Abstract
The prognostic role of the transcription factor SOX11 in mantle cell lymphoma (MCL) is controversial. We investigated prognostic markers in a population-based cohort of 186 MCL cases. Seventeen patients (9%) did not require any therapy within the first 2 years after diagnosis and were retrospectively defined as having an indolent disease. As expected, indolent MCL had less frequent B symptoms and extensive nodal involvement and 88% of these cases expressed SOX11. In our cohort 13 cases (7.5%) lacked nuclear SOX11 at diagnosis. SOX11− MCL had a higher frequency of lymphocytosis, elevated level of lactate dehydrogenase (LDH), and p53 positivity. The overall survival in the whole cohort, excluding 37 patients receiving autologous stem cell transplantation, was 3.1 year and in patients with indolent or nonindolent disease, 5.9 and 2.8 years, respectively (P = .004). SOX11− cases had a shorter overall survival, compared with SOX11+ cases, 1.5 and 3.2 years, respectively (P = .014). In multivariate analysis of overall survival, age > 65 (P = .001), Eastern Cooperative Oncology Group score ≥ 2 (P = .022), elevated LDH level (P = .001), and p53 expression (P = .001) remained significant, and SOX11 lost significance. We conclude that most indolent MCLs are SOX11+ and that SOX11 cannot be used for predicting an indolent disease course.
Introduction
Patients with mantle cell lymphoma (MCL) have a shorter median survival than many other lymphomas because of frequent relapses despite good initial response to conventional therapy. Patients < 65 years of age may benefit from aggressive chemotherapy and autologous stem cell transplantation (ASCT), but, because of the high median age at presentation, this treatment option is mostly not available.1 A subgroup of MCL cases, ∼ 10%-15% of cases, has an indolent clinical course and may not need therapy for several years.1-9
The initial event in MCL biology is the translocation of the cyclin D1 gene (CCND1) to the immunoglobulin heavy chain locus, t(11;14)(q13;q32),10 resulting in aberrant expression of cyclin D1. A small number of cases have CCND1 translocation to the light chain loci11,12 or lack CCND1 translocation.13 In addition to the t(11;14) translocation several secondary genetic events are necessary for lymphomagenesis,1,14-16 and some of these have been associated with clinical outcome. Features associated with worse prognosis include genetic aberrations that lead to further disturbances of cell cycle regulation, including truncation of the cyclin D1 transcript, mutation of genes involved in DNA damage response (deletions and mutations of ATM and/or TP53), and dysregulated cell survival pathways.17,18
CCND1 gene translocation and cyclin D1 nuclear protein expression are used for diagnostic purposes and distinguish MCL from other types of lymphomas.10 Although in most cases it is easy to diagnose MCL, it is more difficult to predict the clinical course of the disease. There is a lack of good biologic and clinical markers for predicting an indolent disease course, and these patients are therefore at risk of overtreatment.
The transcription factor SRY (sex-determining region Y)–box 11 (SOX11) is expressed during fetal life and has several important roles during organogenesis.19,20 Gene expression profiling and immunohistochemistry (IHC) have identified SOX11 as highly expressed in 90%-95% of MCL, including the rare cyclin D1− cases.21-23 SOX11 has been suggested to carry prognostic information in MCL, but the results are conflicting. Wang et al reported that in MCL with lymph node presentation (nodal MCL), absence of nuclear SOX11 (SOX11− MCL) was associated with a shorter overall survival (OS).21 In contrast, others have reported that a subset of SOX11− MCL, carrying the typical t(11;14) translocation but with a non-nodal, leukemic presentation has an indolent clinical course.4,7 These cases were sometimes initially misdiagnosed as other lymphoma subtypes. Whether these apparently conflicting results are influenced by selection criteria of lymphomas included in these studies or reflect differences between subsets of MCL with nodal versus non-nodal presentation remains to be investigated.
The aim of this study was to characterize the features of MCL with an indolent clinical course and of SOX11− MCL in a population-based cohort. We identified all patients diagnosed with MCL in the Stockholm region between January 1998 and June 2010 (n = 186). We reviewed clinical data, diagnostic biopsies, and flow cytometric data. Here, we compare clinical and pathologic findings in MCL with an indolent or nonindolent disease course and presence or absence of SOX11 expression at diagnosis. On the basis of our results we conclude that SOX11 is not a predictive marker for indolent MCL.
Methods
Patients and clinical data
Diagnostic samples from all patients with newly diagnosed MCL between January 1998 and June 2010 at the Pathology Department of Karolinska University Hospital and St Göran Hospital (together covering the entire Stockholm region) were subject to a central review. In total, 186 MCL cases were confirmed. These patients constitute a heterogeneously treated, unselected, population-based cohort with a median follow-up time of 32.6 months (range, 0-157.1 months). Patients < 65 years of age and without comorbidities were included in clinical protocols with ASCT (n = 37). The median follow-up, excluding patients undergoing ASCT, was 28.2 months (range, 0-148.6 months). Baseline and follow-up clinical data as well as cause of death were obtained from hospital files. The MCL International Prognostic Index (MIPI)24 could be evaluated in 143 patients.
Tumor specimens, IHC analysis, and flow cytometry
Lymphomas were diagnosed according to World Health Organization.10 All cases were positive for cyclin D1 by IHC and/or for t(11;14)(q13;q32) by interphase FISH or cytogenetic analysis. Blastoid or pleomorphic morphology (blastoid MCL) was seen in 21 of 166 cases (12.6%) at diagnosis.
IHC staining for cyclin D1, Ki-67, p53, and SOX11 was done on whole sections of paraffin-embedded diagnostic biopsies (lymph node, n = 94; BM, n = 45, gastrointestinal tissue, n = 10; spleen, n = 10; tonsil, n = 9; other extranodal sites, n = 11). In cases that were SOX11− at diagnosis, the staining was repeated ≥ 1 time. When available, consecutive biopsies taken at various times during the disease course were also stained for cyclin D1, p53, and SOX11. In a case with leukemic MCL, SOX11 staining was also performed on cytospins of blood mononuclear cells.
IHC was semiautomated and performed on a Bond Max robot with the use of the Vision Biosystems TM bond Polymer Refine and Bond DAB Enhance, as recommended by the manufacturer (Leica Microsystems). Primary Abs were cyclin D1 clone SP4 from NEOMarkers (Lab Vision), Ki-67 clone MIB1 (DakoCytomation), and p53 clone DO-7 (Novocastra), and for SOX11 the SOX11 Ab HPA000536 (Atlas Antibodies AB) was used with Bond primary Antibody Diluent (AR 9352; Vision Biosystems). For SOX11, Ag retrieval Retriever 2100 (Histolab Products AB) with a pH of 9 or TargetRetrieval Solution S2367 (DakoCytomation) was used.
The evaluation of SOX11 IHC was independently done by 2 hematopathologists (M.K. and B.S.), and the concordance at the initial evaluation was 96.5% (167 of 173 cases). On reevaluation, by the 2 pathologists together, there was full agreement. All SOX11− cases were also evaluated and confirmed by a third hematopathologist (B.C.). Presence of nuclear staining for SOX11 in lymphoma cells was defined as SOX11 positivity. The staining was categorized as weak when it was only discerned at 40× magnification. For enumerating Ki-67+ cells, ≥ 1000 cells were counted, in accordance with previous recommendations.25 In BM biopsies or clots, Ki-67 counting could only be performed if there were well-defined nodular infiltrates of MCL. For evaluating p53 positivity we used a cutoff of > 20% strongly p53+ tumor cells.5 Expression of CD20, CD5, CD23, and light chain restriction was evaluated by multicolor flow cytometry or by IHC.
Statistical methods
OS was calculated from the date of MCL diagnosis to the date of death. Patients were censored at last follow-up (May 1, 2011). Lymphoma-specific survival (LSS) was calculated from the time of diagnosis to death from lymphoma. Death from other reasons was censored. Associations with survival were evaluated with the use of Kaplan-Meier curves and the log-rank test. Fisher exact test or chi-square test (MIPI) was used for comparison between groups. All P values are 2-tailed. P < .05 was considered significant.
Ethical permission
The Regional Central Ethical Review Board at Karolinska Institutet has approved the research and given all necessary ethical permissions, and informed consent was given according to the Helsinki Protocol.
Results
Clinical and pathologic features of indolent versus nonindolent MCL
Patients (n = 186) were diagnosed with MCL in the Stockholm region between January 1998 and June 2010. Seventeen of the 186 patients with MCL in this population-based cohort were retrospectively defined as having an indolent clinical course, not requiring radiotherapy, chemotherapy, rituximab, or splenectomy within the first 2 years after diagnosis. Three patients could not be evaluated for clinical course because of treatment for other malignancies (n = 2) or inadequate follow-up time (n = 1).
One hundred sixty-six patients did not fulfill the criterion for indolent disease (nonindolent MCL). Comparisons of clinical (age, sex, presence of B symptoms, Eastern Cooperative Oncology Group [ECOG] score ≥ 2, nodal presentation, splenomegaly, Ann Arbor stage, white blood cell [WBC] count > 10 × 109/L, lymphocyte count > 5 × 109/L, high serum lactate dehydrogenase [LDH], and MIPI score) and pathologic data (proliferation index ≥ 30% or ≥ 50%, blastoid structure, strong p53 positivity in > 20% of tumor cells, CD23 positivity) and treatment (ASCT or intensive chemotherapy as part of first-line treatment without ASCT) in the indolent and nonindolent MCL subsets are presented in Table 1.
No significant differences were observed for leukemic presentation (WBC count > 10 × 109 or lymphocytosis > 5 × 109) or splenomegaly among patients with an indolent disease and nonindolent disease course. However, as expected, patients with indolent disease had significantly less often B symptoms at diagnosis than patients with a nonindolent disease, 0 of 17 (0%) compared with 71 of 163 (44%; P < .001) and less frequently extensive nodal disease, 6 of 17 (35%) compared with 107 of 163 (66%; P = .018). The cases with indolent disease were less proliferative, although this was only significant with the use of a cutoff of ≥ 30% (P = .048). Analysis for other tumor markers previously described to correlate with better (CD23 expression) or adverse (blastoid morphology, p53 positivity) prognosis showed no significant differences among indolent and nonindolent MCL. SOX11 was expressed in 15 of 17 (88%) of the indolent cases and in 142 of 153 (93%) of the nonindolent cases (NS).
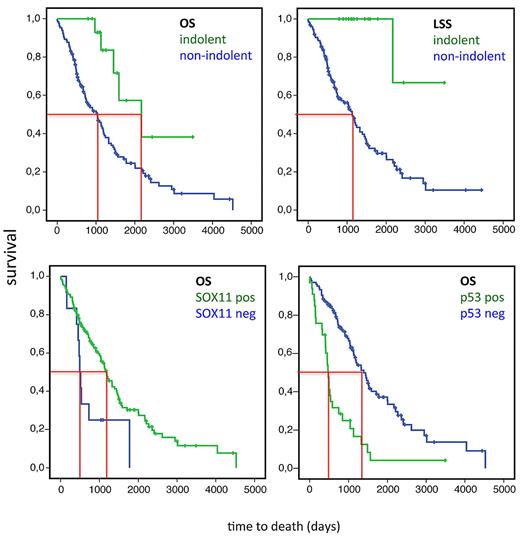
The median follow-up time was 2.7 years (range, 0-13.1 year) in the whole cohort and 2.3 years (range, 0-12.4 years) among patients not receiving ASCT. At last follow-up 5 of 17 (29%) patients with indolent MCL died compared with 105 of 166 (63%) in the nonindolent group. In only 1 of 5 patients with indolent MCL the cause of death was lymphoma related compared with 87 of 105 patients in the nonindolent group. Median OS among indolent and nonindolent MCL was compared, excluding patients who were treated with high-dose chemotherapy and ASCT (1 patient in the indolent group underwent ASCT 70.5 months after diagnosis and 36 patients in the nonindolent group had received ASCT as part of first-line therapy). The 16 patients with indolent MCL had a longer median OS than the nonindolent cases, 5.9 years (2186 days) versus 2.8 years (1031 days), respectively (P = .004; Figure 1). The median LSS time was not reached for the indolent MCL and was 3.1 year (1133 days) for the nonindolent MCL (Figure 1).
OS in MCL (excluding patients who had undergone ASCT as first-line therapy). (Top left) Median OS for MCL with an indolent clinical course (n = 16, defined as not requiring treatment within the first 2 years after diagnosis) and nonindolent MCL (n = 130) were 2168 days (5.9 years) and 1031 days (2.8 years), respectively (P = .004). (Top right) Median LSS for nonindolent MCL was 1133 days (3.1 years) and was not reached for indolent MCL. (Bottom left) Median OS for non-ASCT treated SOX11+ (n = 129) and SOX11− (n = 12) cases were 1180 days (3.2 years) and 494 days (1.5 years), respectively (P = .014). (Bottom right) Median OS for non-ASCT treated p53− cases (n = 104) and p53+ (n = 33) cases were 1391 days (3.8 years) and 477 days (1.3 years), respectively (P < .001).
OS in MCL (excluding patients who had undergone ASCT as first-line therapy). (Top left) Median OS for MCL with an indolent clinical course (n = 16, defined as not requiring treatment within the first 2 years after diagnosis) and nonindolent MCL (n = 130) were 2168 days (5.9 years) and 1031 days (2.8 years), respectively (P = .004). (Top right) Median LSS for nonindolent MCL was 1133 days (3.1 years) and was not reached for indolent MCL. (Bottom left) Median OS for non-ASCT treated SOX11+ (n = 129) and SOX11− (n = 12) cases were 1180 days (3.2 years) and 494 days (1.5 years), respectively (P = .014). (Bottom right) Median OS for non-ASCT treated p53− cases (n = 104) and p53+ (n = 33) cases were 1391 days (3.8 years) and 477 days (1.3 years), respectively (P < .001).
A considerable phenotypic heterogeneity was observed among indolent MCL cases, and the final diagnosis was based on cyclin D1 expression and in most cases on interphase FISH. Only 3 of 17 cases with indolent MCL had a characteristic MCL phenotype by flow cytometry (light chain restriction, CD19+, CD20+, CD5+, CD23−), whereas 2 cases were CD5+/CD23+ and 9 cases were CD5+/CD23dim (Table 2). In one case the tumor cells expressed CD23 in blood but not in flow cytometry of cells from colon biopsy (case 132). Two cases were CD5−/CD23− (Table 2). None of the cases with indolent disease course had blastoid morphology. Furthermore, in most cases with diagnostic material from lymphatic tissue there was a mantle zone growth pattern in at least a part of the sample. Most cases investigated for BM involvement were characterized by sparse to moderate interstitial engagement (Table 2). Leukemic disease was present in 3 of 17 cases. Tumor cell proliferation could be investigated in 14 cases and was < 30% in all but 2. Strong IHC positivity for p53 in > 20% of the tumor cells was detected in 2 cases. Both of these were diagnosed in the BM and had a sparse, interstitial involvement and a classic MCL phenotype by flow cytometry. Both these cases also had a leukemic, non-nodal presentation. Tumor cell proliferation could not be assessed in these cases because of high proliferation in the background BM cells.
Clinical and pathologic characteristics of SOX11− and SOX11+ MCLs
SOX11 expression could be evaluated in the initial diagnostic tissue in 173 cases, 160 of these were SOX11+ (93%). Thirteen cases (7.5%; biopsies from lymph node, n = 4; BM, n = 5; spleen, n = 2; gastrointestinal tract, n = 1; and prostate, n = 1) lacked expression of nuclear SOX11. To evaluate the reproducibility of the SOX11 stainings these were repeated ≥ 1 time in all SOX11− cases, and similar results were obtained.
A detailed comparison between clinical, pathologic, and treatment data in MCL according to SOX11 expression at diagnosis is given in Table 3. Patients with SOX11− MCL had more frequently lymphocytosis > 5 × 109 (P = .045) and elevated levels of serum LDH (P = .029). In addition, MIPI high risk was more frequent among patients with SOX11− tumors but did not reach statistical significance. Strong p53 positivity (Figure 2) was seen in 69% of SOX11− cases and in 16% of SOX11+ cases (P < .001) and was strongly correlated to shorter OS (non-ASCT cases; median OS for p53− MCL, 3.8 years (1391 days) and for p53+ MCL, 1.3 years (477 days; P < .001; Figure 1).
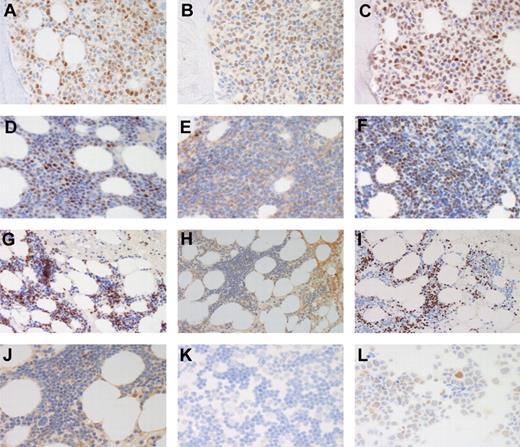
Immunostainings for cyclin D1, SOX11, and p53. Stainings for cyclin D1 (A,D,G), SOX11 (B,E,H,J-L), and p53 (C,F,I). BM of a case (case 80) of SOX11+, p53+ MCL (A-C). BM of SOX11−, p53+ MCL cases 125 (D-F) and 139 (G-I, higher magnification of SOX11 staining in panel J). For case 139 SOX11 was also negative in cells prepared from leukemic cells in peripheral blood (K) compared with the MCL cell line Rec1 (L). Original magnification, ×40 (A-F,J,K-L) and ×20 (G-I). Olympus BX45 microscope, (Olympus), DXC-S500 camera, (Sony), Picsara software (Euromed Network).
Immunostainings for cyclin D1, SOX11, and p53. Stainings for cyclin D1 (A,D,G), SOX11 (B,E,H,J-L), and p53 (C,F,I). BM of a case (case 80) of SOX11+, p53+ MCL (A-C). BM of SOX11−, p53+ MCL cases 125 (D-F) and 139 (G-I, higher magnification of SOX11 staining in panel J). For case 139 SOX11 was also negative in cells prepared from leukemic cells in peripheral blood (K) compared with the MCL cell line Rec1 (L). Original magnification, ×40 (A-F,J,K-L) and ×20 (G-I). Olympus BX45 microscope, (Olympus), DXC-S500 camera, (Sony), Picsara software (Euromed Network).
In our cohort most patients with SOX11− MCL (13 of 15) required therapy at diagnosis and were treated within 42 days. Median OS was 1.5 years (494 days) for patients with SOX11− tumors and 3.2 years (1180 days) for patients with SOX11+ tumors (P = .014), excluding 32 patients (1 SOX11−, 31 SOX11+) receiving ASCT as part of first-line therapy (Figure 1). LSS among SOX11− and SOX11+ cases was 1.5 years (536 days) and 3.8 years (1391 days), respectively (P = .023). In multivariate analysis of the whole cohort (excluding patients who had undergone ASCT) factors significantly associated with shorter OS was age > 65 (P = .001), ECOG score ≥ 2 (P = .022), elevated LDH level (P = .001), and p53 expression (P = .001), whereas SOX11 lost significance. The MIPI is based on age, ECOG score, LDH level, and WBC count.24 The MIPI has mainly been used in the setting of clinical trials. It is therefore interesting that 3 of the MIPI factors were significantly associated with OS survival in this population-based cohort.
The clinical and pathologic data of SOX11− cases are presented in Table 4. Seven of 12 of these cases had leukemic disease at diagnosis, 3 of these also had splenomegaly (Table 4). Six of 12 cases were CD20+, CD5+, and CD23− by flow cytometry, whereas 1 case was CD23+, 6 cases were CD23dim, 1 of which was CD23dim and CD5−. Thus, only 50% of the cases had a classic MCL phenotype by flow cytometry. However, as presented in Table 1 also many SOX11+ cases were dim positive for CD23 by flow cytometry, which underpins the need for IHC and/or genetic analysis for establishing the MCL diagnosis. Two SOX11− MCL cases were of blastoid morphology and 5 cases had a proliferation of > 30%. Two of the SOX11− cases were retrospectively defined as having an indolent clinical course (case 15 and case 139). Both these cases had a non-nodal presentation and were diagnosed on tissue other than lymph node (gastrointestinal biopsy and BM, respectively). None of these 2 patients had bulky disease or splenomegaly, and both cases were leukemic. Nine of 13 patients with SOX11− MCL expressed p53 by IHC.
For most patients consecutive diagnostic samples could also be retrieved and stained for SOX11 and p53 (Table 5). The diagnostic biopsy was restained at the same time for comparison. For patients 2, 15, 101, 139, and 167 the tumor cells in the consecutive samples were negative. For 2 patients occasional weakly positive cells (only discerned at 40× magnification) were detected, and still the majority of tumor cells remained SOX11− (patients 30 and 104). However for 3 patients the frequency of SOX11+ cells in the consecutive samples were such that the case could be considered SOX11+, albeit with weak expression (patients 32, 92, and 123). The p53 status did not in any case change in consecutive biopsies.
Discussion
The key findings in this consecutive MCL cohort analysis are the following: (1) the presence of a subgroup of MCL with a clinically indolent course; (2) the presence of a subgroup of MCL cases lacking SOX11 expression; and (3) the absence of SOX11 protein expression does not correlate to a clinically indolent disease.
It has since long been recognized that a subset of patients with MCL may have a long survival time on conventional therapy or even without treatment.1,6,9,26-29 The choice to treat or not to treat a patient with MCL may therefore have changed over time because of the increased awareness that not all MCLs are aggressive.29 In our population-based cohort the SOX11− cases and cases with an indolent clinical disease course were, however, not enriched in the later years of the study but were rather present at similar frequencies in the early and late years of the study. It is often difficult to predict the clinical course for a given patient at the time of diagnosis, and the decision to wait with treatment is often based on the judgment of the treating clinician.26,27 Among tumor markers, low proliferation as measured by Ki-6730,31 or as a proliferation index that is based on gene expression profiling32 has been associated with longer OS. Deletions of 17p and TP53 mutations are negative prognostic markers.28,33-36
Recently, gene expression profiling and IHC have identified the transcription factor SOX11 as a new tumor marker in MCL, being positive in ∼ 90% of MCL cases,21,22,37 including the rare cyclin D1− tumors22,23 but not in other small-cell lymphomas; B-cell chronic lymphocytic lymphoma/small cell lymphoma, marginal zone lymphoma, and follicular lymphoma. Moreover, nonmalignant lymphocytes lack SOX11 expression.21-23,37 With the use of IHC only nuclear SOX11 staining is considered specific, and the weak cytoplasmic staining occasionally seen does not correlate with SOX11 expression at the mRNA level.22,23,37 The fraction of cyclin D1+ MCL lacking nuclear SOX11 expression has in different series been 10% (1 of 10),22 9% (5 of 53),21 7% (4 of 54),23 and 13% (15 of 112).4 In our population-based cohort we found 7.5% (13 of 173) SOX11− MCL, a figure similar to previous reports.
Although little is known on the functional role of SOX11 in MCL, considerable interest has been raised because of suggestions that its expression may correlate to disease course. Several independent groups have suggested that certain SOX11− MCL may represent a distinctive clinical and biologic subtype with a non-nodal, leukemic disease presentation, characterized by predominantly mutated IGVH genes, non–complex karyotypes, and an indolent clinical course4,7 with a small cell structure.6 Kimura et al investigated SOX11 expression in 15 cases of MCL with a small cell structure, of which 3 did not receive chemotherapy at diagnosis because of either an indolent presentation or misdiagnosis as another indolent lymphoma subtype.6 In total, 66% (8 of 12) of these small cell variant MCLs were SOX11−, whereas 33% (4 of 12) were SOX11+.6 Fernandez et al studied a subset of t(11;14)(q13;q32) positive MCL (n = 12), characterized by an indolent clinical course for > 2 years.4 Ten of these patients had earlier been misdiagnosed as other types of lymphoma or not fully classified, and 2 cases were incidentally found in lymph nodes. Gene expression and genomic analysis were done on 7 of these cases. Six were SOX11− as analyzed by gene expression profiling and IHC. However, weak expression of SOX11 mRNA and protein was found in one case.4 In addition, 2 of 15 classic MCLs analyzed in a similar way lacked SOX11. A validation analysis of 112 other MCL cases identified 15 SOX11− or weakly expressing cases (13%). The SOX11− cases mostly had a non-nodal presentation with splenomegaly and high WBC and lymphocyte counts compared with patients with SOX11+ tumors, but there was no significant difference in Ki-67 proliferative index. Furthermore, in this study SOX11− MCLs were characterized by lack of genomic complexity, predominantly hypermutated immunoglobulin genes and longer survival compared with conventional MCL.4 Ondrejka et al described a series of 8 MCL cases with a leukemic, non-nodal presentation, with a lack of involvement of the spleen and gastrointestinal tract, and with interstitial lymphoma infiltration in the BM.7 These cases represented 3% of all MCL cases diagnosed during the studied time period. SOX11 was negative in 4 of 5 cases investigated by IHC.
Thus, several publications have suggested that lack of SOX11 expression is a feature of MCL with a nonaggressive clinical course, even though exceptions were present in all above-mentioned studies. In contrast, a previous study on MCL from our group suggested that lack of SOX11 expression was associated with shorter OS despite that there were no significant differences in tumor cell proliferation between SOX11− and SOX11+ cases.21 However, since this study was small and not population based, the results might have been influenced by a selection bias because mostly patients with nodal presentation were investigated. We therefore extended the study to all cases of MCL diagnosed in the Stockholm region during the period January 1998 to June 2010 (n = 186). The frequency of SOX11− cases was 7.5%. For most of the SOX11− cases, consecutive samples were also negative or with only occasional positive cells. However, for 3 patients the SOX11 status changed to positive, albeit with a weak staining intensity.
Among the 13 SOX11− cases 2 were retrospectively defined as having an indolent disease course, still alive without treatment for 3.0 and 3.2 years, respectively. Both these cases had a non-nodal presentation and lacked B symptoms. One of these cases (case 15) was confined to the gastrointestinal tract and BM, whereas the other (case 139) involved blood and BM. These 2 cases could correspond to this SOX11−, indolent subtype, as described by Fernandez et al4 and Ondrejka et al,7 which thus constitutes ∼ 1% of cases in our cohort.
In our population-based MCL cohort, 20% (34 of 170) of cases strongly expressed p53 protein. This frequency is similar to the published results from Katzenberger et al that report strong p53 expression by IHC in > 20% of tumor cells in 24 of 123 cases (20%); 42% of cases with high p53 IHC expression had deletions of 17p compared with 20% of p53 IHC-negative cases.5 In that MCL series, an indolent clinical course was associated with fewer secondary and fewer high-risk chromosomal aberrations.5 17p deletions, however, were equally distributed among cases with many or few secondary genomic aberrations.5 Among our MCL with an indolent clinical course, 2 of 17 cases were strongly p53+. Thus, although p53 protein expression in our cohort was strongly associated with a shorter survival, a few cases may be p53+ already from the time of diagnosis and still have an indolent clinical course.
Interestingly, the majority of the SOX11− cases in our cohort, 9 of 13, strongly expressed p53. This finding has not been reported previously. Strong expression of p53 is often an indication of a mutation in the TP53 gene, resulting in a nonfunctional p53 protein that is not properly degraded. However, other mechanisms of p53 protein stabilization may also be operational.38 Another member of the SOXC group of transcription factors, SOX4, is closely related to SOX11. SOX4 and SOX11 compete for transcribing of the same target genes.20,39 SOX4 has been suggested to interact with p53,40,41 and it could be hypothesized that SOX11 would have similar functions but this remains to be investigated. Because we found a strong correlation between p53 overexpression and impaired survival in our cohort, it is probable that the high p53 expression among SOX11− cases contributed to the shorter survival in this group.
In conclusion, in this population-based cohort we retrospectively identified a subgroup of patients with a clinically indolent disease, constituting ∼ 9% of MCL cases. These patients did not require treatment until > 2 years after diagnosis, and some of these patients have stable disease for a prolonged time. SOX11 did not identify these indolent cases because only 12% of these lacked SOX11 expression, whereas the majority was SOX11+, thus in this respect similar to nonindolent MCL. We can conclude that SOX11 negativity is not associated with an indolent clinical course in this population-based MCL cohort, and, in fact, most indolent MCL cases are SOX11+. Thus, all available clinical and pathologic data should be integrated before initiating therapy. However, we still need reliable predictive markers for indolent disease.
Presented in abstract form (Abstract 3651) at the 53rd Annual Meeting of the American Society of Hematology, San Diego, CA, December 12, 2011.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the Swedish Cancer Society, the Swedish Research Council, the Cancer Society in Stockholm, the Karolinska Institutet Funds, and the Stockholm County Council.
Authorship
Contribution: L.N. retrieved clinical data and participated in pathology review; S.B.W. retrieved clinical data; M.K. and B.C. retrieved and evaluated pathology data; E.K. designed the research and retrieved clinical data; B.S. designed the research, retrieved pathology data, and wrote the manuscript; and all authors participated in data analysis and read the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Birgitta Sander, Department of Pathology, Karolinska Institutet and Karolinska University Hospital Huddinge, SE 141 86 Stockholm, Sweden; e-mail: birgitta.sander@ki.se.
References
Author notes
L.N. and S.B.W. contributed equally to this study.