Plasmacytoid dendritic cells (pDCs) produce large amounts of type I interferons (IFN-α/β) in response to viral or endogenous nucleic acids through activation of their endosomal Toll-like receptors (TLR-7 and TLR-9). Enhanced TLR-7–mediated IFN-α production by pDCs in women, compared with men, has been reported, but whether sex hormones, such as estrogens, are involved in this sex-based difference is unknown. Here we show, in humanized mice, that the TLR-7–mediated response of human pDCs is increased in female host mice relative to male. In a clinical trial, we establish that treatment of postmenopausal women with 17β-estradiol markedly enhances TLR-7– and TLR-9–dependent production of IFN-α by pDCs stimulated by synthetic ligands or by nucleic acid-containing immune complexes. In mice, we found exogenous and endogenous estrogens to promote the TLR-mediated cytokine secretion by pDCs through hematopoietic expression of estrogen receptor (ER) α. Genetic ablation of ERα gene in the DC lineage abrogated the enhancing effect of 17β-estradiol on their TLR-mediated production of IFN-α, showing that estrogens directly target pDCs in vivo. Our results uncover a previously unappreciated role for estrogens in regulating the innate functions of pDCs, which may account for sex-based differences in autoimmune and infectious diseases.
Introduction
Dendritic cells (DCs) are specialized sentinels in the immune system that detect invading pathogens and play a crucial role in orchestrating the immune responses. In response to viral infection, a specialized DC subset, plasmacytoid DCs (pDCs), produces a large amount of type I IFNs (IFN-α/β), which are potent anti–viral and immunostimulatory cytokines.1 pDCs become activated to produce IFN-α/β through Toll-like receptors (TLR-7 and TLR-9) within endosomal compartments that can sense viral nucleic acids. In the context of autoimmune diseases, such as systemic lupus erythematosus (SLE), these TLRs can also be inappropriately activated by self-nucleic acids complexed with autoreactive antibodies, resulting in IFN-α production by pDCs.2 Activation of pDCs by endogenous DNA and RNA has been suggested to play a critical role in promoting and exacerbating SLE.2,–4 SLE patients show increased serum levels of IFN-α and overexpression of IFN-α-regulated genes in blood cells, suggesting a central role for type I IFNs in disease pathogenesis.5,,–8 This is supported by the observation that antinuclear antibody and SLE syndrome can develop during IFN-α treatment in patients with nonautoimmune disorders.9 Likewise, IFN-α administration accelerates disease development and enhances disease severity in lupus-prone mouse strains.10,11 In addition to IFN-α, TNF-α has been shown to be increased in the serum of patients with active SLE disease and correlates with IFN-α levels.12,13 Although pDCs can also produce TNF-α, it is not clear whether they represent the unique source of this cytokine in SLE.14
Cumulative evidence supports a role for sex-based differences in the pathogenesis of autoimmune and infectious diseases, which may be the result of sex hormones through their effects on innate and adaptive immunity.15,16 A strong sex bias is observed in SLE, whose incidence is approximately 9 times higher in women relative to men.15 Because disease onset is much more frequent in women of childbearing age, it has been hypothesized that sex steroid hormones, such as estrogens, could be responsible for the sex bias in lupus susceptibility.15,17 In support of this, it has been shown in murine models of SLE that administration of 17β-estradiol (E2) accelerated disease onset and increased its severity.18,19 Interestingly, marked sex differences have been also reported in susceptibility to HIV-1 pathogenesis, with women having a higher risk of developing AIDS compared with men infected with a similar viral load.20,21 Given the central role of pDCs in these diseases, it is probable that common sex-linked factors influencing pDC innate functions could contribute to this major sexual dimorphism. In agreement with this hypothesis, a marked increase in the TLR-7–mediated IFN-α production by women pDCs, compared with men, has been recently reported in healthy subjects.22,23 Interestingly, in the study by Meier et al,23 there was a trend toward a lower frequency of IFN-α–producing pDCs in postmenopausal women compared with premenopausal ones, suggesting that female sex hormones could regulate the TLR-mediated responses of pDCs. However, direct evidence for a role of female steroid hormones in the regulation of pDC innate functions is still lacking.
In this study, we investigated whether estradiol could regulate the innate functions of human and mouse pDCs in vivo. Altogether, our results highlight a new property of estrogens in promoting the TLR-mediated innate functions of pDCs in both humans and mice in vivo, through pDC-intrinsic estrogen receptor (ER) α signaling.
Methods
Subjects and study design
Postmenopausal volunteers (46-59 years old) were included in the study after they had given their written informed consent in accordance with the Declaration of Helsinki. The study was reviewed and approved by a national institutional review committee and the regional ethical committee (Affsaaps 060149–00; CPPRB 2–06-09). Three blood samples were collected. The 2 first samples were collected within a 2- to 3-week interval before estrogen treatment. The women were then randomized into 2 groups to receive either transdermal E2 (Estrapatch, 60 μg/24 hours; n = 15) or oral E2 (Estrofem, 2 mg/day; n = 13) for 30 days. The last blood sample was collected at the end of the E2 treatment period. For the comparison between men and women, fresh blood samples from healthy donors (18-45 years) were obtained from Etablissement Français du Sang.
Mice
NOD/SCID/β2m−/− mice, obtained from Plateforme de Haute Technologie (Université Joseph Fourier), were sublethally irradiated (120 cGy) at the age of 4 weeks and were injected intravenously with 1 to 2 × 105 CD34+ hematopoietic progenitor cells purified from umbilical cord blood as described.24 Eight weeks after reconstitution, human bone marrow cells were negatively enriched by magnetic depletion of mouse cells using anti–mouse CD45.1-biotin mAb (A20) and anti–biotin microbeads (Miltenyi Biotec). The frequency of human pDCs producing IFN-α and TNF-α in response to the TLR-7/TLR-8 ligand R-848 was then determined as detailed in “Intracellular cytokine staining of pDCs.”
Female C57BL/6 (B6) mice were purchased from the Center d'Elevage R. Janvier. ERα-deficient (ERα−/−) B6 mice (CD45.2), which have a deletion in exon 2 of the ERα gene (Esr1), have been previously described25 and backcrossed into the B6 background for at least 10 generations. Mice selectively lacking ERα in the hematopoietic compartment or in the DC lineage were generated by crossing B6 mice carrying an Esr1 gene in which exon 2 was flanked by loxP sites (ERαfl/fl) with B6 mice expressing the Cre recombinase under the control of the Tie2 promoter-enhancer (Tie2-ERαKO) or the CD11c promoter (CD11c-ERαKO), respectively, as described elsewhere.26 Mice were maintained in our animal facilities under pathogen-free conditions. Unless otherwise stated, 8- to 12-week-old mice were used in all experiments. All protocols used in mice experiments were approved by the Inserm U1043 Institutional Review Board for animal experimentation.
Where indicated, animals were castrated or sham-operated at 4 weeks. Eight-week-old mice that received exogenous E2 were treated for 2 to 3 weeks by subcutaneous implantation of low-dose E2 pellets (0.05 mg, 60-day release, Innovative Research of America). These E2 pellets have been shown to result in the maintenance of a constant serum E2 concentration of 34 pg/mL, corresponding to estrus levels.27
IFN-α production by human PBMCs and mouse pDCs.
Human PBMCs (5 × 105 cells/well) isolated from whole blood were suspended in X-Vivo 15 medium (Cambrex) and stimulated with titrated amounts of TLR-9 ligand CpG-2216 (Invivogen). For some subjects, PBMCs were stimulated with SLE serum in the presence of necrotic supernatant from PBMCs as described.28 Human IFN-α was measured by ELISA (PBL InterferonSource) in 24-hour culture supernatants. Data from the ELISA tests were normalized on the basis of the respective percentage of pDCs present in the PBMC fraction to show IFN-α production on a per-pDC basis. These results were used to compute at each time point an IFN-α score by summing the data obtained for the different doses of CpG-2216 tested (supplemental Methods, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Mouse pDCs (B220+, mPDCA-1+) were sorted using a FACSAria (BD Biosciences) and were stimulated in 96-well plates (40-75 × 103 cells/well) for 24 hours with CpG-2216. All stimulations were made in RPMI 1640 (Eurobio) complete medium supplemented with 10% heat-inactivated FCS (ATGC Biotechnologie). To measure the pDC-mediated IFN-α response in vivo,29 mice were injected intravenously with 2 μg CpG-2216 mixed with 1,2-dioleoyloxy-3-trimethylammonium-propane (Roche Diagnostics). Serum IFN-α production was assessed by ELISA (PBL InterferonSource).
Intracellular cytokine staining of pDCs.
Human PBMCs and mouse cell-depleted bone marrow cells from humanized mice were stimulated with 1 to 3 μg/mL R-848 (Invivogen) during 5 hours. Brefeldin A (eBioscience) was added for the last 3 hours of culture. Human PBMCs were surface labeled with anti–BDCA2-APC (Miltenyi Biotec) and anti–Lin-FITC antibodies and BM cells from humanized mice with anti–human CD45-PE–Cy7, anti–CD123-PE–Cy5, and anti–BDCA4-APC. Cells were then fixed, permeabilized, and stained for intracellular cytokine production using anti–IFN-α–PE (Miltenyi Biotec) or anti–TNF-α–Alexa700 (BD Biosciences) antibodies.
Mouse bone marrow cells suspensions were activated with 2 μg/mL R848 (Invivogen), or preparation of oligonucleotides PolyU (Sigma-Aldrich) or CpG-2216 (Invivogen) mixed with 1,2-dioleoyloxy-3-trimethylammonium-propane for 3 to 4 hours. Brefeldin A (eBioscience) was added for the last 2 hours of culture. Bone marrow cell suspensions were then stained with PECy7-labeled antibodies specific for mouse CD11c (N418) or B220 (RA3–6B2), and mPDCA1-APC (all from eBioscience). Cells were then fixed and stained intracellularly with mixed FITC-labeled IFN-α/β-specific antibodies (RMMA-1/RMMB-1; PBL), PE-labeled TNF-α (MP6-XT2; BD Biosciences), and anti–IL-12p40–PerCP-Cy5 (C15.6; BD Biosciences). Data were acquired on a LSR II (BD Biosciences).
Statistical analysis
Data were analyzed using GraphPad Prism Version 4.03 (GraphPad Software). The effect of E2 treatment on cytokine production by TLR-stimulated pDCs from postmenopausal women was assessed by the 2-tailed Wilcoxon signed rank test. Differences between groups were otherwise analyzed by the 2-tailed Mann-Whitney U test.
Results
Sex-based differences determine the TLR-7–mediated response of human pDCs in vivo
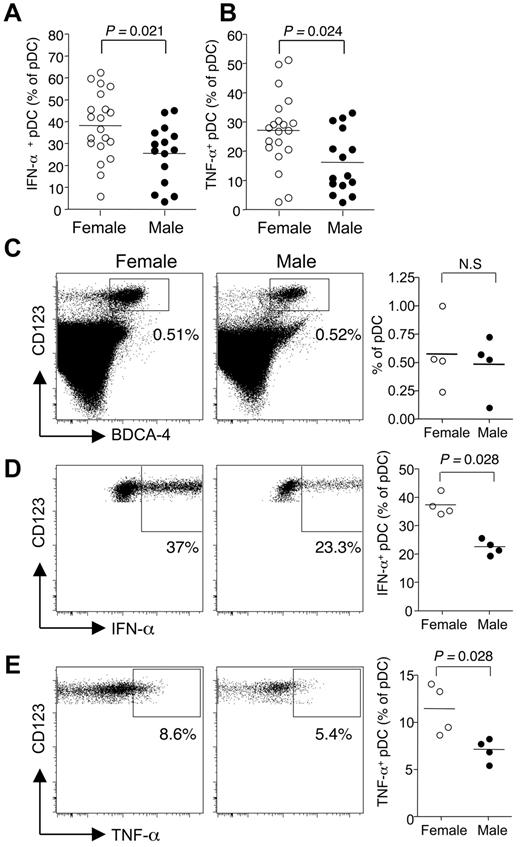
It has been recently reported that pDCs from women compared with men exhibited an enhanced capacity to produce type I IFNs when stimulated with TLR-7/TLR-8 ligands.22,23 We first confirmed these results by measuring the frequency of IFN-α– and TNF-α–producing pDCs in response to short term ex vivo stimulation of PBMCs from healthy female and male donors with the TLR-7/TLR-8 ligand R-848. In agreement with previous works,23 we observed an increased frequency of pDCs producing not only IFN-α but also TNF-α in response to TLR-7 stimulation in women of child-bearing potential compared with age-matched men (Figure 1A-B).
Sex-based differences control the TLR-7-mediated response of human pDCs in vivo. (A-B) pDCs derived from premenopausal women (18-45 years old, n = 20) or age-matched male donors (n = 15) were stimulated with TLR-7/TLR-8 ligand R-848 and stained for IFN-α and TNF-α. Percentage of IFN-α (A) and TNF-α (B) positive pDCs in premenopausal women (18-45 years old, n = 20) compared with men (n = 15). (C-E) Female or male NOD/SCID/β2m−/− were sublethally irradiated and transplanted with human CD34+ hematopoietic progenitor cells from 3 different female donors. (C) After 8 weeks, human pDCs were identified as the CD45+ CD123+ BDCA-4+ compartment in bone marrow cells. (D-E) Bone marrow cells were restimulated ex vivo as in panel A. The frequencies of IFN-α (D) and TNF-α (E) producing pDCs were determined by flow cytometry. Horizontal lines in scatter plots on the right panels indicate mean values. Statistical difference between groups was assessed using the Mann-Whitney U test.
Sex-based differences control the TLR-7-mediated response of human pDCs in vivo. (A-B) pDCs derived from premenopausal women (18-45 years old, n = 20) or age-matched male donors (n = 15) were stimulated with TLR-7/TLR-8 ligand R-848 and stained for IFN-α and TNF-α. Percentage of IFN-α (A) and TNF-α (B) positive pDCs in premenopausal women (18-45 years old, n = 20) compared with men (n = 15). (C-E) Female or male NOD/SCID/β2m−/− were sublethally irradiated and transplanted with human CD34+ hematopoietic progenitor cells from 3 different female donors. (C) After 8 weeks, human pDCs were identified as the CD45+ CD123+ BDCA-4+ compartment in bone marrow cells. (D-E) Bone marrow cells were restimulated ex vivo as in panel A. The frequencies of IFN-α (D) and TNF-α (E) producing pDCs were determined by flow cytometry. Horizontal lines in scatter plots on the right panels indicate mean values. Statistical difference between groups was assessed using the Mann-Whitney U test.
However, it is not clear whether this bias is the result of gene dosage effect because TLR-7 is encoded on the X chromosome, differences in other X chromosome-linked genetic factors, or differences in the production of sex steroid hormones. To address this issue, we evaluated the TLR-mediated response of human pDCs from different female donors that developed in a male or female environment using a well-established model of humanized mice.24 Sublethally irradiated NOD/SCID/β2m−/− mice of either sex were transplanted with CD34+ hematopoietic progenitor cells from healthy female donors. Eight weeks after transplantation, bone marrow cells from these humanized mice were depleted of hematopoietic cells of mouse origin and stimulated in vitro with a TLR-7/TLR-8 ligand to assess the frequency of IFN-α– or TNF-α–producing pDCs as described in Figure 1A-B. The frequency of CD123+ BDCA-4+ pDCs among human CD45+ cells in the bone marrow was not affected by the sex of the recipient mice (Figure 1C). In striking contrast, the frequency of IFN-α+ pDCs was significantly increased in female compared with male mice (Figure 1D). Interestingly, the mean frequencies of IFN-α+ pDCs measured in humanized male and female mice were similar to those obtained in sex-matched human donors, with female pDCs exhibiting a 1.8-fold increase frequency of IFN-α–producing cells compared with male ones (Figure 1A,D). Similar results were obtained by analyzing the production of TNF-α (Figure 1E). Again, pDCs that developed in a female environment exhibited an increased frequency of TNF-α–producing cells compared with male ones (Figure 1E). A similar tendency was observed when we analyzed the murine pDC response after R-848 stimulation in the same humanized NOD/SCID/β2m−/− mice (not shown). However, this sex-dependent difference was not found in C57BL/6 mice (data not shown). Altogether, these data show that the TLR-7–mediated response of human pDCs is shaped by the sex of the host rather than cell-intrinsic X-linked genetic factors.
Exogenous and endogenous estrogens selectively enhance the R-848–mediated responses of human pDCs but not monocytes
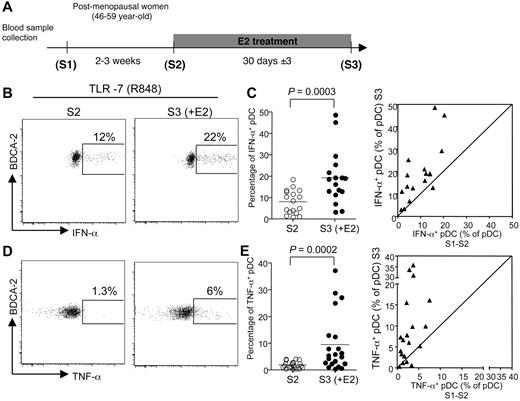
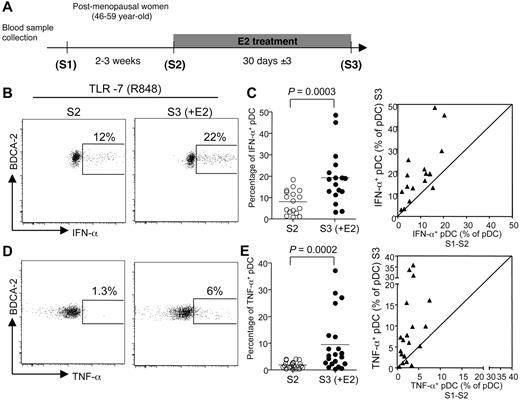
To investigate whether estrogens were responsible for this sexual dimorphism, we evaluated the effect of E2 treatment on the TLR-mediated response of pDCs in early postmenopausal women in a longitudinal clinical trial. In this study, blood samples were obtained at 3 time points over a period of up to 2 months. To evaluate the intraindividual variability of the different parameters analyzed, 2 PBMC samples (S1 and S2) were collected before treatment, 2 to 3 weeks apart. Estrogen therapy was initiated just after the S2 collection, and the last blood sample (S3) was taken one month later (Figure 2A). The absolute numbers and relative abundances of conventional DCs (cDCs) and pDCs were constant in samples S1 and S2 from each person before treatment and were not modified after one month of E2 administration (supplemental Figure 1A-B). In addition, the percentages of distinct DC subsets among purified PBMCs were stable (supplemental Figure 1C). Thus, administration of E2 in postmenopausal women did not affect blood DC subset counts, including pDCs.
E2 administration increases the frequency of pDC producing IFN-α and TNF-α after TLR-7 stimulation. (A) The first 2 blood samples (S1 and S2) from early postmenopausal women volunteers were collected before E2 treatment by oral or transdermal route with standard substitutive posology. The last blood sample (S3) was collected after 30 ± 3 days of treatment. (B,D) Representative flow cytometry plots showing IFN-α (B) and TNF-α (D) production by BDCA-2+ pDCs derived from postmenopausal women before (left) or after E2 treatment (right), after 5 hours of in vitro stimulation with TLR-7/TLR-8 ligand R-848. (C,E) Percentage of pDCs from postmenopausal women before (S2) and after E2 treatment (S3), producing IFN-α (C, n = 17) or TNF-α (E, n = 21) on in vitro culture in the presence of R848. Horizontal bars indicate mean values, and P values were determined using the Wilcoxon signed rank test. (C,E) Dot plot panels show the correspondence between the values for each patient between mean S1-S2 versus S3.
E2 administration increases the frequency of pDC producing IFN-α and TNF-α after TLR-7 stimulation. (A) The first 2 blood samples (S1 and S2) from early postmenopausal women volunteers were collected before E2 treatment by oral or transdermal route with standard substitutive posology. The last blood sample (S3) was collected after 30 ± 3 days of treatment. (B,D) Representative flow cytometry plots showing IFN-α (B) and TNF-α (D) production by BDCA-2+ pDCs derived from postmenopausal women before (left) or after E2 treatment (right), after 5 hours of in vitro stimulation with TLR-7/TLR-8 ligand R-848. (C,E) Percentage of pDCs from postmenopausal women before (S2) and after E2 treatment (S3), producing IFN-α (C, n = 17) or TNF-α (E, n = 21) on in vitro culture in the presence of R848. Horizontal bars indicate mean values, and P values were determined using the Wilcoxon signed rank test. (C,E) Dot plot panels show the correspondence between the values for each patient between mean S1-S2 versus S3.
At each time point, we stimulated PBMCs with the TLR-7/TLR-8 ligand R-848 and analyzed IFN-α and TNF-α production in Lin− BDCA-2+ pDCs (supplemental Figure 2A). Before E2 treatment, the average frequencies of IFN-α- and TNF-α–producing cells among pDCs were 7.7% ± 5% and 2.1% ± 1.5%, respectively (mean ± SEM, Figure 2; supplemental Figure 2). There was no difference in the percentage of cytokine-producing pDCs at the 2 time points tested before E2 therapy (supplemental Figure 2). Of note, the percentages of IFN-α– or TNF-α–producing pDCs in TLR-7–stimulated PBMCs were significantly higher (P < .001) in premenopausal women (Figure 1A-B) compared with postmenopausal women (Figure 2C,E). These differences were probably the result of estrogen deprivation because the proportions of TLR-7–stimulated pDC-producing IFN-α (Figure 2B-C) or TNF-α (Figure 2D-E) were significantly increased after one month of E2 treatment, with a mean fold increase of 2.8 and 5.2, respectively. This enhancing effect of estrogens on the TLR-7–mediated response of pDCs did not require the presence of the hormone in vitro because addition of physiologic concentrations of E2 to PBMC cultures did not modify the frequency of pDCs producing either IFN-α or TNF-α on stimulation via TLR-7 (supplemental Figure 3). Because this result was obtained with PBMCs from both untreated and E2-treated postmenopausal women (supplemental Figure 3), we can conclude that the enhancing effect of estrogens on cytokine production by TLR-stimulated pDCs was established in vivo.
Because the TLR-7/TLR-8 ligand R-848 is also capable of triggering TNF-α production in monocytes through TLR-8,30 we also analyzed the impact of menopause and E2 supplementation on endosomal TLR responsiveness in this cell population (supplemental Figure 4). We focused our analysis on the FSChi SSChi flow cytometric fraction that is mainly composed of large monocytes expressing CD11c and CD14 (supplemental Figure 4A). In premenopausal women, the proportion of TNF-α–producing monocytes was similar to that of TNF-α-producing pDCs (mean ± SEM, 34.8% ± 13.4% vs 27.1% ± 12.7%, respectively). Interestingly, whereas the frequency of TNF-α+ pDCs dropped in postmenopausal women (from 27.1% ± 12.7% to 3.73% ± 4.7%), the percentage of TNF-α–producing monocytes in response to R-848 stimulation remained at a comparable level between premenopausal and postmenopausal women and was not affected by E2 therapy in postmenopausal women (supplemental Figure 4C). Altogether, these data demonstrate that estrogens selectively up-regulate endosomal TLR-responsiveness in pDCs but not in monocytes.
E2 treatment enhances TLR-9–mediated IFN-α production by pDCs in postmenopausal women
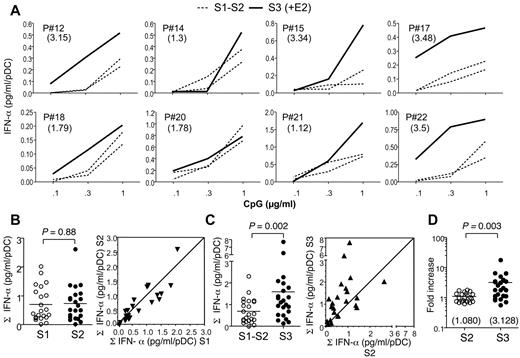
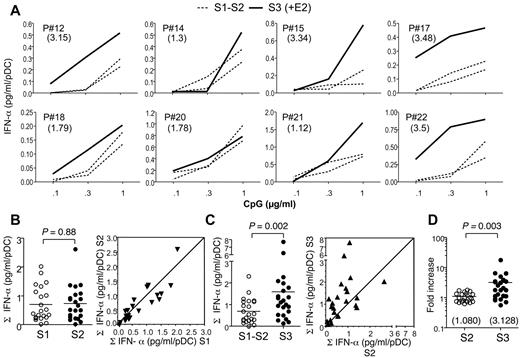
We next investigated whether the enhancing effect of E2 on IFN-α production by pDCs was specific of TLR-7–mediated activation or could also be observed in response to TLR-9 stimulation of PBMCs using CpG-2216 oligonucleotides. CpG-2216 is primarily acting on pDCs and has been shown to trigger the production of IFN-α in whole PBMCs but not in PBMCs depleted of pDCs.31 Before E2 treatment, CpG-2216 triggered the production of IFN-α in a dose-dependent manner, with no significant differences between the S1 and S2 samples (Figure 3A-B). Interestingly, after one month of E2 treatment, a strong increase in IFN-α production by CpG-stimulated PBMCs was observed in the majority of women (Figure 3C). The analysis of the TLR-9–responsiveness in 23 subjects demonstrated a highly significant enhancing effect of E2 therapy (Figure 3C). When all data were normalized to S1 values, there was a significant 3-fold increase on average in IFN-α scores after E2-treatment (Figure 3D).
E2 treatment enhances IFN-α production by TLR-9 ligand-stimulated pDCs in postmenopausal women. (A) PBMCs purified from whole blood were cultured at 5 × 105 cells/well in the presence of indicated concentrations of CpG-2216. IFN-α concentration was measured by ELISA in 24-hour culture supernatants. The IFN-α range measured for 1 μg/mL of CpG was from 21 pg/mL to 2914 pg/mL. Data were normalized to the number of pDCs present in PBMCs, as determined by flow cytometry. Data from 8 representative subjects are shown, before (dashed lines) or after E2 treatment (solid line). Numbers in parentheses show the fold increase of IFN-α production as calculated in panel D. (B-C) Normalized IFN-α production before (B) and after (C) E2 treatment was calculated by summing the concentrations of IFN-α measured in response to the different concentrations of CpG as shown in panel A (n = 23). Dot plot panels show the correspondence between the values for each patient in samples S1 versus S2 (B) and S2 versus S3 (C). (D) Data from samples S2 and S3 were expressed as fold increase of normalized IFN-α production relative to the S1 and mean S1-S2 values, respectively. Individual data are shown, and horizontal bars indicate mean values. P values were determined using the Wilcoxon signed rank test.
E2 treatment enhances IFN-α production by TLR-9 ligand-stimulated pDCs in postmenopausal women. (A) PBMCs purified from whole blood were cultured at 5 × 105 cells/well in the presence of indicated concentrations of CpG-2216. IFN-α concentration was measured by ELISA in 24-hour culture supernatants. The IFN-α range measured for 1 μg/mL of CpG was from 21 pg/mL to 2914 pg/mL. Data were normalized to the number of pDCs present in PBMCs, as determined by flow cytometry. Data from 8 representative subjects are shown, before (dashed lines) or after E2 treatment (solid line). Numbers in parentheses show the fold increase of IFN-α production as calculated in panel D. (B-C) Normalized IFN-α production before (B) and after (C) E2 treatment was calculated by summing the concentrations of IFN-α measured in response to the different concentrations of CpG as shown in panel A (n = 23). Dot plot panels show the correspondence between the values for each patient in samples S1 versus S2 (B) and S2 versus S3 (C). (D) Data from samples S2 and S3 were expressed as fold increase of normalized IFN-α production relative to the S1 and mean S1-S2 values, respectively. Individual data are shown, and horizontal bars indicate mean values. P values were determined using the Wilcoxon signed rank test.
E2 enhances IFN-α production by pDCs stimulated with self-nucleic acid-containing immune complexes
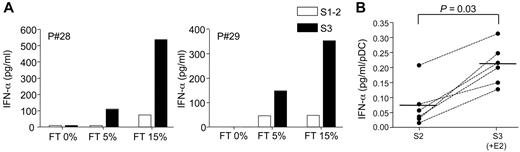
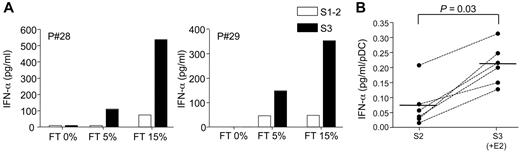
Autoantibodies found in the serum of SLE patients in the form of immune complexes containing self-DNA or RNA are potent inducers of type I IFN production by pDCs through activation of TLR-9 or -7, respectively.32,33 We also analyzed the effect of E2 treatment on the production of IFN-α by PBMCs from postmenopausal women stimulated with SLE sera. In agreement with previous work by others,28 SLE sera could induce IFN-α secretion by PBMCs only in the presence of necrotic supernatants (Figure 4A). In postmenopausal women, the production of IFN-α in response to SLE serum was usually low and, when normalized to the number of pDC, represented in average (± SD) 0.068 ± 0.06 pg/mL/pDC (Figure 4B). Interestingly, after E2-treatment we observed a marked up-regulation of IFN-α production by PBMCs cocultured with SLE sera (Figure 4A). After normalization to pDC numbers, we found a significant stimulatory effect of E2 administration on the IFN-α response of PBMCs stimulated by SLE sera in the 6 subjects tested in this assay (Figure 4B). The increase in IFN-α production (to 0.21 ± 0.06 pg/mL/pDC) corresponded to a 1.7- to 15-fold change relative to the response obtained before E2 treatment (Figure 4B). These data show that in vivo exposure to E2 conditions blood pDCs to enhanced TLR-dependent production of IFN-α in response to nucleic acid containing immune complexes.
E2 treatment enhances IFN-α production induced by nucleic acid-containing immune complexes present in sera from SLE patients. PBMCs from postmenopausal women (5 × 105 cells/well) were stimulated with 1% SLE serum in the presence of different concentrations of necrotic supernatant from frozen-thawed (FT) PBMCs. IFN-α concentration in 24-hour culture supernatants was measured by ELISA. (A) IFN-α production by PBMCs from 2 representative postmenopausal women before (open bar) or after transdermal E2-therapy (solid bar). (B) Data obtained from 6 subjects before (S2) and after (S3) E2 treatment. PBMCs were stimulated as in panel A with 1% SLE serum with 15% FT, and IFN-α production was normalized to pDC numbers and expressed as picograms per milliliter pDCs. Individual data are shown, and bars represent mean values. P values were determined using the Wilcoxon signed rank test.
E2 treatment enhances IFN-α production induced by nucleic acid-containing immune complexes present in sera from SLE patients. PBMCs from postmenopausal women (5 × 105 cells/well) were stimulated with 1% SLE serum in the presence of different concentrations of necrotic supernatant from frozen-thawed (FT) PBMCs. IFN-α concentration in 24-hour culture supernatants was measured by ELISA. (A) IFN-α production by PBMCs from 2 representative postmenopausal women before (open bar) or after transdermal E2-therapy (solid bar). (B) Data obtained from 6 subjects before (S2) and after (S3) E2 treatment. PBMCs were stimulated as in panel A with 1% SLE serum with 15% FT, and IFN-α production was normalized to pDC numbers and expressed as picograms per milliliter pDCs. Individual data are shown, and bars represent mean values. P values were determined using the Wilcoxon signed rank test.
Endogenous and exogenous estrogens enhance cytokine production by mouse pDCs after TLR-7/TLR-9 stimulation
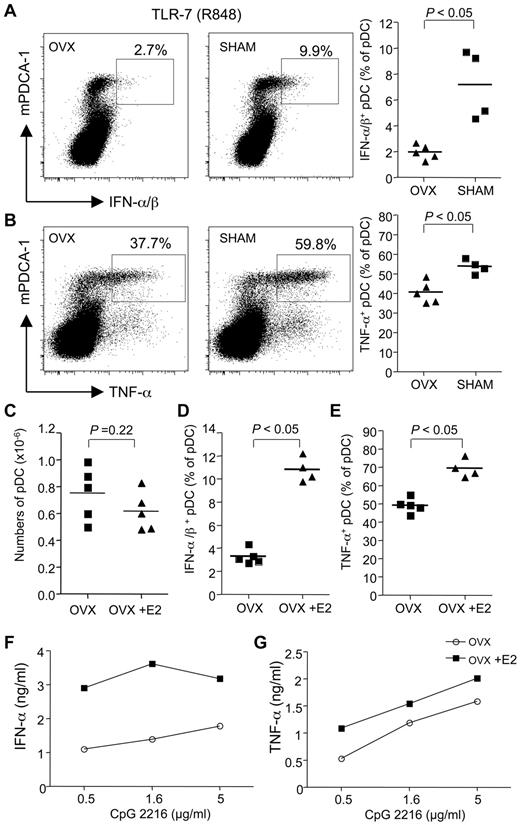
We next sought to understand the mechanisms involved in the E2-amplifying effect on the TLR-dependent response of pDCs in mice. We first analyzed whether endogenous estrogens could influence pDC response to TLR stimulation by comparing female mice that were ovariectomized (Ovx) or not before their sexual maturity. We found that ovariectomy was associated with a 3-fold decrease in the frequency of bone marrow pDCs producing type I IFNs (Figure 5A) and with a significant decrease in pDCs producing TNF-α (Figure 5B) in response to TLR-7 triggering. These results suggested that endogenous estrogens enhanced the TLR-mediated response in mouse pDCs as well. To directly demonstrate this point, Ovx mice were supplemented or not with E2 before the TLR-dependent responses of pDCs were examined. Although E2 supplementation decreased the overall bone marrow cellularity (not shown), the absolute number of pDCs was not significantly different between E2-treated and control Ovx mice (Figure 5C). In response to TLR-7 stimulation, we found an enhanced frequency of pDCs producing not only IFN-α (Figure 5D) but also TNF-α (Figure 5E) in E2-supplemented Ovx mice.
Endogenous and exogenous estrogens enhance pDC cytokine production after TLR-7/TLR-9 stimulation. Female WT C57BL/6 (B6) mice were ovariectomized (OVX) or not (Sham) before sexual maturity (3-4 weeks). In adulthood (7-8 weeks), bone marrow cells were restimulated ex vivo in the presence of R-848 for 3 hours. Dot plot showing the frequency of pDCs (mPDCA1+) producing IFN-α/β (A) and TNF-α (B). The lines on scatter plots indicate mean values. Results from individual mice are shown. P values were determined using the Mann-Whitney U test. (C-E) Ovariectomized mice were treaded or not with E2 (0.05-mg E2 pellet, 60-day release) for 2 weeks. pDCs were stimulated with R-848 and analyzed for IFN-α (C) and TNF-α (D). (E) Purified bone marrow pDCs were stimulated with the TLR-9 ligand CpG-2216. Culture supernatant was collected after 24 hours of stimulation, and IFN-α (F) and TNF-α (G) were measured by ELISA. Data are representative of 2 or 3 independent experiments.
Endogenous and exogenous estrogens enhance pDC cytokine production after TLR-7/TLR-9 stimulation. Female WT C57BL/6 (B6) mice were ovariectomized (OVX) or not (Sham) before sexual maturity (3-4 weeks). In adulthood (7-8 weeks), bone marrow cells were restimulated ex vivo in the presence of R-848 for 3 hours. Dot plot showing the frequency of pDCs (mPDCA1+) producing IFN-α/β (A) and TNF-α (B). The lines on scatter plots indicate mean values. Results from individual mice are shown. P values were determined using the Mann-Whitney U test. (C-E) Ovariectomized mice were treaded or not with E2 (0.05-mg E2 pellet, 60-day release) for 2 weeks. pDCs were stimulated with R-848 and analyzed for IFN-α (C) and TNF-α (D). (E) Purified bone marrow pDCs were stimulated with the TLR-9 ligand CpG-2216. Culture supernatant was collected after 24 hours of stimulation, and IFN-α (F) and TNF-α (G) were measured by ELISA. Data are representative of 2 or 3 independent experiments.
To make sure that these functional differences were the result of changes in the cell-intrinsic properties of pDCs, the specific population was isolated out of bone marrow cells before testing them for TLR activation in vitro using the TLR-9 ligand CpG-2216. We found a marked increase in the production of IFN-α (Figure 5F) and TNF-α (Figure 5G) by TLR-9–stimulated pDCs from E2-treated mice. No cytokines were produced in the absence of TLR ligand (not shown). Together, these data showed that both endogenous and exogenous estrogens increase endosomal TLR-7 and TLR-9 responsiveness of mouse pDCs in vivo.
ERα-deficiency in bone marrow cells decreases pro-inflammatory cytokine production of pDCs after TLR-7/TLR-9 stimulation
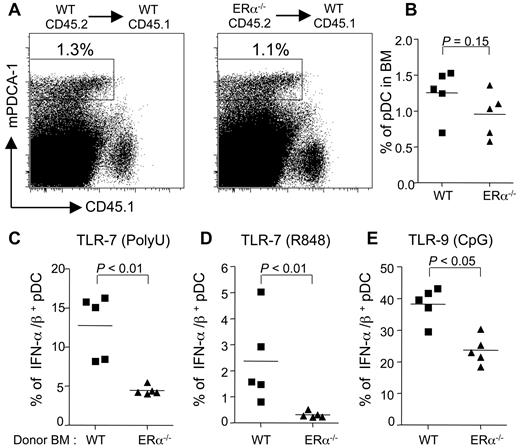
To gain insight into the mechanisms involved, we determined whether the effect of estrogens on pDC innate functions was mediated through ERα signaling within the hematopoietic compartment. Because ERβ is not expressed in DC progenitors,34 we decided to focus our analysis on ERα. To this end, female CD45.1 mice were sublethally irradiated and then reconstituted with either CD45.2 wild-type (WT) or ERα−/− bone marrow cells. After 3 weeks of reconstitution, we analyzed the pDC response after TLR-7– or TLR-9–triggering in the bone marrow of these chimeras. We found that ERα deficiency did not modify the percentage of pDCs derived from the grafted cells (Figure 6A-B). When bone marrow cells were stimulated with TLR-7 ligands, poly(U) (Figure 6C), R-848 (Figure 6D), or TLR-9 ligand CpG (Figure 6E), the frequencies of IFN-α/β–producing pDCs were significantly reduced in bone marrow cells derived from ERα−/− compared with WT donors. This impaired pDC responsiveness in the absence of ERα was not limited to type I IFN but was also observed when we analyzed the frequency of pDCs producing TNF-α or IL-12p40 (supplemental Figure 5). Altogether, our results show that hematopoietic expression of ERα is required to mediate the in vivo enhancing effect of endogenous estrogens on the TLR-mediated responses of mouse pDCs in the bone marrow.
The enhancing effect of E2 on the TLR-mediated responses of pDC is mediated through hematopoietic ERα. WT or ERα−/− B6 bone marrow cells were injected into lethally irradiated CD45.1 WT female B6 mice. After 3 weeks of reconstitution, bone marrow cells were stimulated with polyU (C), R848 (D), or GpG-2216 (E). (A) Dot plots showing the frequency of WT or ERα−/− pDCs (mPDCA1+CD45.1−). (B) Percentage of pDCs from individual mice. (C-E) Intracellular IFN-α/β staining of pDCs derived from ERα+/+ or ERα−/− bone marrow cells. Stimulated pDCs were stained for IFN-α/β and analyzed by flow cytometry. Results from individual mice are shown. The lines on scatter plots indicate mean values. P values were determined using the Mann-Whitney U test. Data are representative of 3 independent experiments.
The enhancing effect of E2 on the TLR-mediated responses of pDC is mediated through hematopoietic ERα. WT or ERα−/− B6 bone marrow cells were injected into lethally irradiated CD45.1 WT female B6 mice. After 3 weeks of reconstitution, bone marrow cells were stimulated with polyU (C), R848 (D), or GpG-2216 (E). (A) Dot plots showing the frequency of WT or ERα−/− pDCs (mPDCA1+CD45.1−). (B) Percentage of pDCs from individual mice. (C-E) Intracellular IFN-α/β staining of pDCs derived from ERα+/+ or ERα−/− bone marrow cells. Stimulated pDCs were stained for IFN-α/β and analyzed by flow cytometry. Results from individual mice are shown. The lines on scatter plots indicate mean values. P values were determined using the Mann-Whitney U test. Data are representative of 3 independent experiments.
E2-mediated up-regulation of IFN-α production by TLR-9–activated pDCs is lost in mice lacking ERα in pDCs
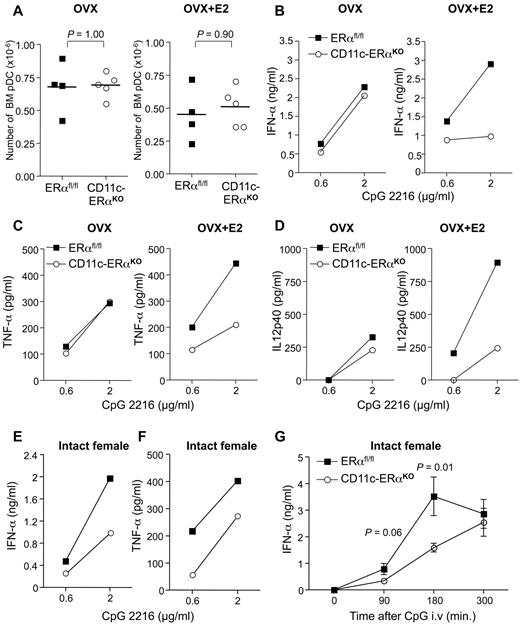
E2-mediated activation of ERα may act at distinct stages of pDC development to modulate their functional properties, either through cell-intrinsic or cell-extrinsic mechanisms within the hematopoietic compartment. To dissect at which stage of pDC development ERα activation is determinant, we compared the effect of E2 treatment on the innate functions of pDCs isolated from mice lacking ERα within the whole hematopoietic compartment (Tie2-ERαKO) or more specifically in the DC lineage (CD11c-ERαKO). In the CD11c-ERαKO mice, Cre-mediated recombination removing the floxed genes has been shown to occur mainly in the DC lineage, with minimal deletion in lymphocytes and myeloid cells.35 Although there was a tendency toward a slight reduction in immature pDC number in the bone marrow of E2-treated mice, this effect was not significant and was not dependent on ERα expression (supplemental Figure 6A; Figure 7A). Absolute numbers of bone marrow pDCs were not altered by Cre-mediated ERα gene deletion whether mice were supplemented or not with E2 (supplemental Figure 6A; Figure 7A). Thus, in agreement with data in Figure 6B, ERα signaling did not modify the absolute numbers of immature pDCs in the bone marrow. Next, pDCs were FACS-sorted (purity > 99%; supplemental Figure 7) from the bone marrow of Ovx mice treated or not with E2 and stimulated in vitro with CpG-2216. Whereas production of IFN-α (Figure 7B), TNF-α (Figure 7C), and IL-12p40 (Figure 7D) was similar between estrogen-deprived Ovx ERαflox/flox control and Ovx CD11c-ERαKO mice, enhanced cytokine production was observed in ERαflox/flox mice compared with CD11c-ERαKO mice in the presence of exogenous E2. Similar results were obtained by comparing the TLR-9–mediated responses of bone marrow pDCs from control and Tie2-ERαKO mice (supplemental Figure 6). Again, a marked difference in the TLR-9–dependent production of IFN-α and TNF-α was seen between control ERαflox/flox and Tie2-ERαKO pDCs from E2-treated mice (supplemental Figure 6B-C). By contrast, when pDCs were obtained from untreated Ovx mice, the cytokine responses were similar between both groups. Analysis of phenotypic markers on CpG-2216–stimulated pDCs did not show overt changes regarding the expression of mPDCA-1 and CD40 between pDCs from control or conditional knock-out mice, whether mice were supplemented or not with E2 (supplemental Figure 7; and data not shown).
ERα signaling in pDCs is required to mediate the enhancing effect of E2 on the TLR-mediated production of IFN-α. (A-D) Ovariectomized CD11c-ERαKO female or ERαfl/fl control littermates on a B6 background were treated or not with E2 as in Figure 5. (A) Absolute numbers of bone marrow pDCs from individual mice. P value was determined using the Mann-Whitney U test. pDCs were FACS-purified from pooled bone marrow cells (3-5 mice per group) and stimulated with the indicated amounts of the TLR-9 ligand CpG-2216. Culture supernatants were collected after 24 hours of stimulation, and IFN-α (B), TNF-α (C), and IL-12p40 (D) concentrations were measured by ELISA. Data are representative of 2 independent experiments. (E-G) Bone marrow pDCs were purified from intact female CD11c-ERαKO or ERαfl/fl control mice, and the production of IFN-α (E) and TNF-α (F) on TLR-9 stimulation was analyzed by ELISA. (G) Intact CD11c-ERαKO (n = 5) or ERαfl/fl (n = 4) female mice were injected intravenously with 2 μg of CpG-2216, and their serum IFNα concentration was then assessed by ELISA at the indicated time points.
ERα signaling in pDCs is required to mediate the enhancing effect of E2 on the TLR-mediated production of IFN-α. (A-D) Ovariectomized CD11c-ERαKO female or ERαfl/fl control littermates on a B6 background were treated or not with E2 as in Figure 5. (A) Absolute numbers of bone marrow pDCs from individual mice. P value was determined using the Mann-Whitney U test. pDCs were FACS-purified from pooled bone marrow cells (3-5 mice per group) and stimulated with the indicated amounts of the TLR-9 ligand CpG-2216. Culture supernatants were collected after 24 hours of stimulation, and IFN-α (B), TNF-α (C), and IL-12p40 (D) concentrations were measured by ELISA. Data are representative of 2 independent experiments. (E-G) Bone marrow pDCs were purified from intact female CD11c-ERαKO or ERαfl/fl control mice, and the production of IFN-α (E) and TNF-α (F) on TLR-9 stimulation was analyzed by ELISA. (G) Intact CD11c-ERαKO (n = 5) or ERαfl/fl (n = 4) female mice were injected intravenously with 2 μg of CpG-2216, and their serum IFNα concentration was then assessed by ELISA at the indicated time points.
Lastly, we analyzed the impact of DC-specific ERα deficiency in intact female mice on the TLR-9–mediated response of bone marrow and splenic pDCs. A reduced production of IFN-α (Figure 7E) and TNF-α (Figure 7F) was observed in CpG-stimulated cultures of purified bone marrow pDCs from CD11c-ERαKO mice compared with their ERαflox/flox littermate controls. We then measured the kinetics of serum IFN-α production after intravenous injection of CpG-2216, which is primarily mediated by splenic pDCs.29 Data in Figure 7G show that the production of IFN-α in CD11c-ERαKO was significantly lower at 3 hours compared with ERαflox/flox mice. Similar results were obtained in intact Tie2-ERαKO female mice injected intravenously with CpG, which exhibited a 2-fold reduction in the serum IFN-α concentration compared with ERαflox/flox control mice (supplemental Figure 6D).
Altogether, these results demonstrate that ERα signaling in pDCs in vivo regulates their capacity to produce type I IFNs on stimulation of their endosomal TLR-9 not only ex vivo but also in situ.
Discussion
In this paper, we have analyzed the role of estrogens in the regulation of the TLR-mediated responses of human and mouse pDCs in vivo. Our data demonstrate that endogenous and exogenous estrogens were able to markedly enhance the endosomal TLR-7– and TLR-9–mediated responses of pDCs during their steady state development. By using gene-targeted mice, we provided direct in vivo evidence for estradiol-dependent modulation of the TLR-mediated responses of pDCs through a mechanism involving pDC-intrinsic expression of ERα.
Previous works by others failed to report significant sex-dependent difference in the TLR-9–mediated response of human pDCs.22,23 In our clinical study in postmenopausal women, titrated amounts of CpG were used to stimulate PBMCs, which are much lower than the doses used in previous studies.22,23 In some patients, the enhancing effect of E2 treatment on IFN-α production was even more evident for the lowest concentration of CpG, which induced barely detectable levels of IFN-α in most PBMCs from untreated donors. Because this effect of E2 treatment was not attributable to an increase of circulating human pDC numbers in PBMCs, our data suggest that E2 treatment had decreased the threshold of TLR-9 responsiveness in human pDCs. Our experiments in mice are consistent with this hypothesis and demonstrate enhanced TLR-9–mediated responses of pDCs from E2-treated mice through a mechanism that required pDC-intrinsic expression of ERα. Thus, the impact of female sex hormones on the TLR-9–mediated response of human pDCs could have been masked in previous studies because of differences in experimental settings.22,23
In line with previous works,22 we did not detect any substantial effect of E2 on the TLR-mediated responses of pDCs on short-term in vitro exposure. This observation suggests that the effect of E2 is somehow imprinted in vivo and independent of the presence of E2 at the time of TLR triggering in vitro. E2 mediates its effect through 2 main nuclear receptors, ERα and ERβ. We present compelling evidence that ERα expression in the hematopoietic compartment is required for the enhancing effect of estrogens on the TLR-mediated responses of pDCs, suggesting a direct action of the hormone on pDCs or their precursors in the bone marrow. pDCs can be produced from the same myeloid progenitors in the bone marrow that give rise to monocytes and DC precursors.36 It has been recently shown that myeloid progenitors defined as Lin− c-kithi Flt3+ cells expressed high levels of ERα, but not ERβ, transcripts34 and responded to E2 by inducing the expression of the transcription factor IRF-4, which is critical for DC development in the presence of GM-CSF.37,38 Indeed, we and others have shown that E2 is required for normal development of murine inflammatory DCs in vitro in the presence of GM-CSF, through ERα, but not ERβ.37,39 In the presence of Flt3L, monocytes and DC precursors give rise to the common DC precursors from which pDCs and pre-cDCs develop in the bone marrow.36,40 We did not notice any deficiency in the homeostatic numbers of pDCs in the bone marrow and spleen in the absence of hematopoietic ERα, suggesting that ERα signaling was dispensable for normal homeostatic pDC development. In striking contrast, we observed that ERα activation in cells already committed to the pDC lineage critically regulated the TLR-mediated response of pDCs, not only in the bone marrow, but also in the periphery. In the CD11c-ERαKO murine model, ERα inactivation probably occurs at a later stage of pDC development, probably after the common DC precursor stage because common DC precursors have been shown to lack CD11c expression.40 Therefore, we postulate that the E2-mediated increase in TLR-mediated responsiveness of pDCs is dependent on ERα activation occurring at a development stage between the putative committed pDC progenitor and terminally differentiated pDC state in the bone marrow41 and still persists in peripheral pDCs.
In vivo enhancing effects of E2 on innate immune functions of myeloid cells have been previously reported in mice.42,43 We and others have shown in vivo E2 administration to increase TLR-4–induced pro-inflammatory cytokine production by murine macrophages and microglia through ERα.42,43 Using conditional ERα-mutant mice, we recently demonstrated this in vivo effect of E2 to be mediated through ERα signaling in macrophages.44 However, in the present study, we did not detect any significant difference in the TLR-7/TLR-8–mediated response of blood monocytes between premenopausal and postmenopausal women, whether they were treated or not with E2. This is in agreement with the previous study by Meier et al, which failed to uncover any changes in the endosomal TLR-7/TLR-8–mediated response of human monocytes or myeloid DCs between women and men.23 These observations suggest that estrogens, at least in human, may selectively condition pDCs in vivo, but not other myeloid cells, such as monocytes, to enhance their capacity to respond to nucleic acids after stimulation of their endosomal TLRs.
Of note, we demonstrate that E2 treatment in vivo increases the production of type I IFNs by human pDCs after TLR-7 or TLR-9 activation, not only by synthetic ligands, but also by nucleic acid-containing immune complexes. This potent regulatory effect of estrogens may account for a large part of the substantial sex-dependent dimorphism in SLE. Indeed, estrogens represent a known risk factor for lupus in both women and mice.15,17,18,45 Our results identify a mechanism by which estrogens could exert such a profound modulatory effect through their action on the innate function of pDCs. A large body of evidence supports a central role for pDC-derived type I IFNs in SLE, and inhibition of TLR signaling in pDCs using TLR antagonists has been shown to represent a promising strategy for SLE treatment.4,46 Our data suggest that antagonizing ER signaling in vivo could represent an alternative approach for the down-regulation of TLR-dependent pDC activation in SLE.
Lastly, because of the pivotal role of pDCs in viral infections, up-regulation of the TLR-7 pathway in pDCs might improve anti–viral immunity in patients chronically infected with hepatitis C virus. Indeed, it has been recently shown that pDCs can sense hepatitis C virus-infected cells in the liver and produce IFN-α/β, thereby inhibiting infection of neighboring hepatocytes.47 We think that the potent regulatory effect of estrogens on TLR-mediated IFN-α production by human pDCs may account for the substantial sex bias observed in the pathogenesis of hepatitis C virus.48 This could also explain the intriguing observation that hormone replacement therapy is beneficial in postmenopausal women with chronic hepatitis C.49,50
In conclusion, our data highlight a previously unappreciated role for estrogens in promoting the innate functions of pDCs. This observation should open avenues to the development of therapeutic approaches aimed at modulating TLR signaling in pDCs, using selective ER modulators, for the optimal management of various diseases where this important DC subset plays a pivotal role.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the subjects who participated in this study, Drs M. Martinez and L. Pelletier for valuable advice, Dr S. Guerder for reviewing the manuscript, Prof J. Pourrat, Toulouse University Hospital, for providing sera from SLE patients, and Prof Pierre Chambon and Dr Boris Reizis for the generous gift of the ERα floxed and the CD11c-Cre mice, respectively.
C.S. was supported by Association pour la Recherche sur le Cancer (fellowship). This clinical study was sponsored by the University Hospital of Toulouse for regulatory and ethic submission (no. 0507402) and supported by the Délégation Régionale à la Recherche Clinique des Hôpitaux de Toulouse and Pierre Fabre laboratories. This work was also supported by Agence Nationale de la Recherche (ANR-06-PHYSIO-010, ANR-09-GENO-008), Arthritis Fondation Courtin, Association pour la Recherche sur le Cancer, Comité Haute-Garonne de la Ligue Nationale Contre le Cancer, and Conseil Régional Midi-Pyrénées. J.-C.G. and P.G. are the recipients of Contrats d'Interface from Toulouse University Hospital and Inserm, respectively.
Authorship
Contribution: C.S., S.L., and N.R. performed research and analyzed data; F.T. and C.R. designed the clinical study and recruited postmenopausal volunteers; V.D.-E., J.-F.A., and P.G. designed the clinical study; C.S., S.L., V.D.-E., and J.-C.G. designed the research; and C.S., S.L., and J.-C.G. analyzed data and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jean-Charles Guéry, Inserm UMR1043, Centre Hospitalier Universitaire Purpan, Place du Dr Baylac, 31024 Toulouse Cedex 3, France; e-mail: jean-charles.guery@inserm.fr.