment (P < .001, data not shown). No thromboembolic events occurred in any of the study patients or controls during the course of the study.
Platelet counts and immature platelets
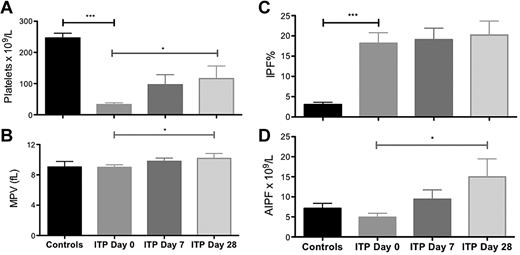
Before treatment with eltrombopag, the ITP patients had, as expected, significantly lower platelet counts than the healthy controls (32.5 ± 5.9 × 109/L vs 246.4 ± 15.2 × 109/L, P < .0001, Figure 1). There was no significant difference in MPV or AIPF between ITP patients and controls, but ITP patients had, as expected, a higher IPF% than controls (18.17 ± 2.61% vs 3.06 ± 0.57%, P < .001; Figure 1).
Platelet parameters in healthy controls and in patients with ITP at 0, 7, and 28 days of eltrombopag treatment. (A) Platelet count, (B) mean platelet volume (MPV), (C) immature platelet fraction (IPF) %, and (D) absolute immature platelet fraction (AIPF). Data are mean ± SEM (*P < .05; **P < .02; ***P < .001).
Platelet parameters in healthy controls and in patients with ITP at 0, 7, and 28 days of eltrombopag treatment. (A) Platelet count, (B) mean platelet volume (MPV), (C) immature platelet fraction (IPF) %, and (D) absolute immature platelet fraction (AIPF). Data are mean ± SEM (*P < .05; **P < .02; ***P < .001).
Treatment of the ITP patients with eltrombopag resulted in an increase in platelet count (day 0, 32.5 ± 5.9 vs day 7, 96.5 ± 32.2 [P = .05] vs day 28, 116.2 ± 40.2 × 109/L [P = .04 for day 28 vs day 0]; Figure 1). Platelet size was increased by day 28 of eltrombopag treatment (MPV day 0, 9.0 ± 0.3 vs day 7, 9.8 ± 0.4 [P = NS] vs day 28, 10.2 ± 0.6 fL [P = .03 for day 28 vs day 0]; Figure 1). There was also a significant increase in the absolute immature platelet fraction by day 28 of treatment (AIPF, day 0, 4906 ± 101 vs day 28, 14 980 ± 449 [P = .04] immature platelets/mL) although no change in the immature platelet fraction (IPF %; Figure 1).
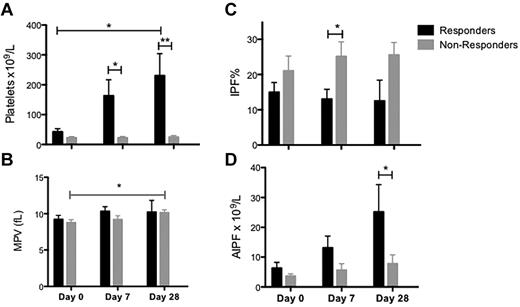
Ten of the 20 patients treated with eltrombopag responded to treatment with a day 28 platelet count that was ≥ 50 × 109/L and ≥ 2-fold higher than on day 0. The patients who responded to eltrombopag had a slightly higher platelet count and lower immature platelet fraction before starting treatment than those who did not respond (Figure 2). There was no difference in platelet size or AIPF between eltrombopag responders and nonresponders (Figure 2). Although the AIPF increased after eltrombopag treatment in both responders and nonresponders, the IPF% did not change significantly in either group of patients (Figure 2).
Platelet parameters in responders to eltrombopag treatment. (A) Platelet count, (B) mean platelet volume (MPV), (C) immature platelet fraction (IPF) %, and (D) absolute immature platelet fraction (AIPF). Responders (black columns) and nonresponders (gray columns). Data are mean ± SEM (*P < .05; **P < .02; ***P < .001).
Platelet parameters in responders to eltrombopag treatment. (A) Platelet count, (B) mean platelet volume (MPV), (C) immature platelet fraction (IPF) %, and (D) absolute immature platelet fraction (AIPF). Responders (black columns) and nonresponders (gray columns). Data are mean ± SEM (*P < .05; **P < .02; ***P < .001).
Platelet surface markers of activation with and without ex vivo agonist stimulation in ITP patients before eltrombopag treatment compared with controls
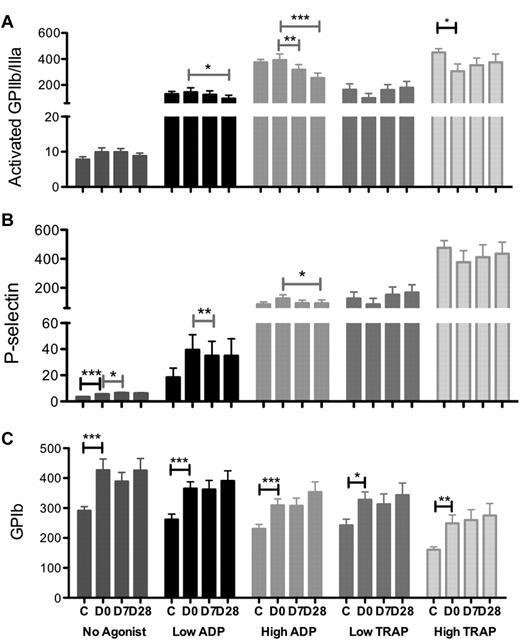
With no addition of agonist ex vivo, platelet surface expression of P-selectin and GPIb (and to a lesser extent activated GPIIb/IIIa) on circulating platelets was higher in ITP patients than in normal controls before eltrombopag treatment (P-selectin, P < .0001; GPIb, P = .001; GPIIb/IIIa, P = .14; Figure 3). As expected, in response to ex vivo stimulation with ADP and TRAP agonists, platelet surface expression of activated GPIIb/IIIa and P-selectin increased, whereas platelet surface GPIb decreased on platelet activation. In response to TRAP stimulation, platelet surface activated GPIIb/IIIa and surface P-selectin tended to be lower among ITP patients than normal controls, whereas platelet response to ADP was similar among ITP patients and controls (Figure 3A-B). Expression of platelet surface GPIb in response to both ADP and TRAP was substantially higher in ITP patients than in controls (Figure 3C).
Platelet surface expression of activation markers in controls and ITP patients at days 0, 7, and 28 of eltrombopag treatment with no added agonist and in response to low and high dose ADP and TRAP stimulation. (A) Activated GPIIb/IIIa (PAC-1), (B) P-selectin, and (C) GPIb. C indicates healthy controls; D0, ITP patients pretreatment with eltrombopag; and D7 and D28, 7 and 28 days after initiation of eltrombopag treatment in ITP patients. Black-capped bars indicate unpaired (ITP at baseline vs controls) and gray bars indicate pairwise comparisons (ITP patients at different study days). Data are mean ± SEM (*P < .05; **P < .02; ***P < .001).
Platelet surface expression of activation markers in controls and ITP patients at days 0, 7, and 28 of eltrombopag treatment with no added agonist and in response to low and high dose ADP and TRAP stimulation. (A) Activated GPIIb/IIIa (PAC-1), (B) P-selectin, and (C) GPIb. C indicates healthy controls; D0, ITP patients pretreatment with eltrombopag; and D7 and D28, 7 and 28 days after initiation of eltrombopag treatment in ITP patients. Black-capped bars indicate unpaired (ITP at baseline vs controls) and gray bars indicate pairwise comparisons (ITP patients at different study days). Data are mean ± SEM (*P < .05; **P < .02; ***P < .001).
Platelet surface markers of activation without ex vivo agonist stimulation in ITP patients after eltrombopag treatment
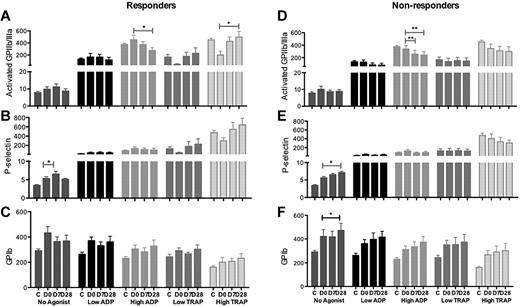
Platelet expression of activated GPIIb/IIIa and GPIb was unchanged after eltrombopag treatment despite increasing platelet counts (Figure 3). A slight increase in P-selectin expression was observed in both responders and nonresponders (P < .05; Figure 4B,E).
Platelet surface markers of activation in responders and nonresponders to eltrombopag treatment with no added agonist and in response to low and high dose ADP and TRAP stimulation. (A-C) Responders and (D-F) nonresponders. (A,D) Activated GPIIb/IIIa (PAC-1), (B,E) P-selectin, and (C,F) GPIb. C indicates healthy controls; D0, ITP patients pretreatment with eltrombopag; D7 and D28, 7 and 28 days after initiation of eltrombopag treatment in ITP patients. Data are mean ± SEM (*P < .05; **P < .02; ***P < .001).
Platelet surface markers of activation in responders and nonresponders to eltrombopag treatment with no added agonist and in response to low and high dose ADP and TRAP stimulation. (A-C) Responders and (D-F) nonresponders. (A,D) Activated GPIIb/IIIa (PAC-1), (B,E) P-selectin, and (C,F) GPIb. C indicates healthy controls; D0, ITP patients pretreatment with eltrombopag; D7 and D28, 7 and 28 days after initiation of eltrombopag treatment in ITP patients. Data are mean ± SEM (*P < .05; **P < .02; ***P < .001).
Platelet surface markers of activation with ex vivo agonist stimulation in ITP patients after eltrombopag treatment
With high-dose ADP stimulation, platelet surface activated GPIIb/IIIa and P-selectin expression was significantly lower after eltrombopag treatment than at baseline as well as lower than that of the healthy controls (GPIIb/IIIa expression day 0 vs day 7, P = .07, day 0 vs day 28, P = .001; P-selectin day 0 vs day 28, P = .04; Figure 3A-B). Eltrombopag treatment lead to a slight but nonsignificant increase in TRAP-induced platelet surface GPIIb/IIIa and P-selectin expression to levels not above those observed in the healthy controls when responders and nonresponders were considered together (Figure 3A-B). Comparing responders with nonresponders, a decrease in activated GPIIb/IIIa expression in response to ADP stimulation was observed during eltrombopag treatment in both groups, but an increase in response to TRAP stimulation occurred only in the responders (Figure 4).
There was no significant change in agonist-induced platelet surface GPIb expression among the ITP patients after eltrombopag treatment when all patients were combined (Figure 3C).
To explore whether the changes in platelet surface expression were related to the increase in platelet count rather than to platelet activation/reactivity per se, control experiments were performed using whole blood from healthy controls in which the platelet count was adjusted (Figure 5). These experiments showed: (1) the ADP-induced increase in platelet surface activated GPIIb/IIIa is slightly, but not significantly increased by increasing platelet count, whereas the TRAP-induced increase was significantly greater with increasing platelet count (Figure 5A); (2) the agonist-induced increase in platelet surface P-selectin is unaffected by platelet count, except for 1.5μM (low dose/submaximal) TRAP which results in an increased platelet surface P-selectin with increasing platelet count (Figure 5B); and (3) the agonist-induced decrease in platelet surface GPIb is unaffected by the platelet count (Figure 5C).
Platelet surface markers of activation in controls following in vitro manipulation of platelet counts. (A) Activated GPIIb/IIIa, (B) P-selectin, and (C) GPIb. Data are mean ± SEM, n = 5 (*P < .05, **P < .02) for agonist-induced platelet integrin expression in thrombocytopenic samples compared with that at “normal” platelet counts (ie, 175 × 109/L).
Platelet surface markers of activation in controls following in vitro manipulation of platelet counts. (A) Activated GPIIb/IIIa, (B) P-selectin, and (C) GPIb. Data are mean ± SEM, n = 5 (*P < .05, **P < .02) for agonist-induced platelet integrin expression in thrombocytopenic samples compared with that at “normal” platelet counts (ie, 175 × 109/L).
Although this study was not powered to formally examine reproducibility of platelet function endpoints, this was tested in 8 patients who were treated with 2 treatment cycles of eltrombopag as part of the REPEAT study and similar trends were seen in the first and second treatment cycles (supplemental Figure 2).
Discussion
Patients with ITP often have few bleeding symptoms despite very low platelet counts, and it is generally accepted that the larger, younger platelets of patients with ITP function better than normal platelets.7-9 Stimulating platelet production using TPO-R agonists is a novel treatment for ITP3,4 ; all other therapies primarily aim to ameliorate the immune-mediated accelerated platelet destruction. Two TPO-R agonists, romiplostim and eltrombopag, are now approved for use in chronic ITP in the United States and at least 50 other countries and can potently increase platelet counts and reduce bleeding symptoms in approximately 60%-90% of patients.3,26-28
In view of the presence of the TPO-R on the platelet surface, it is important to define the possible effects of TPO-R agonists on platelet function in patients with ITP because of potential safety concerns as a result of any additional platelet activation beyond that which may already be present in patients with ITP.7-9 Specifically, the incidence of thromboembolic events in patients with chronic ITP is thought to be higher than in the general population independent of TPO-R agonist therapy.29-31
In this study, we therefore examined the in vivo activation state of circulating platelets (no added agonist) and the platelet response to agonist stimulation ex vivo in ITP patients with marked thrombocytopenia before and after 1 and 4 weeks of treatment with eltrombopag. These time points were selected to assess the impact of eltrombopag administration on platelets already in circulation as well as platelets produced in response to eltrombopag treatment. These parameters were compared with those in healthy controls, and in addition, control experiments were performed to explore the effect of the platelet count per se (ie, independent of eltrombopag treatment) on platelet function.
IPF% was higher in patients with ITP than the controls (Figure 1C), reflecting the increased platelet production. Treatment with eltrombopag led to increased platelet counts, platelet size, and AIPF, but no significant change in IPF% (Figure 1). The higher pretreatment platelet counts and lower IPF% among patients who responded to eltrombopag treatment (Figure 2) suggests that a higher preexisting rate of immune platelet clearance may be associated with failure to respond to eltrombopag treatment.
Before treatment with eltrombopag, expression of P-selectin, GPIb, and activated GPIIb/IIIa on circulating platelets (no added agonist) was higher among ITP patients than controls, which suggests that a higher level of platelet activation and platelet surface GPIb contributes to maintaining vascular integrity at lower platelet counts. The platelet surface expression of activated GPIIb/IIIa and P-selectin in response to TRAP stimulation was lower for ITP patients than controls, although there was no difference in response to ADP (Figure 3). Therefore, although the activation state of circulating platelets was higher and the platelets are younger and larger, the capacity for further activation of these platelets on thrombin stimulation appears impaired in severely thrombocytopenic patients with ITP compared with control individuals.
Because eltrombopag restored normal responsiveness to TRAP stimulation and did not result in abnormal reactivity to ADP, there was no evidence of platelet hyperreactivity after eltrombopag treatment. Indeed, a progressive decrease in platelet surface expression of P-selectin and activated GPIIb/IIIa in response to ADP stimulation was observed at 7 and 28 days after initiation of eltrombopag treatment (Figure 3). Although increased platelet reactivity to TRAP stimulation was observed in the patients whose platelet counts increased after eltrombopag treatment, it was not to a level beyond that of the control subjects (Figure 4A). Furthermore, the whole blood experiments in which the platelet count was adjusted (Figure 5) demonstrated that this increase was consistent with higher platelet count rather than higher platelet reactivity per se. TRAP is a strong degranulator of platelets, inducing release of endogenous ADP and serotonin from dense granules, resulting in further platelet activation. As the platelet count increases, more endogenous ADP and serotonin is available for further platelet stimulation. The platelet responses to ADP, unlike TRAP, were unaffected by the platelet count (Figure 5), perhaps because ADP is a much weaker degranulator of platelets than TRAP and therefore there is much less endogenous secretion of ADP and serotonin.
Therefore, the marginal increase in platelet surface activated GPIIb/IIIa and P-selectin in response to TRAP stimulation after eltrombopag treatment may be explained by the eltrombopag-induced increase in platelet count. In support of these in vitro data, no increase in platelet P-selectin nor activated GPIIb/IIIa in response to TRAP stimulation was observed in the patients whose platelet counts did not increase after eltrombopag treatment (Figure 4D-E).
In contrast to the increase in platelet surface P-selectin and activated GPIIb/IIIa on platelet activation, platelet surface GPIb expression decreases on platelet activation, as a result of both proteolysis and internalization.19,32 Before eltrombopag treatment, expression of platelet surface GPIb in response to both ADP and TRAP was substantially higher in ITP patients than in controls (Figure 3C), presumably reflecting the larger platelet size and therefore surface area in ITP patients. GPIb expression on circulating platelets was not reduced after eltrombopag treatment (Figure 3C). There was therefore no evidence that eltrombopag resulted in platelet activation in patients with ITP, irrespective of whether the patients were responders or nonresponders to eltrombopag.
This study is the first detailed examination of platelet activation and platelet reactivity in thrombocytopenic patients with ITP receiving TPO-R agonists. Phase 1 studies in healthy individuals with normal platelet counts indicated that there was no change in the platelet surface expression of activated GPIIb/IIIa or platelet aggregation in response to ADP stimulation after eltrombopag treatment.14 In a study using platelets isolated from healthy volunteers, the addition of eltrombopag in vitro had no effect on platelet aggregation and α-granule release in response to ADP and collagen, whereas platelet reactivity was enhanced after treatment with recombinant TPO.33 A report of 2 patients who received continuous eltrombopag treatment for 12 weeks and 1 patient treated intermittently found that the reduced responsiveness to TRAP stimulation seen pretreatment in ITP patients appeared to “normalize” after eltrombopag treatment,34 in accordance with our findings in this study of 19 patients with 27 eltrombopag treatment courses.
In conclusion, although thrombocytopenic ITP patients have a higher level of baseline platelet activation than controls, eltrombopag does not result in additional platelet activation or hyperreactivity in patients with chronic ITP, irrespective of whether the patients were responders or nonresponders to eltrombopag. Consistent with our findings, there has been no clear increase in thromboembolic events in the clinical trials of TPO-R agonists in chronic ITP compared with placebo controls.23,35
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank M. Lesser and M. Kline at the Biostatistics Unit, Feinstein Institute, North Shore LIJ for assisting with statistical analysis and data interpretation.
B.P. is the recipient of a Kay Kendall Leukaemia Fund traveling fellowship and a Fulbright Scholarship for Cancer Research, and J.B.B. receives funding from National Institutes of Health grant 1U01 HL72196-01-05. This study was partly supported by unrestricted grants from GlaxoSmithKline and Sysmex.
National Institutes of Health
Authorship
Contribution: B.P., J.B.B., A.L.F., and A.D.M. participated in study design, sample collection and processing, data analysis, and cowrote the paper; B.B., C.T., and K.M. recruited patients and collected samples; and M.D.L., Y.L., and M.R.B. processed samples and participated in data analysis.
Conflict-of-interest disclosure: Funding was provided by an unrestricted grant from GlaxoSmithKline; J.B.B. receives clinical research support from Amgen, Cangene, GlaxoSmithKline, Genzyme, IgG of America, Immunomedics, Ligand, Eisai Inc, Shionogi, and Sysmex. His family owns stock in Amgen and GlaxoSmithKline and he has participated in advisory boards/consults for Amgen, GlaxoSmithKline, Ligand, Shionogi, Eisai, and Portola. A.D.M. has consulted with GlaxoSmithKine. The remaining authors declare no competing financial interests.
Correspondence: Alan D. Michelson, Center for Platelet Research Studies, Division of Hematology/Oncology, Children's Hospital Boston, 300 Longwood Ave, Karp 08213, Boston, MA; e-mail: alan.michelson@childrens.harvard.edu.