Abstract
Galectin-9 (Gal-9) is a tandem repeat-type member of the galectin family and is a ligand for T-cell immunoglobulin mucin domain 3 (Tim-3), a type-I glycoprotein that is persistently expressed on dysfunctional T cells during chronic infection. Studies in autoimmune diseases and chronic viral infections show that Tim-3 is a regulatory molecule that inhibits Th1 type immune responses. Here we show that soluble Gal-9 interacts with Tim-3 expressed on the surface of activated CD4+ T cells and renders them less susceptible to HIV-1 infection and replication. The Gal-9/Tim-3 interaction on activated CD4+ T cells, leads to down-regulation of HIV-1 coreceptors and up-regulation of the cyclin-dependent kinase inhibitor p21 (also known as cip-1 and waf-1). We suggest that higher expression of Tim-3 during chronic infection has evolved to limit persistent immune activation and associated tissue damage. These data demonstrate a novel mechanism for Gal-9/Tim-3 interactions to induce resistance of activated CD4+ T cells to HIV-1 infection and suggest that Gal-9 may play a role in HIV-1 pathogenesis and could be used as a novel microbicide to prevent HIV-1 infection.
Introduction
Prophylactic interventions against HIV-1 acquisition, such as vaccine and microbicide candidates, have not proved efficacious or even enhanced acquisition in previous human clinical trials.1,2 Even the most promising vaccine to date, which involved a canarypox prime followed by a gp120 protein boost, only showed limited efficacy that waned over time.3 More effective strategies that block initial HIV-1 acquisition at the site of exposure are required. Interestingly, deletion of 32 base pairs in the ccr5 gene4 and selective up-regulation of p21 in CD4+ T cells from elite controllers5 render some individuals naturally resistant to HIV-1 infection. The mechanism(s) responsible for resistance of CD4+ T cells to HIV-1 infection are not well known, but defining them is vital for designing prophylactic interventions.
Galectin-9 (Gal-9), a member of the β-galactoside–binding animal lectin family, was originally characterized as an eosinophil chemoattractant.6 Subsequent studies determined that it is a versatile immunomodulator involved in a wide range of biologic activities, such as cell adhesion and migration, proliferation and apoptosis, interaction of host cells with microbial pathogens, regulatory T-cell (Treg) differentiation and function, dendritic cell (DC) maturation, and antimicrobial immunity.7-13 Gal-9 is expressed by eosinophils, endothelial cells, T lymphocytes, DCs, macrophages, lymphoid cells, Kupffer cells, intestinal epithelial cells, and vascular endothelial cells.10,14-19 Wide distribution of Gal-9 on host cells demonstrates an important but complex role for this lectin, whose biologic effects are exerted by 2 receptors with distinct, and often opposing effects: TIM-3 (T-cell immunoglobulin [Ig] and mucin domain-containing molecule 3)20 and cell surface protein disulfide isomerase (PDI).21
Tim-3 negatively regulates Th1 responses on interaction with Gal-9.20 In humans, defects in Tim-3 expression contribute to multiple sclerosis pathology,22 suggesting that expression of Tim-3 on effector T cells is involved in inducing/maintaining peripheral tolerance of these T cells. Furthermore, sustained Tim-3 expression by effector CD4+ and CD8+ T cells during HIV-1 and hepatitis C virus (HCV) infection defines a distinct population of dysfunctional T cells and correlates with disease progression.23,24 Tregs constitutively express Gal-9,25 and thus could be providing the ligand for inducing tolerance in Tim-3–expressing effectors. Recently we demonstrated that Tregs suppress proliferation of nonprotective HIV-specific CD8+ CTL through Tim-3/Gal-9 interactions during chronic infection.7 Apparently, under these conditions, Tim3:Gal9 interactions lead to inappropriate suppression or apoptosis of Tim-3+ effector T cells,11 thereby limiting their antiviral activity. Such a response may be an adaptation to chronic infection or inflammation to prevent the development of immunopathology.
Although the Tim-3/Gal-9 interaction in autoimmunity and tolerance induction has been extensively studied, the role of this receptor/ligand interaction in antiviral immunity has not been fully elucidated. Data suggest that Tim-3/Gal-9 interactions in the context of microbial infection leads to a dual outcome, either enhancement of innate immunity and clearance of the pathogen10 or termination of adaptive immunity and reduction in inflammation-related tissue damage.26 This may be related to the fact that Tim-3 ligation on DCs and macrophages leads to their activation, whereas Tim-3 ligation on T cells results in their inhibition.13 Although many Gal-9 functions, in particular on T cells, are regulated via Tim-3 binding, various Tim-3–independent effects of Gal-9 have been reported.27 These observations indicate that the effects of Gal-9 on T cells are mediated by additional receptors apart from Tim-3.
A recent study revealed the cell surface PDI as a novel T-cell glycoprotein receptor for Gal-9, and demonstrated that the Gal-9/PDI interaction enhanced HIV-1 entry and infection in CD4+ T cells.21 As a result, interaction of Gal-9 with different receptors can have either positive or negative effects on different cell types or the same cell type in different inflammatory settings.
In this study, we demonstrate a novel role for Tim-3 in the context of HIV-1 infection and show that Gal-9/Tim-3 interactions reduce HIV-1 infection in activated CD4+ T cells. The mechanisms by which Gal-9 acted in vitro are probably complex and involve down-regulation of HIV-1 coreceptors, such as CXCR4, CCR-5, and α4β7, and up-regulation of p21 (Waf1/Cip1) in CD4+ T cells, which has been reported to be associated with control of HIV-1 replication.5 Therefore, our data suggest that Gal-9/Tim-3 interactions are beneficial in the context of HIV-1 acquisition (by inhibiting HIV-1 replication in activated CD4+ T cells) but detrimental in the context of chronic infection (leading to HIV-specific CD8+ T-cell suppression by Tregs, as we previously described7 ). Taken together, influencing the function of the Tim-3/Gal-9 pathway holds promise as a means to prevent HIV-1 infection in activated CD4+ T cells.
Methods
Study population
PBMC samples from 12 HIV− and 4 HIV+ individuals were used for this study. Plasma samples for Gal-9 detection were obtained from 30 chronically HIV+ individuals. The appropriate Institutional Review Boards at the University of Washington (UW), Fred Hutchinson Cancer Research Center (FHCRC), and Seattle BioMed approved the studies. All study participants gave written informed consent to participate in this study in accordance with the Declaration of Helsinki.
HIV-1 viruses
The CXCR4-using isolate LAI and the CCR5-using HIV-1 strain CSF-Jr were obtained from the AIDS Research and Reagent Program at the National Institutes of Health. Green fluorescent protein (GFP)–labeled T-cell tropic single cycle HIV-1SF2 was kindly provided by Leonidas Stamatatos (Seattle Biomed).
Ex vivo infection assays
CD4+ T cells were cultured in RPMI supplemented with 10% fetal bovine serum, recombinant IL-2 (50 U/mL), and phytohemagglutinin (PHA; 2 μg/mL). After 3 days, cells were washed and treated with different concentrations of stable Gal-9 (sGal-9 or G9NC[null] provided by GalPharma)28 for 1 to 2 hours before infection with HIV-1. Gal-9–treated cells and untreated cells were infected with HIV-1 viruses at multiplicity of infection (MOI) of 0.1 using magnetofection.29 However, in some experiments, CD4+ T cells were infected first, washed extensively and then Gal-9 was added into the wells for 2 hours. Every other day, one-half of the culture supernatant was replaced with fresh medium supplemented with 50 U/mL IL-2. Four to 5 days after infection CD4+ T cells were subjected to flow cytometric p24 analysis using KC57-PE anti-p24 antibody (Beckman Coulter). Parallel cultures of CD4+ T cells infected with the virus in the absence of Gal-9 served as negative controls.
Blocking assays
PHA stimulated CD4+ T cells were incubated with 2.5 to 10 μg/mL anti–Tim-3 polyclonal antibody or IgG isotype control (R&D Systems) or 10 μg/mL LEAF anti–Tim-3 (clone F38-2E2; BioLegend) at 37°C for +30 hours to block the interactions of Tim-3 with Gal-9. In addition, in some other assays anti-PDI monoclonal antibody (mAb; clones RL77, RL90) from Novus Biologicals (1:3000) was used to block the interactions of PDI and Gal-9.
Cell separation and flow cytometry
CD4+ T cells were isolated by negative selection using a magnetic separation system (StemCell Technologies). Purity was assessed as > 97% by flow cytometry using a LSRII cytometer (BD Bioscience). Th2 cells were isolated from enriched CD4+ T cells by positive selection using CD294 MicroBeads (Miltenyi Biotec). Purity of monocytes (> 96% pure as determined by flow cytometry) were obtained by positive selection of CD14+ cells using magnetic separation (StemCell Technologies). For phenotypic characterization, CD4+ T cells were stained with appropriate antibodies as indicated for each assay. Viability dye and antibodies directed against CD3, CD4, CCR5, CXCR4, α4β7, CD25, CD38, HLA-DR, and Ki67 (BD Bioscience), Tim-3 (R&D Systems), and Gal-9 (Biolegend) were used in these studies. Twenty-five minutes after staining cells were washed, fixed in paraformaldehyde and acquired by flow cytometry using a LSRII flow cytometer (BD Bioscience), and then analyzed with FlowJo Version 7.2.2 software (TreeStar).
RT-PCR assays
Expression of CDKN1A and p53 mRNA were analyzed by reverse transcription-polymerase chain reaction (RT-PCR) using standard methods.5 Total RNA was extracted from CD4+ T cells using RNeasy Mini kit (QIAGEN) RNA isolation system followed by one step RT-PCR using SuperScript (Invitrogen). The primers to detect p53 cDNA sequence, sense primer 5′GTTCCGAGAGCTGAATGAGG3′ and antisense primer 5′TCTGAGTCAGGCCCTTCTGT3′; primers to detect cDNA sequence of p21 sense primer 5-GACAGCAGAGGAAGACCAT-3 and antisense primer 5-TGGAGTGGTAGAA ATCTGTCAT-3; and primers to detect cDNA sequences of GAPDH (glyceraldehyde-3-phosphate dehydrogenase) sense primer 5′-CCACCCATGGCAAATTCCATGGCA-3′ and antisense primer 5′-TCTAGACG GCAGGTCAGGTCCACC-3′ as a housekeeping gene, were synthesized by the instrument manufacturer (Sigma-Aldrich).
p21 expression assay by real-time PCR
Total RNA was extracted from CD4+ T cells and cDNA was synthesized using SuperScript VILO cDNA Synthesis Kit (Applied Biosystems). CDKN1A mRNA expression was analyzed by quantitative RT-PCR using the standard TaqMan expression assay with primers and probes synthesized by the instrument manufacturer (Applied Biosystems). Actb (encoding β-actin) was used as a housekeeping gene. Real-time PCR was conducted using the following conditions: 95°C for 10 minutes, and 95°C for 15 seconds, 60°C 15 seconds, 72°C 30 seconds for 50 cycles, followed by 72°C 10 minutes. Quantification of the genes was performed using the comparative threshold method. The expression level of each mRNA was normalized to Actb mRNA and represented as n-fold difference relative to the calibrator untreated control. All PCR assays were performed in duplicate and the results are represented by the mean ± SD.
siRNA transduction
CD4+ T cells were isolated using magnetic separation (StemCell Technologies) and RNA interference experiments were performed using a nucleofector device according to the manufacturer's protocol (Lonza). Briefly, CD4+ T cells were suspended in transfection reagent (Lonza) and p21-specific or control siRNA (Dharmacon-Thermo Scientific) was added at a concentration of 10nM. CD4+ T cells were then transfected using program V-024. Cells were resuspended in culture medium, 4 hours after transfection medium was replaced with fresh medium containing IL-2 (50 U/mL). Twenty-four hours after transfection, expression of p21 was assessed using RT-PCR (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), and cells were infected with HIV-1. Knockdown of p21 gene expression in CD4+ T cells is shown in supplemental Figure 1. Control CD4+ T cells received a scrambled I duplex RNA (Dharmacon-Thermo Scientific).
Gal-9 detection by ELISA
The Gal-9 concentration in plasma, as previously described,30 was quantified by ELISA (Galpharma).
Results
Reduced susceptibility of CD4+ T cells to HIV-1 infection in the presence of Gal-9
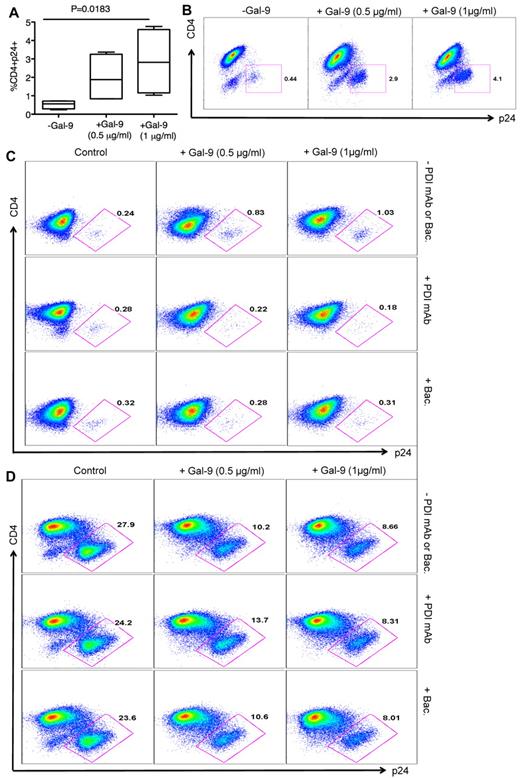
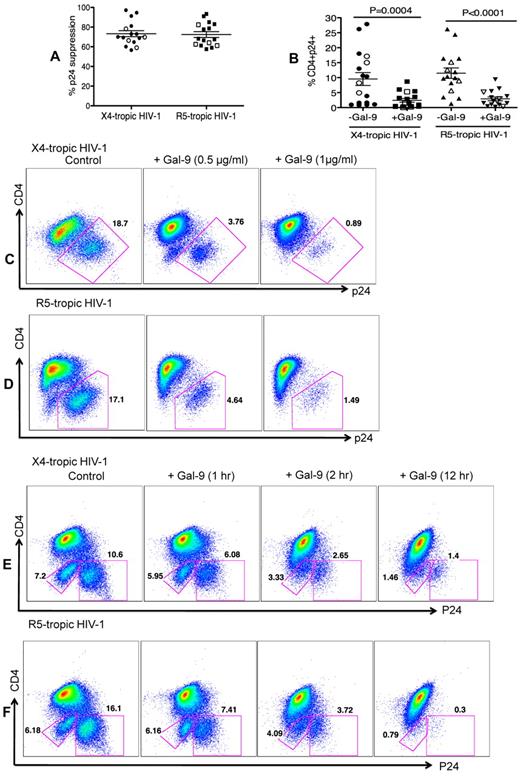
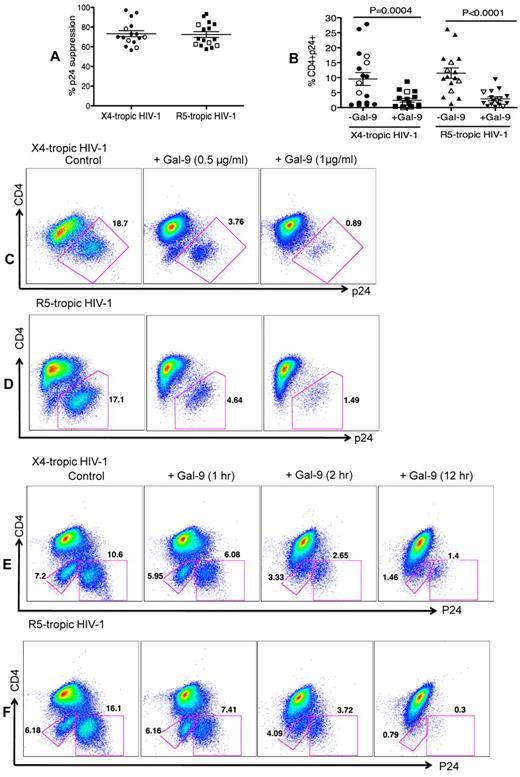
The impact of Gal-9 on HIV-1 infection of CD4+ T cells was evaluated in an HIV-1 ex vivo infection assay. CD4+ T cells were isolated and made susceptible to HIV-1 infection by in vitro culture with exogenous IL-2 and PHA stimulation. PHA activation resulted in significant up-regulation of Tim-3 expression on the surface of CD4+ T cells (both CD4+ and CD294+CD4+ cells; supplemental Figure 2). Subsequently, CD4+ T cells were infected with either the laboratory-adapted X4-tropic isolate (HIV-1LAI; MOI 0.1) or R5-tropic HIV-1 isolate (HIV-1JR-CSF; MOI 0.1) in the presence or absence of Gal-9 (0.5-1 μg/mL; 2 hours before infection). Viral replication was analyzed by intracellular p24 staining using flow cytometry on day 4 to 5 after infection. Using these culture conditions, we consistently observed that Gal-9 induced up to > 90%, and an average of 75%, inhibition of infection in CD4+ T cells with both X4-tropic and/or R5-tropic HIV-1 viruses (Figure 1A). Gal-9 induced inhibition of HIV-1 infection in CD4+ T cells was significant for both X4-tropic (P = .0004) and R5-tropic isolates (P < .0001; Figure 1B). Interestingly, Gal-9 exhibited dose-dependent suppressive effects on HIV-1 infection (HIV-1LAI, Figure 1C; HIV-1JR-CSF, Figure 1D) and the inhibitory effects were time-dependent, with a max effect at 12 hours stimulation (Figure 1E-F). However, 2 hours of stimulation provided the optimal inhibitory effects on HIV-1 infection and minimal CD4+ T-cell death (supplemental Figure 3). Therefore, 2 hours of Gal-9 stimulation was used in all subsequent experiments. Although it was reported that Gal-9 induces apoptosis of both CD4+ and CD8+ T cells in vitro through the calcium-calpain caspase-1 pathway,11 it does not induce cell death in activated CD8+ T cells in vivo.13 Importantly, Gal-9 induced minimal apoptosis of CD4+ T cells after 2 hours incubation (supplemental Figure 3) demonstrating that the apoptotic effects of Gal-9 on CD4+ T cells does not have a significant impact on the frequency of CD4+ T cells in Gal-9–treated compared with nontreated wells. However, exposure of CD4+ T cells to Gal-9 for a longer period of time (12 hours) results in more death in CD4− p24− T cells (Figures 1E-F).
Reduced susceptibility of CD4+ T cells to HIV-1 infection in the presence of Gal-9. (A) Percentages of p24 suppression in activated CD4+ T cells stimulated with Gal-9 (1 μg/mL) 2 hours before HIV-1 infection with X4-tropic (HIV-1LAI) and R-5 (HIV-1JR-CSF) isolates. CD4+ T cells from 12 HIV-1 seronegative (filled symbols) and 4 HIV-1 seropositive (open symbols) were studied. (B) Percentages of CD4+p24+ cells in activated CD4+ T cells in the presence of Gal-9 (1 μg/mL) 2 hours before HIV-1 infection with X4-tropic (HIV-1LAI) and R-5 (HIV-1JR-CSF) isolates. Significance was tested using paired t test. (C-D) Representative flow cytometry dot plots from CD4+ T cells stimulated with 0.5 μg/mL or 1 μg/mL Gal-9 before infection with X4-tropic or R5-tropic HIV-1 isolates, respectively. (E-F) Representative flow cytometry dot plots from CD4+ T cells stimulated with Gal-9 (1 μg/mL) for 1, 2, and 12 hours before infection with X4-tropic or R5-tropic HIV-1 isolates, respectively. The number of infected cells was quantified by intracellular viral p24 antigen staining using flow cytometry on day 5 after infection. Error bars indicate mean ± SEM from duplicate cell cultures from 12 HIV-1 seronegative and 4 HIV+ individuals infected 4 separate times with both X4-and R5-tropic viral isolates in vitro. Each point represents an individual.
Reduced susceptibility of CD4+ T cells to HIV-1 infection in the presence of Gal-9. (A) Percentages of p24 suppression in activated CD4+ T cells stimulated with Gal-9 (1 μg/mL) 2 hours before HIV-1 infection with X4-tropic (HIV-1LAI) and R-5 (HIV-1JR-CSF) isolates. CD4+ T cells from 12 HIV-1 seronegative (filled symbols) and 4 HIV-1 seropositive (open symbols) were studied. (B) Percentages of CD4+p24+ cells in activated CD4+ T cells in the presence of Gal-9 (1 μg/mL) 2 hours before HIV-1 infection with X4-tropic (HIV-1LAI) and R-5 (HIV-1JR-CSF) isolates. Significance was tested using paired t test. (C-D) Representative flow cytometry dot plots from CD4+ T cells stimulated with 0.5 μg/mL or 1 μg/mL Gal-9 before infection with X4-tropic or R5-tropic HIV-1 isolates, respectively. (E-F) Representative flow cytometry dot plots from CD4+ T cells stimulated with Gal-9 (1 μg/mL) for 1, 2, and 12 hours before infection with X4-tropic or R5-tropic HIV-1 isolates, respectively. The number of infected cells was quantified by intracellular viral p24 antigen staining using flow cytometry on day 5 after infection. Error bars indicate mean ± SEM from duplicate cell cultures from 12 HIV-1 seronegative and 4 HIV+ individuals infected 4 separate times with both X4-and R5-tropic viral isolates in vitro. Each point represents an individual.
CD14+ cells (monocytes) were also treated with Gal-9 for 2 hours and then infected with HIV-1JR-CSF(MOI 0.1). Gal-9 inhibited infection of monocytes in a dose-dependent manner (supplemental Figure 4), which suggests that the increased resistance to HIV-1 infection induced by Gal-9 occurs in both activated CD4+ T cells and monocytes. Because CD4+ T cells are the primary initial targets of infection,31 the remaining studies were performed only on CD4+ T cells. Overall, these results demonstrate that Gal-9 induces significant resistance of in vitro-stimulated CD4+ T cells to HIV-1 infection in a time and dose-dependent manner.
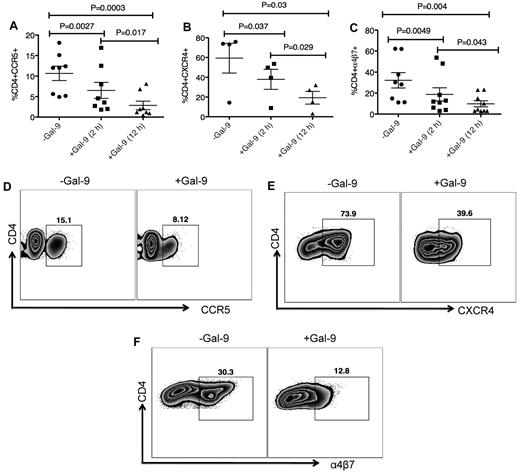
Gal-9 down-regulates CCR5, CXCR4, and α4β7 on the surface of activated CD4+ T cells
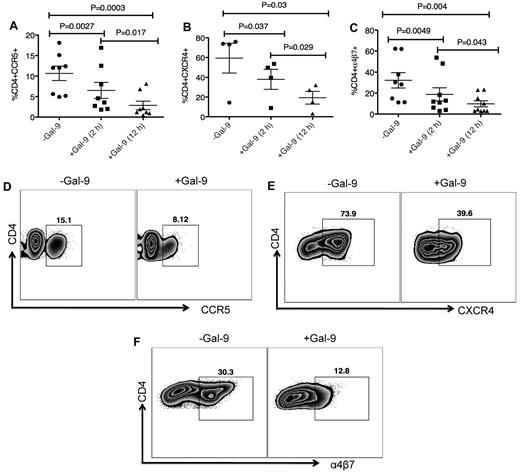
We investigated whether Gal-9 affects cell surface levels of the major HIV coreceptors. Because CD4+ T cells from some donors do not express substantial levels of HIV coreceptors, we induced up-regulation of these ex vivo. CD4+ T cells were cultured in the presence of PHA and IL-2 for 3 to 4 days to up-regulate CCR5.32 After activation, CD4+ T cells were cultured in the presence of Gal-9 for up to 12 hours. Gal-9 (1 μg/mL) significantly down-regulated expression of CCR5 approximately 50% to 75% compared with untreated cultures after 2 (P = .0027) and 12 hours (P = .0003), respectively (Figure 2 A-D). The effects of Gal-9 on down regulation of CCR5 were time dependent (Figures 2A; P = .017). CD4+ T cells were cultured with IL-4 and/or dexamethasone for 3 days to induce expression of CXCR4.33 Gal-9 (1 μg/mL) significantly down-regulated surface expression of CXCR4 approximately 25% and 50% at 2 and 12 hours incubation compared with untreated cultures (P = .037 and P = .03, respectively; Figures 2B-E). The inhibitory effects of Gal-9 on CXCR4 expression were significantly higher after 12 hours incubation (P = .029; Figure 2B). α4β7 demarcates a highly susceptible subset of CD4+ T cells.34 We cultured CD4+ T cells in the presence of retinoic acid for 3 days to express α4β735 (Figure 2F) before culturing in the presence or absence of Gal-9 (1 μg/mL). The presence of Gal-9 resulted in a significant decline in α4β7 surface expression (average 30% in 2 hours and > 50% after 12 hour incubation) compared with untreated cultures (P = .0049 and P = .004, respectively; Figures 2C-F). The suppressive effects of Gal-9 on α4β7 expression were significantly more evident when CD4+ T cells remained in the presence of Gal-9 for 12 hours (P = .043; Figure 2C). These data collectively indicated that Gal-9 significantly reduces surface expression of CCR5, CXCR4, and α4β7 on activated CD4+ T cells in a time-dependent manner and suggested that down regulation of HIV-1 coreceptors could be a mechanism by which Gal-9 reduces HIV-1 infection in CD4+ T cells. However, many of these markers (eg, CCR5 and α4β7) are also activation markers,34 and thus the effects of Gal-9 on surface expression could simply be a result of the ability of Gal-9 to suppress T-cell proliferation and activation.20 Indeed, Gal-9 also decreased expression of other known activation markers (CD38, HLA-DR, and Ki-67; supplemental Figure 5). Interestingly, Gal-9 up-regulates expression of CD25 on CD4+ T cells (supplemental Figure 5), which is in agreement with its known ability to expand Tregs.36
Gal-9 down-regulates CCR5, CXCR4 and α4β7 on the surface of activated CD4+ T cells. (A) Percentage of CCR5 suppression in PHA activated CD4+ T cells after stimulation with Gal-9 (1 μg/mL) for 2 and 12 hours. Data obtained from 8 HIV-1 seronegative individuals. (B) Percentage of CXCR4 suppression in IL-4/dexamethasone activated CD4+ T cells after stimulation with Gal-9 (1 μg/mL) for 2 and 12 hours. Data obtained from 4 HIV-1 seronegative individuals. (C) Percentage of α4β7 suppression in retinoic acid activated CD4+ T cells after stimulation with Gal-9 (1 μg/mL) for 2 and 12 hours. Data obtained from 9 HIV-1 seronegative individuals. Significance was tested using paired t test. (D-F) Representative flow cytometry dot plots from CD4+ T cells in the absence of Gal-9 (−Gal-9; left flow panel) or in the presence of Gal-9 (+Gal-9; right flow panel) for CCR5, CXCR4, and α4β7, respectively. Data obtained 2 hours after stimulation with Gal-9. Error bars indicate mean ± SEM from duplicate cell cultures from 4 to 9 independent experiments performed in vitro. Each point represents an individual.
Gal-9 down-regulates CCR5, CXCR4 and α4β7 on the surface of activated CD4+ T cells. (A) Percentage of CCR5 suppression in PHA activated CD4+ T cells after stimulation with Gal-9 (1 μg/mL) for 2 and 12 hours. Data obtained from 8 HIV-1 seronegative individuals. (B) Percentage of CXCR4 suppression in IL-4/dexamethasone activated CD4+ T cells after stimulation with Gal-9 (1 μg/mL) for 2 and 12 hours. Data obtained from 4 HIV-1 seronegative individuals. (C) Percentage of α4β7 suppression in retinoic acid activated CD4+ T cells after stimulation with Gal-9 (1 μg/mL) for 2 and 12 hours. Data obtained from 9 HIV-1 seronegative individuals. Significance was tested using paired t test. (D-F) Representative flow cytometry dot plots from CD4+ T cells in the absence of Gal-9 (−Gal-9; left flow panel) or in the presence of Gal-9 (+Gal-9; right flow panel) for CCR5, CXCR4, and α4β7, respectively. Data obtained 2 hours after stimulation with Gal-9. Error bars indicate mean ± SEM from duplicate cell cultures from 4 to 9 independent experiments performed in vitro. Each point represents an individual.
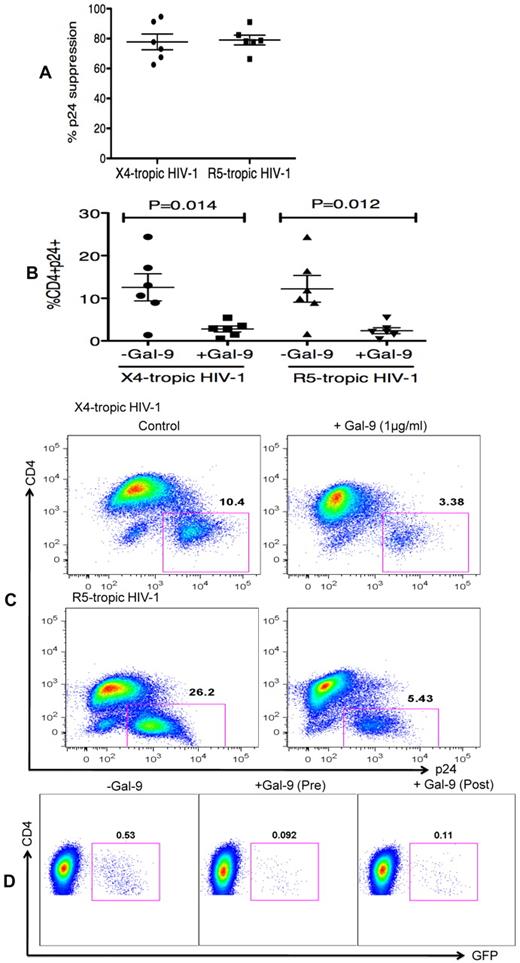
Gal-9 reduces HIV-1 infection in already infected CD4+ T cells
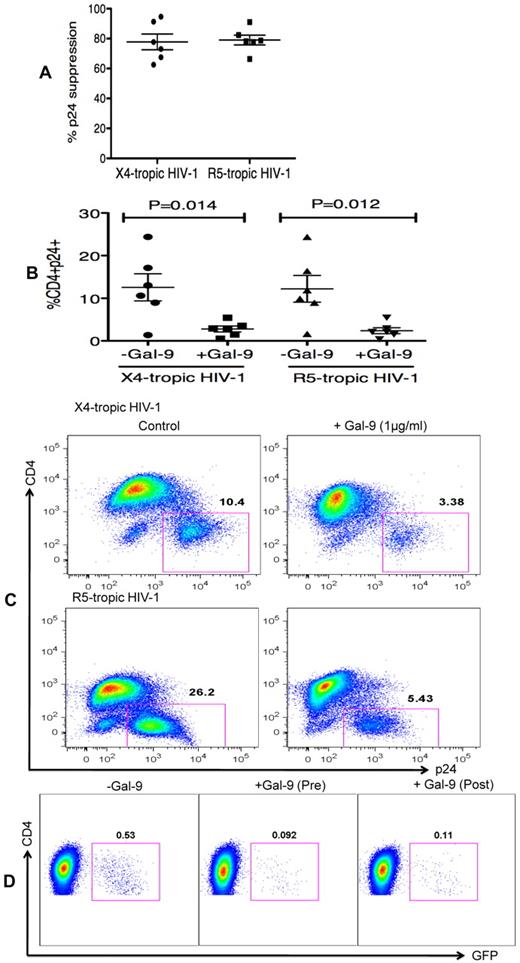
In the previous experiments, Gal-9 was added before HIV-1 infection of activated target cells. To determine whether Gal-9 could limit HIV-1 infection in cells that were already infected, CD4+ T cells were incubated with Gal-9 for 2 hours after infection with HIV-1. Surprisingly, Gal-9 significantly reduced viral infectivity ≥ 75% by both X4-tropic and R5-tropic viral isolates (P = .014 and P = .012, respectively; Figures 3A-C). Furthermore, to dissect whether the activation status of CD4+ T cells or downstream infection-related changes in cytokine milieu were responsible for the observed effects of Gal-9, cells were infected by a GFP-labeled single-cycle virus before or after treatment with Gal-9 (0.5 μg/mL 2 hours). Gal-9 reduced viral infection with this single-cycle virus, which suggests that the suppressive effects of Gal-9 were directly because of reducing susceptibility of the target cell rather than from altering downstream infection-related events (Figure 3D). These data demonstrated that Gal-9 both prevents HIV-1 infection in activated CD4+ T cells but also limits viral replication in CD4+ T cells that are already infected.
Gal-9 reduces HIV-1 infection in CD4+ T cells that are already HIV-infected. (A) Percentages of p24 suppression in activated CD4+ T cells that were exposed to Gal-9 (1 μg/mL) for 2 hours after HIV-1 infection with X-4 and R-5-tropic isolates. (B) Percentages of CD4+p24+ cells in activated CD4+ T cells in the presence of Gal-9 (1 μg/mL) 2 hours after HIV-1 infection with X-4-tropic (HIV-1LAI) and R-5 (HIV-1JR-CSF) isolates. Significance was tested using paired t test. (C) Representative flow cytometry dot plots from CD4+ T cells infected, and then stimulated with 1 μg/mL Gal-9 after infection with X-4-tropic or R5-tropic HIV-1 isolates, respectively. (D) Representative flow cytometry dot plots from CD4+ T cells incubated for 2 hours with 0.5 μg/mL Gal-9 prior or after infection with GFP-labeled T-cell tropic single-cycle HIV-1SF2. CD4+ T cells from 6 HIV-1 seronegative donors were used for each viral isolate. The number of infected cells was quantified by intracellular GFP detection 40 hours after infection for SF2 isolate and viral p24 antigen staining using flow cytometry on day 5 after infection for other viral isolates. Data are from 6 HIV-1 seronegative individuals infected with both X4-and R5-tropic viral isolates in vitro with duplicate cultures per condition. Error bars indicate mean ± SEM from duplicate cell cultures from 6 HIV-1 seronegative individuals infected 4 separate times with both X4-and R5-tropic viral isolates in vitro. Each point represents an individual.
Gal-9 reduces HIV-1 infection in CD4+ T cells that are already HIV-infected. (A) Percentages of p24 suppression in activated CD4+ T cells that were exposed to Gal-9 (1 μg/mL) for 2 hours after HIV-1 infection with X-4 and R-5-tropic isolates. (B) Percentages of CD4+p24+ cells in activated CD4+ T cells in the presence of Gal-9 (1 μg/mL) 2 hours after HIV-1 infection with X-4-tropic (HIV-1LAI) and R-5 (HIV-1JR-CSF) isolates. Significance was tested using paired t test. (C) Representative flow cytometry dot plots from CD4+ T cells infected, and then stimulated with 1 μg/mL Gal-9 after infection with X-4-tropic or R5-tropic HIV-1 isolates, respectively. (D) Representative flow cytometry dot plots from CD4+ T cells incubated for 2 hours with 0.5 μg/mL Gal-9 prior or after infection with GFP-labeled T-cell tropic single-cycle HIV-1SF2. CD4+ T cells from 6 HIV-1 seronegative donors were used for each viral isolate. The number of infected cells was quantified by intracellular GFP detection 40 hours after infection for SF2 isolate and viral p24 antigen staining using flow cytometry on day 5 after infection for other viral isolates. Data are from 6 HIV-1 seronegative individuals infected with both X4-and R5-tropic viral isolates in vitro with duplicate cultures per condition. Error bars indicate mean ± SEM from duplicate cell cultures from 6 HIV-1 seronegative individuals infected 4 separate times with both X4-and R5-tropic viral isolates in vitro. Each point represents an individual.
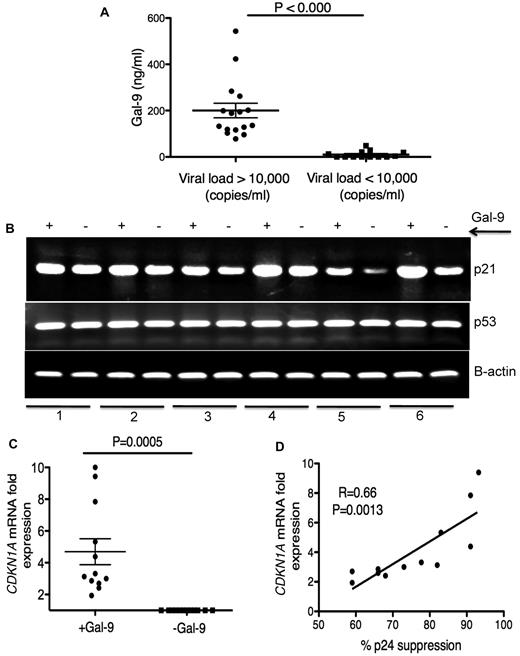
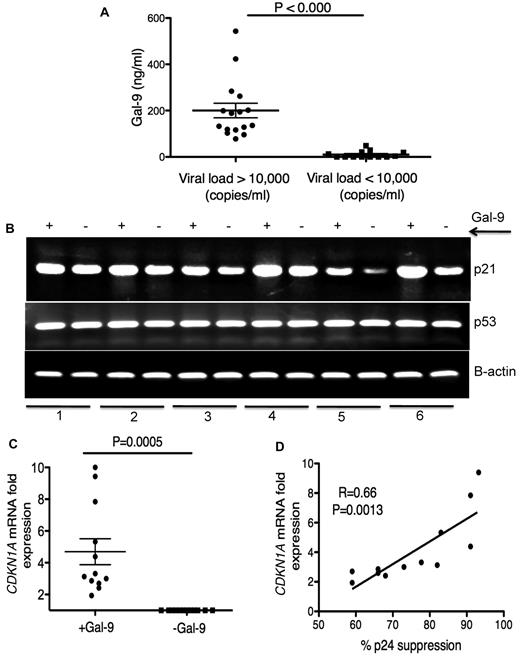
Elevation of Gal-9 in plasma of chronically HIV-1–infected individuals
Plasma Gal-9 concentrations in HIV+ individuals were measured by ELISA. We observed a significant increase (P < .0001) in plasma Gal-9 levels in HIV-1–infected individuals with > 10 000 copies/mL viral load than those with < 10 000 copies/mL (Figure 4A). HIV-1− and healthy controls had very low to undetectable levels of Gal-9 in their plasma (data not shown). Although, the average concentration of plasma Gal-9 was < 200 ng/mL, it could be detected at > 500 ng/mL in the plasma of some chronically infected individuals (Figure 4A). Gal-9, even at a concentration of 0.25 μg/mL and MOI = 0.025, reduced the infection of activated CD4+ T cells by approximatley 50% (supplemental Figure 6), which suggested that the Gal-9 concentrations we used in the previous experiments were physiologically relevant.
Elevation of Gal-9 in plasma of HIV+ individuals and up-regulation of p21 in CD4+ T cells after stimulation with Gal-9. (A) Detected levels of plasma Gal-9 from 30 HIV+ individuals with a viral load of less or more than 10 000 copies/mL. (B) RT-PCR reflecting β-actin, p53, and p21 gene expression in activated CD4+ T cells stimulated with Gal-9 (1 μg/mL; +Gal-9) or in the absence of Gal-9 (−Gal-9) for 2 hours before total RNA isolation. (C) Quantitative CDKN1A mRNA fold expression from activated CD4+ T cells after stimulation with Gal-9 (1 μg/mL) for 2 hours in vitro compared with CDKN1A mRNA expression in CD4+ T cells in the absence of Gal-9 from the same individual. Significance was tested using Wilcoxon signed rank test. (D) Correlation between CDKN1A mRNA fold expression from CD4+ T cells in the presence of Gal-9 (1 μg/mL) and resistance to HIV-1 infection. Resistance to infection was measured by intracellular staining for viral p24 antigen using flow cytometry on CD4+ T cells infected with R5-tropic HIV-1 isolate in the presence of Gal-9 (1 μg/mL). Pearson correlation coefficient is shown. Data were obtained from 12 different individuals and each point represents 1 individual.
Elevation of Gal-9 in plasma of HIV+ individuals and up-regulation of p21 in CD4+ T cells after stimulation with Gal-9. (A) Detected levels of plasma Gal-9 from 30 HIV+ individuals with a viral load of less or more than 10 000 copies/mL. (B) RT-PCR reflecting β-actin, p53, and p21 gene expression in activated CD4+ T cells stimulated with Gal-9 (1 μg/mL; +Gal-9) or in the absence of Gal-9 (−Gal-9) for 2 hours before total RNA isolation. (C) Quantitative CDKN1A mRNA fold expression from activated CD4+ T cells after stimulation with Gal-9 (1 μg/mL) for 2 hours in vitro compared with CDKN1A mRNA expression in CD4+ T cells in the absence of Gal-9 from the same individual. Significance was tested using Wilcoxon signed rank test. (D) Correlation between CDKN1A mRNA fold expression from CD4+ T cells in the presence of Gal-9 (1 μg/mL) and resistance to HIV-1 infection. Resistance to infection was measured by intracellular staining for viral p24 antigen using flow cytometry on CD4+ T cells infected with R5-tropic HIV-1 isolate in the presence of Gal-9 (1 μg/mL). Pearson correlation coefficient is shown. Data were obtained from 12 different individuals and each point represents 1 individual.
Up-regulation of p21 in CD4+ T cells after stimulation with Gal-9
We have shown that Gal-9 reduces expression of HIV-1 coreceptors as a possible inhibitory barrier against HIV-1 infection (Figure 2). Recently, the cyclin-dependent kinase inhibitor, p21 has been shown to be strongly up-regulated in CD4+ T cells from elite controllers. Furthermore, a highly significant inverse correlation was found between CDKN1A (the gene name for p21) mRNA expression levels in CD4+ T cells and susceptibility to HIV-1 infection.5 To determine whether p21 was involved in the reduced HIV-1 susceptibility of Gal-9 treated CD4+ T cells, we analyzed CDKN1A mRNA expression in Gal-9–treated CD4+ T cells by RT-PCR and quantitative RT-PCR. We observed strong up-regulation of CDKN1A mRNA as measured by RT-PCR (Figure 4B). Quantitative RT-PCR confirmed that Gal-9 induced a 2- to 10-fold (average > 4-fold) increase in mRNA expression of CDKN1A in Gal-9 treated CD4+ T cells compared with nontreated controls (P = .0005; Figure 4C). In addition, we compared the expression of p21 mRNA in CD4+ T cells in the presence of Gal-9 with the inhibitory effects of Gal-9 on HIV-1 infection in CD4+ T cells. A significant positive correlation between CDKN1A mRNA expression levels in Gal-9–treated CD4+ T cells and suppression of HIV-1 infection in CD4+ T cells, as determined by p24 expression after infection of ex vivo Gal-9–treated CD4+ T cells with HIV-1JR-CSF was observed (r2 = 0.66, P = .0013; Figure 4D). The expression of p21 is regulated by expression of p53.37 Therefore, we analyzed p53 mRNA expression by RT-PCR after treatment of CD4+ T cells with Gal-9. There were no obvious changes in the expression of p53 (Figure 4B), which suggests that Gal-9 causes p21 up-regulation in a p53-independent manner. Taken together, these data demonstrated that CD4+ T cells treated with Gal-9 expressed higher levels of p21 than did untreated cells and that levels of p21 expression were inversely correlated to CD4+T cell susceptibility to HIV-1 infection.
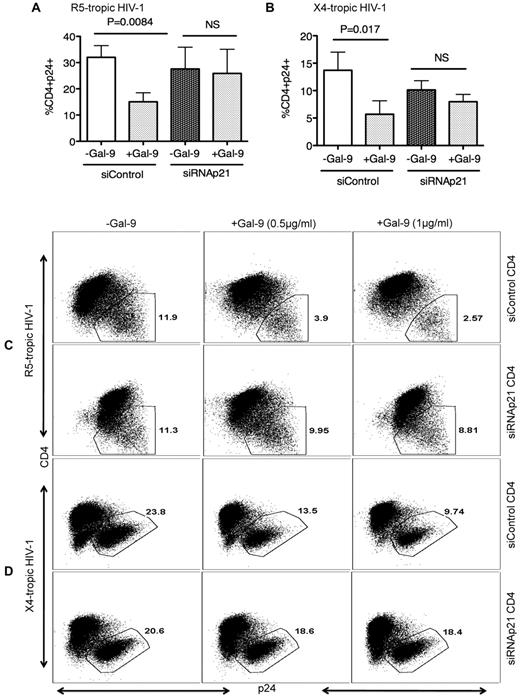
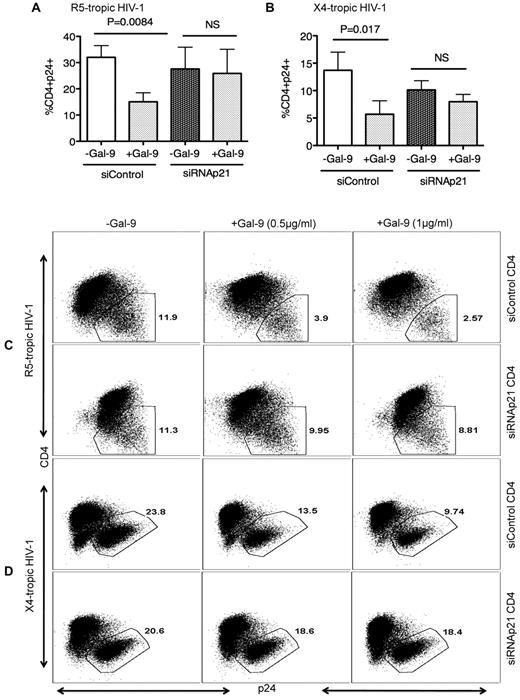
To test whether the elevated p21 levels in Gal-9–treated CD4+ T cells functionally contribute to resistance to HIV-1 infection, we performed ex vivo infection assays of CD4+ T cells transfected with siRNA, inducing effective down-regulation of p21 expression, or with control siRNA that does not affect CDKN1A gene expression (supplemental Figure 1) in the presence or absence of Gal-9. In the absence of p21 expression, the Gal-9–induced resistance of activated CD4+ T cells to HIV-1 infection was significantly abrogated for both X-4 tropic (P = .008; Figure 5A-C) and R-5 tropic (P = .017) viruses (Figure 5B-D) indicating that induction of p21 is critical for Gal-9 enhanced resistance of CD4+ T cells to HIV-1 infection.
Gal-9–mediated up-regulation of p21 reduces the susceptibility of CD4+ T cells to HIV-1 infection. (A) Percentages of CD4+p24+ cells in activated CD4+ T cells in the presence of control or p21-specific siRNA; then stimulated with Gal-9 (1 μg/mL) 2 hours before HIV-1 infection with R-5-tropic (HIV-1JR-CSF) or (B) X-4 tropic (HIV-1LAI) isolate. (C) Representative example of dot plots from activated CD4+ T cells infected in the presence of p21-specific or control siRNA with R5-tropic HIV-1 isolate or (D) X4-tropic HIV-1 isolate in the presence (0.5 μg/mL or 1 μg/mL) or absence of Gal-9. Viral infection was measured by viral p24 antigen staining on CD4+ T cells using flow cytometry. Plots are representative of independent experiments performed on 3 different donors for each viral isolate. Significance was tested using paired t test.
Gal-9–mediated up-regulation of p21 reduces the susceptibility of CD4+ T cells to HIV-1 infection. (A) Percentages of CD4+p24+ cells in activated CD4+ T cells in the presence of control or p21-specific siRNA; then stimulated with Gal-9 (1 μg/mL) 2 hours before HIV-1 infection with R-5-tropic (HIV-1JR-CSF) or (B) X-4 tropic (HIV-1LAI) isolate. (C) Representative example of dot plots from activated CD4+ T cells infected in the presence of p21-specific or control siRNA with R5-tropic HIV-1 isolate or (D) X4-tropic HIV-1 isolate in the presence (0.5 μg/mL or 1 μg/mL) or absence of Gal-9. Viral infection was measured by viral p24 antigen staining on CD4+ T cells using flow cytometry. Plots are representative of independent experiments performed on 3 different donors for each viral isolate. Significance was tested using paired t test.
Gal-9 up-regulates p21 and reduces infection of CD4+ T cells to HIV-1 infection through interaction with Tim-3
To determine whether the observed effects of Gal-9 on decreasing susceptibility to HIV-1 infection were mediated through its interaction with Tim-3, PHA activated CD4+ T cells were incubated with blocking anti–Tim-3 antibodies38 before incubation with Gal-9 and HIV-1 infection. Tim-3 blockade abrogated the effect of Gal-9 to prevent HIV-1 infection in a dose-dependent manner (Figure 6A). Consistent with this, we also blocked Tim-3 by incubating PHA activated CD4+ T cells with anti–Tim-3 blocking antibody and then stimulated them with or without Gal-9 for 2 hours and the expression of p21 mRNA by RT-PCR was examined. We observed no changes in the expression of p21after Gal-9 stimulation in the presence of Tim-3 blockade (Figure 6B). In contrast, the presence of Gal-9, in the absence of Tim-3 blockade, up-regulated p21 expression in PHA-activated CD4+ T cells (Figures 4A and 6B). These data indicated that the up-regulation of p21 by Gal-9 was mediated through Tim-3 ligation on activated CD4+ T cells.
Gal-9 reduces infection of CD4+ T cells to HIV-1 infection and up-regulates p21 through interaction with Tim-3. (A) Representative dot plots of activated CD4+ T cells in the presence of Gal-9 (1 μg/mL) for 2 hours and also in the presence or absence of anti–Tim-3 antibodies (2.5, 5, and 7.5 μg/mL anti–Tim-3 antibody [clone F38-2E2]) before in vitro infection with X4-Tropic isolate. Data obtained by flow cytometry 5 days after infection. (B) RT-PCR reflecting GAPDH and p21 gene expression in activated CD4+ T cells in the absence of Gal-9 (−), presence of Gal-9 (+), and presence of both anti–Tim-3 antibody and Gal-9 (++). SNO2, SN06, and SN011 are 3 HIV-1 seronegative individuals. These data are representative of 3 independent experiments performed on 3 different individuals.
Gal-9 reduces infection of CD4+ T cells to HIV-1 infection and up-regulates p21 through interaction with Tim-3. (A) Representative dot plots of activated CD4+ T cells in the presence of Gal-9 (1 μg/mL) for 2 hours and also in the presence or absence of anti–Tim-3 antibodies (2.5, 5, and 7.5 μg/mL anti–Tim-3 antibody [clone F38-2E2]) before in vitro infection with X4-Tropic isolate. Data obtained by flow cytometry 5 days after infection. (B) RT-PCR reflecting GAPDH and p21 gene expression in activated CD4+ T cells in the absence of Gal-9 (−), presence of Gal-9 (+), and presence of both anti–Tim-3 antibody and Gal-9 (++). SNO2, SN06, and SN011 are 3 HIV-1 seronegative individuals. These data are representative of 3 independent experiments performed on 3 different individuals.
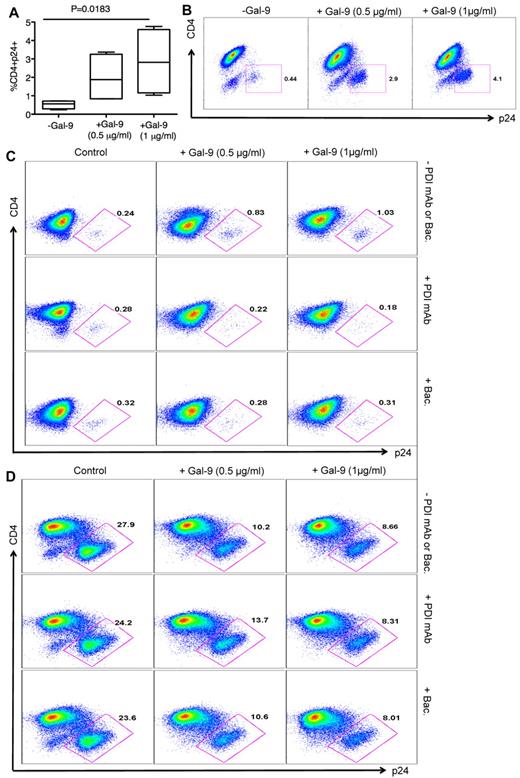
Gal-9 enhances HIV-1 infection of resting CD4+ T cells
Recent studies have shown that PDIs are activating T-cell surface receptors for Gal-9.21 PDI can directly form a complex with HIV gp120, CD4, and CXCR4 on the surface of T cells39,40 and Gal-9 enhances HIV-1 viral entry in a PDI-dependent manner.21 To confirm that Gal-9 enhances HIV-1 infection in resting CD4+ T cells that lack substantial Tim-3 expression (supplemental Figure 2), we incubated resting, nonactivated CD4+ T cells in the presence and absence of Gal-9 for 2 hours, and cells were then infected with HIV-1LAI and analyzed for p24 by flow cytometry at 4 days after infection. Presence of Gal-9 enhanced HIV-1 infection in resting CD4+ T cells in a dose-dependent manner (P = .0183; Figure 7A-B), consistent with a previous report.21 To confirm that Gal-9 enhances HIV-1 infection in a PDI-dependent manner, we blocked PDI with an anti-PDI antibody and bacitracin, an inhibitor of PDI enzymatic activity.21,41 Gal-9–induced enhancement of HIV-1 infection in resting nonactivated CD4+ T cells was reversed by addition of anti-PDI antibody and bacitracin (Figure 7C). To determine whether PDI inhibitors interfere with Gal-9/Tim-3 interactions on activated CD4+ T cells, we performed HIV-1 infection assays in the presence of Gal-9 and with and without anti-PDI antibody or bacitracin (Figure 7D). The presence of PDI inhibitors in culture did not have a significant impact on the interaction of Gal-9 with Tim-3. Thus, Gal-9–induced inhibitory effects on HIV-1 infection in activated CD4+ T cells were similar in the presence and absence of PDI inhibitors (Figure 7D).
Gal-9 enhances HIV-1 infection of resting CD4+ T cells. (A) Percentages of CD4+p24+ in nonactivated CD4+ T cells in the absence or presence of Gal-9 (0.5 μg/mL or 1 μg/mL) 2 hours before HIV-1 infection with X-4-tropic (HIV-1LAI) isolates. Bounds of boxes denote interquartile range; lines within boxes denote median; and whiskers indicate range. Significance was tested using Kruskal-Wallis test. (B) Representative flow cytometry dot plots from CD4+ T cells stimulated with 0.5 μg/mL or 1 μg/mL Gal-9 before infection with X-4-tropic HIV-1 isolate in vitro. (C) Rested CD4+ T cells were incubated with bacitracin (+Bac), or anti-PDI mAb RL77 (1:3000 D) or in the absence of both (−PDI mAb or Bac). Then Gal-9 (0.5 μg/mL or 1 μg/mL) as indicated was added for 2 hours and cells were washed. Five days after infection, the number of infected cells with X4-tropic HIV-1 isolate was quantified by intracellular viral p24 antigen staining using flow cytometry. (D) PHA activated CD4+ T cells were incubated with bacitracin (+Bac), anti-PDI mAb RL77 (1:3000 D), or in the absence of both (−PDI mAb or Bac). Then Gal-9 (0.5 μg/mL or 1 μg/mL) as indicated was added for 2 hours and cells were washed. Five days after infection, the number of infected cells with X4-tropic HIV-1 isolate was quantified by intracellular viral p24 antigen staining using flow cytometry.
Gal-9 enhances HIV-1 infection of resting CD4+ T cells. (A) Percentages of CD4+p24+ in nonactivated CD4+ T cells in the absence or presence of Gal-9 (0.5 μg/mL or 1 μg/mL) 2 hours before HIV-1 infection with X-4-tropic (HIV-1LAI) isolates. Bounds of boxes denote interquartile range; lines within boxes denote median; and whiskers indicate range. Significance was tested using Kruskal-Wallis test. (B) Representative flow cytometry dot plots from CD4+ T cells stimulated with 0.5 μg/mL or 1 μg/mL Gal-9 before infection with X-4-tropic HIV-1 isolate in vitro. (C) Rested CD4+ T cells were incubated with bacitracin (+Bac), or anti-PDI mAb RL77 (1:3000 D) or in the absence of both (−PDI mAb or Bac). Then Gal-9 (0.5 μg/mL or 1 μg/mL) as indicated was added for 2 hours and cells were washed. Five days after infection, the number of infected cells with X4-tropic HIV-1 isolate was quantified by intracellular viral p24 antigen staining using flow cytometry. (D) PHA activated CD4+ T cells were incubated with bacitracin (+Bac), anti-PDI mAb RL77 (1:3000 D), or in the absence of both (−PDI mAb or Bac). Then Gal-9 (0.5 μg/mL or 1 μg/mL) as indicated was added for 2 hours and cells were washed. Five days after infection, the number of infected cells with X4-tropic HIV-1 isolate was quantified by intracellular viral p24 antigen staining using flow cytometry.
Discussion
Gal-9 has diverse effects in the immune system. It plays a crucial role in the regulation of various T cell–mediated conditions, such as autoimmunity, transplantation, and asthma (reviewed by Wiersma et al19 ). In addition, Gal-9 can stimulate innate immunity, playing a protective role in viral, bacterial, and parasite infections10,17,42 and effectively limits tumor progression.11,12 Here for the first time, we report a novel function for Gal-9 via Tim-3 in regulating CD4+ T cell susceptibility to infection by HIV-1 in vitro. Multiple effects of Gal-9 have been reported in different in vitro and in vivo systems; whereas Gal-9 affects many cellular process, different effects result from specific binding interactions with different receptors.27,43
Although numerous biologic activities of Gal-9 have been attributed to its interaction with Tim-3, a more recent study has identified that Gal-9 can also regulate the T-cell surface redox environment by binding to PDI and β3 integrin (CD61), potentiating infection with HIV-1.21 Similarly, we found Gal-9/PDI interaction on nonstimulated CD4+ T cells enhanced HIV-1 entry and infection. Gal-9 dependent augmentation of surface PDI can change the conformation/activity of many cell surface molecules including integrins and activates migration of T cells.21 We already reported the activation of eosinophil migration6 in which PDI might also be involved. In addition, we have also seen suppression of VLA-4 (α-4/β-1)–VCAM-1 interactions and suppression of metastasis in a murine model of cancer.44 Thus, the function of Gal-9 could be contextual and different depending on the microenvironment, and the physiologic concentration of Gal-9 in different microenvironments in vivo is not known.
In this study, we found Gal-9 to be circulating at significantly higher levels in the serum of HIV-1–infected individuals with a viral load of more than 10 000 copies/mL compared with those with less than 10 000 copies/mL and normal healthy controls. Although the source of Gal-9 needs to be determined, elevated levels of Gal-9 in chronically HIV-1–infected individuals suggest that it may play an important role in HIV-1 pathogenesis.
We found that interaction of Gal-9 with Tim-3 on CD4+ T cells significantly inhibited HIV-1 infection by both X4-and R5-tropic isolates. These suppressive effects of Gal-9 were mediated through Tim-3 interactions as blocking Tim-3/Gal-9 interactions with anti–Tim-3 antibodies abolished the Gal-9 mediated resistance of CD4+ T cells to HIV-1 infection. Tim-3 is also a receptor for phosphatidylserine (PtdSer), which binds on its N-terminal IgV domain.45 Although PtdSer is involved in phagocytic abilities of macrophages and DCs, T cells expressing Tim-3 do not engulf apoptotic cells45 and therefore, we believe that Tim-3/PtdSer interactions did not play a significant role in our observations using enriched CD4+ T cells.
We found that Gal-9 significantly, and in a time-dependent manner, reduced surface expression of CCR5, CXCR4, and α4β7 HIV-1 coreceptors on activated CD4+ T cells, providing a potential mechanism for rendering CD4+ T cells less prone to HIV-1 infection. Interestingly, we also observed that Gal-9/Tim-3 interactions significantly reduced viral replication in CD4+ T cells that were already HIV-1 infected. Although, reduction in HIV-1 coreceptors on CD4+ T cells may render cells less permissive to infection, we also showed that p21, which has recently been recognized to be directly involved in reducing the susceptibility of CD4+ T cells to HIV-1 by inhibiting viral reverse transcription and mRNA transcription in elite controllers,5 was strongly up-regulated in Gal-9 treated CD4+ T cells. Furthermore, we demonstrated that Gal-9–mediated up-regulation of p21 occurs through Tim-3 as blocking Tim-3 interaction with its ligand, Gal-9, abolished the effects of Gal-9–mediated up-regulation of p21.
The role of p21 to modulate the susceptibility of human cells to HIV-1 infection by interfering with individual viral replication steps has already been documented. In macrophages, p21 can reduce the efficacy of reverse transcription and integration, which restricts the replication of HIV and related primate lentiviruses,46 and earlier studies in lymphocytic cell lines suggested that HIV-1 gene expression from proviral HIV-1 DNA can be suppressed by pharmaceutical cyclin-dependent kinase inhibitors.47 In hematopoietic stem cells, resistance to HIV-1 infection was shown to be selectively associated with p21 expression48 ; this could represent a stem cell–specific unique molecular barrier against HIV-1 infection.49
To our knowledge, the data presented here are the first to show that Gal-9 is directly involved in reducing the susceptibility of activated CD4+ T cells to HIV-1 infection in vitro. Studies to investigate whether Gal-9 can be used as a microbicide in vivo and whether Gal-9 provides a unique molecular barrier to HIV-1 infection in different stages of HIV-1 infection will be important. The use of Gal-9 at the mucosal surfaces can provide additional benefits for microbicides: because Gal-9 can be naturally found either in the cytoplasm or the extracellular matrix,50 we would anticipate limited adverse effects with its administration. In addition, because the effects of Gal-9 on HIV-1 infection of CD4+ T cells are not virus-specific, we would anticipate no problems related to drug resistance of HIV-1, which is a potential problem with using antiviral drugs as microbicides. However, careful studies will need to be undertaken as Gal-9 is a potent suppressor of T-cell activation. Thus, administering it as a microbicide may result in untoward suppression of protective antimicrobial T-cell responses in the genital mucosa.
Mucosal tissues play important roles in the earliest phases of infection after sexual transmission of HIV-1. For instance, higher expression of HIV-1 coreceptors on CD4+ T cells in particular α4β7, the gut mucosal homing receptor, facilitates infection by HIV-1.34 α4β7 facilitates the migration of lymphocytes from gut-inductive sites where immune responses are first induced (Peyer patches and mesenteric lymph nodes) to the lamina propria.51,52 These 3 gut-associated lymphoid tissues (GALT) play central roles in the initial phases of infection after sexual transmission. Therefore, local expression of Gal-9 at the mucosal surfaces, such as genital and gut tissues could reduce infection of CD4+ T cells via down regulation of HIV-1 coreceptor expression, especially α4β7.
Despite the promising effects of Gal-9 on decreasing susceptibility of activated CD4 T cells to infection, binding of Gal-9 to PDI facilitates HIV-1 entry and subsequently enhances infection in resting CD4 T cells. We demonstrated that using anti-PDI antibody or PDI inhibitors can block the interaction of Gal-9 with PDI, and as a result Gal-9 would only bind to Tim-3, thereby providing resistance against infection of activated CD4+ T cells with HIV-1.
In summary, whether Gal-9/Tim-3 interactions are beneficial or detrimental for HIV-1 control will probably depend on the cell type and stage of infection. During initial infection Tim-3/Gal-9 interactions are probably beneficial on activated CD4+ T cells, as these are the primary targets. However, in chronic infection Tim-3/Gal-9 interactions are probably detrimental on CD8+ T cells because of suppression of HIV-specific T-cell responses. Further study is warranted to clarify the function of Gal-9 in HIV-1 infection and to consider its clinical potential to prevent /reduce HIV infection.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank our study volunteers for providing samples and supporting this work and the clinical staff for their dedication to this research.
This work was supported by National Institutes of Health (NIH) grants R01 AI65328, R21 AI089373, P30 AI127757, a New Investigator Award (for S.E.) from the University of Washington/Fred Hutchinson Cancer Research Center for AIDS Research (CFAR), and M01-RR-00037 (the University of Washington General Clinical Research Center). This research was supported by the University of Washington Center for AIDS Research (CFAR), an NIH funded program (P30 AI027757) which is supported by the NIH Institutes Institute of Allergy and Infectious Diseases, National Cancer Institute, National Institute of Mental Health, National Institute of Drug Abuse, National Institute of Child Health and Human Development, National Heart, Lung, and Blood Institute and National Center for Complementary and Alternative Medicine). We also acknowledge the support of the James B. Pendleton Charitable Trust.
National Institutes of Health
Authorship
Contribution: S.E. designed, performed all the research, analyzed the data, and wrote the paper; T.N. and M.H. provided new reagents and advised on the experiments; H.H. supervised all of the research and revised/corrected the paper; and all authors read and edited the paper.
Conflicts-of-interest disclosure: All authors of this paper are listed as inventors on a provisional patent application relevant to this work. T.N. and M.H. have each received more than $10 000 compensation as board members of GalPharma in the calendar year preceding the date of submission of this paper.
Correspondence: Helen Horton, Seattle Biomedical Research Institute, 307 Westlake Ave N, Seattle, WA, 98109; e-mail: helen.horton@seattlebiomed.org.

![Figure 6. Gal-9 reduces infection of CD4+ T cells to HIV-1 infection and up-regulates p21 through interaction with Tim-3. (A) Representative dot plots of activated CD4+ T cells in the presence of Gal-9 (1 μg/mL) for 2 hours and also in the presence or absence of anti–Tim-3 antibodies (2.5, 5, and 7.5 μg/mL anti–Tim-3 antibody [clone F38-2E2]) before in vitro infection with X4-Tropic isolate. Data obtained by flow cytometry 5 days after infection. (B) RT-PCR reflecting GAPDH and p21 gene expression in activated CD4+ T cells in the absence of Gal-9 (−), presence of Gal-9 (+), and presence of both anti–Tim-3 antibody and Gal-9 (++). SNO2, SN06, and SN011 are 3 HIV-1 seronegative individuals. These data are representative of 3 independent experiments performed on 3 different individuals.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/119/18/10.1182_blood-2011-11-389585/4/m_zh89991290470006.jpeg?Expires=1769587096&Signature=lNJW4o2pAD3IknXkiC2mk73OPo8TZtEMAuREnZDSwXO8IeSdfsjAHgQV6Mvk4cYcc3yNgYlR5EhFC5CiGA1BWGLrp4xeDeBY5KbBZ4ksPngYDySggc2o798zUCq6RkvwmjxsTjDwkNaQp~bAYH-nuf0iSN4r0fXIlPiyIt1RLZt7USPF2iy1wEXxi1VPp49WCCek3Srg5f7vDAm-mTIDUBPyp-0kZq0tsgNGenrxGgOnUbMEWZRLG6PETep9HdUPDVjL5fgnSO2JRZsj8si4hZTh8~cgcbjTjY0AblaWzl8eE2LcsN09s5u9dde~5pWQfA27fKbAHcNwX6yUr6S-NQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 6. Gal-9 reduces infection of CD4+ T cells to HIV-1 infection and up-regulates p21 through interaction with Tim-3. (A) Representative dot plots of activated CD4+ T cells in the presence of Gal-9 (1 μg/mL) for 2 hours and also in the presence or absence of anti–Tim-3 antibodies (2.5, 5, and 7.5 μg/mL anti–Tim-3 antibody [clone F38-2E2]) before in vitro infection with X4-Tropic isolate. Data obtained by flow cytometry 5 days after infection. (B) RT-PCR reflecting GAPDH and p21 gene expression in activated CD4+ T cells in the absence of Gal-9 (−), presence of Gal-9 (+), and presence of both anti–Tim-3 antibody and Gal-9 (++). SNO2, SN06, and SN011 are 3 HIV-1 seronegative individuals. These data are representative of 3 independent experiments performed on 3 different individuals.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/119/18/10.1182_blood-2011-11-389585/4/m_zh89991290470006.jpeg?Expires=1769613619&Signature=w~O5G8KokBFHBFU3inHtyhiKHYRQk~-NJQ0Oz0mEpOBir1np~7pcAwCHEqcKSvdJzNGW4kRrPxQsgTDABUfquorBW4Ycs0QHuybuRFIDgT8TB7OaX6NrcJLG4aLDHA5YKRwOalmOiKl-pn0Sp~aq57RWapAOADQHkCG9b0lWEhb2RmtKBMM8aq4J-tRhy23bNhABYFVUbr5HVDwdCWQfDvATkHtGeUeQ2fxHO3O0MZLPoH3GnRUb7Kj5BPPvRvBmry7bkpmrGkyqjboHP~AfSqFVydABEB4up8VjWjs0fILIUntNSILCQgUR1xgVo3StTInp3r88Mko0hPjfC11GcA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)