In two independent retrospective studies, Mikhael et al and Venner et al report unprecedentedly high hematologic response rates, 94% and 81%, including complete response (CR) in 71% and 42%, respectively, to the combination of cyclophosphamide-bortezomib-dexamethasone in patients with either naive or relapsed light chain (AL) amyloidosis.1,2
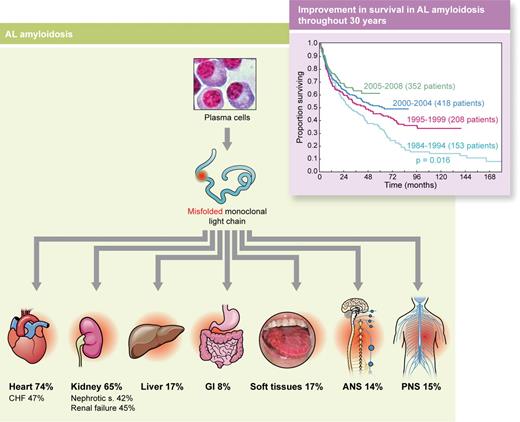
AL amyloidosis is caused by a small or modest, relatively indolent, plasma cell clone secreting light chains (ratio of λ to κ light chains, 4:1) with abnormal folding (misfolded) caused by mutations (symbolized by red dot) that affect critical structural sites. These light chains are prone to aggregation, form amyloid fibrils, and become toxic for cells and tissues, producing failure of vital organs. Heart involvement is the most clinically relevant because it is the cause of death in virtually all patients. Inset: Survival of 1131 patients with AL amyloidosis according to the year of diagnosis followed at the Pavia Amyloid Research and Treatment Center. Although survival continues to improve, the slope of the first part of the curves has not changed during almost 30 years, mostly because of early death of patients with severe cardiac involvement. ANS indicates autonomous nervous system; CHF, congestive heart failure defined as New York Heart Association class III or IV; GI, gastrointestinal tract; and PNS, peripheral nervous system. Professional illustration by Kenneth X. Probst.
AL amyloidosis is caused by a small or modest, relatively indolent, plasma cell clone secreting light chains (ratio of λ to κ light chains, 4:1) with abnormal folding (misfolded) caused by mutations (symbolized by red dot) that affect critical structural sites. These light chains are prone to aggregation, form amyloid fibrils, and become toxic for cells and tissues, producing failure of vital organs. Heart involvement is the most clinically relevant because it is the cause of death in virtually all patients. Inset: Survival of 1131 patients with AL amyloidosis according to the year of diagnosis followed at the Pavia Amyloid Research and Treatment Center. Although survival continues to improve, the slope of the first part of the curves has not changed during almost 30 years, mostly because of early death of patients with severe cardiac involvement. ANS indicates autonomous nervous system; CHF, congestive heart failure defined as New York Heart Association class III or IV; GI, gastrointestinal tract; and PNS, peripheral nervous system. Professional illustration by Kenneth X. Probst.
Light chain amyloidosis is caused by a small or modest, relatively indolent, plasma cell clone synthesizing misfolded light chains. These abnormal light chains behave like “sick molecules,” a term coined by Jan Waldenström, because they display a pathologic conformation prone to aggregation and become toxic for cells and tissues, producing devastating systemic damage (see figure). λ light chains are more prone to misfold than are κ light chains, and certain subgroups have been reported to be preferentially associated with specific organ targeting (eg, IGVL1-44 with heart involvement).3 The misfolded monoclonal light chain can be directly cytotoxic to cardiomyocytes,4 and the extent of cardiac damage is a major prognostic determinant because heart failure and fatal arrhythmias are the cause of death in a substantial number of patients. Cardiac biomarkers, troponins, and particularly the natriuretic peptide type B (NT-proBNP), are very sensitive and reliable sensors of light chain cardiotoxicity and allow early assessment of cardiac response to therapy. They are also powerful prognostic determinants used to stratify the risk according to the Mayo cardiac staging system.5 The light chain–induced cardiac damage is progressive, but potentially reversible, unless the myocardial lesions are advanced. Improvement of cardiac function after therapy, assessed by NT-proBNP, translates into improved survival.6,7
Thus, the strategy for improving survival in AL amyloidosis should be based on early detection of amyloid cardiac involvement while the damage is still reversible, and on the rapid and profound reduction of the amyloid light chain concentration to arrest the progression of the cardiac damage and rescue the heart's function. The most sensitive, although not specific, marker of early cardiac involvement is NT-proBNP, which can anticipate the onset of symptoms related to heart failure by several months.8 Because patients with monoclonal gammopathy of undetermined significance (MGUS) are at risk, albeit low, of developing AL amyloidosis it would be advisable that periodic checkups of individuals with this condition include measurement of NT-proBNP, to detect and treat amyloid cardiomyopathy promptly.
The goal of chemotherapy is rapid, profound, and persistent reduction of amyloid light chain concentration to rescue organ function and improve survival. Although significant progress has been made toward this goal (see figure inset) standard therapy based on melphalan and dexamethasone9,10 is not effective in patients with severe cardiac involvement (Mayo cardiac stage III). The dismal outcome is mainly due to the high early death rate (23%-33%), from advanced cardiac amyloidosis, in the first 2 to 3 months after starting therapy.9,10 These patients do not have enough time to respond to this therapy.
Since the very first investigations, it was evident that bortezomib might represent a breakthrough in the care of AL amyloidosis, producing prompt and profound responses even in pretreated patients. The molecular basis of the high sensitivity of amyloidogenic plasma cells to proteasome inhibition is still under intense investigation. A prospective phase 1/2 study on bortezomib as a single agent in relapsed patients using either a weekly or a twice-weekly schedule resulted in hematologic response rates of 68.8% and 66.7%, respectively, including 37.5% and 24.2% CR. Bortezomib showed limited cardiac toxicity. Importantly, the median time to first response for the twice-weekly schedule was just 1 cycle.
The addition of bortezomib to an alkylating agent, melphalan, and dexamethasone further improved outcome. A phase 2 prospective study including 26 patients with AL amyloidosis showed that this regimen produced a hematologic response in 94% of patients, with 56% achieving a CR.11
After impressive response rates observed in multiple myeloma, the combination of bortezomib, cyclophosphamide, and dexamethasone (CyBorD, or CVD) has been explored in AL amyloidosis. Here, Mikhael et al report the retrospective analysis of 17 patients with AL amyloidosis, 10 of whom were treatment-naive, who were treated with a median of 3 cycles (range, 2-6) of weekly administration of CyBorD before autologous stem cell transplantation (ASCT), as an alternative to high-dose therapy for those deemed ineligible, and as salvage for relapsing patients.1 The reported 94% of hematologic responses is excellent and the 71% CR rate unprecedented in AL amyloidosis. The estimated median duration of CR was 22 months (range, 5-30 months). Organ response was observed in 50% of patients with renal involvement. Notably, 3 patients originally not eligible for ASCT became eligible after treatment with CyBorD. Treatment was well tolerated, with only 2 patients experiencing grade 1 or 2 peripheral neuropathy, possibly thanks to the weekly schedule of bortezomib. In the London National Amyloidosis Center study by Venner et al, 43 patients (20 treatment-naive) were treated with biweekly bortezomib and dexamethasone.2 Because 46% of patients had Mayo cardiac stage III disease, both bortezomib and dexamethasone dosages were attenuated. The hematologic response rate was 81.4% with a CR rate of 39.5%. As expected, neuropathy was more frequent in this study using biweekly bortezomib, occurring in 30% of patients and resulting in discontinuation of therapy in 14%. The remarkable finding of this study was that in the 20 patients with Mayo cardiac stage III disease, the estimated overall survival at 2 years was 94.4%, thus strikingly better than the median survival with melphalan and dexamethasone, which is ∼ 10 months from diagnosis.9 This is the first hint that the profound and rapid hematologic response induced by bortezomib may modify the course of severe amyloid cardiomyopathy.
Although the results of these 2 studies should be interpreted with caution given their retrospective design with possible selection bias, limited number, variable entry criteria, and, above all, short follow-up (median 21 months1 and 14 months,2 respectively), they clearly show that CyBorD/CVD has a profound and rapid effect on the amyloidogenic clone. Because there is strong evidence linking the quality of response to survival in AL amyloidosis,12 CyBorD/CVD has great potential to change the natural history of the disease. The availability of this powerful regimen opens new perspectives in the care of AL amyloidosis and raises several challenging questions that should be addressed through controlled, prospective trials. First, given the sparing of stem cells with this regimen, it is uncertain whether patients who achieve CR should proceed with ASCT, in light of the risk of the procedure in these fragile individuals. Second, the rapid responses produced by CyBorD/CVD open up the possibility of thwarting the high rate of early mortality in patients with advanced cardiac amyloidosis. A European phase 2 trial using attenuated doses is being developed for patients with Mayo cardiac stage III disease. Third, the overall impact of adding bortezomib to alkylating agents front-line on the natural history of the disease should also be assessed in prospective randomized trials. Because “time is life” in this frequently rapidly progressive disease, we need to answer these important questions quickly. AL amyloidosis is a rare disease and international collaboration is needed to accrue patients in clinical trials. Through an unprecedented international effort, a randomized trial comparing the present standard of care, melphalan-dexamethasone, versus bortezomib-melphalan-dexamethasone is now under way in Europe, Australia, and in the United States. This recently established network of referral centers dedicated to AL amyloidosis is a precious resource for conducting future trials. Furthermore, investigators working in the field of AL amyloidosis have recently defined a framework for clinical research including defining clinically relevant end points, study populations, and other criteria for collaborative clinical research that should encourage rapid testing of therapies and expedite new drug development. This framework will be formally presented in May 2012 at the XIII International Symposium on Amyloidosis at the University Medical Center Groningen before being published.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■