Hyperresponsiveness to growth factors underlies a wide variety of human diseases, including hemangiomas, the most common tumor of childhood. Hemangiomas have been found to be clonal neoplasms of endothelial cells, with somatic mutations in unknown genes likely to be responsible for their development.
In this issue of Blood, Bhattacharya et al discover a novel mechanism of endothelial quiescence mediated by the Na+/H+ exchanger regulatory factor-2 (NHERF-2) gene, which might play a role in regulating responses to exogenous growth factors.1 Specifically, they describe that loss of NHERF-2 leads to a loss of endothelial quiescence and an enhanced response to exogenous growth factors. This is of clinical interest because previous studies have shown that hemangiomas do not make large amounts of endogenous VEGF. A major source of VEGF in hemangiomas is paracrine, namely overlying keratinocytes and stromal cells. Thus, controlling responsiveness to exogenous growth factors is a key to the pathogenesis and treatment of hemangiomas.
Bhattacharya et al discovered that NHERF-2 expression is relatively enriched in endothelial cells compared with the closely related gene NHERF-1, which exhibits a wider tissue distribution.1 SIRNA-mediated knockdown of NHERF-2 resulted in a significant increase in trititated thymidine uptake in cultured endothelial cells. Intriguingly, knockdown of NHERF-2 did not affect the phosphorylation of canonical VEGFR2 sites, including Y951, Y1059, and Y1175, and appears to down-regulate phosphorylation of Y416 Src. MAPK phosphorylation does not appear to be appreciably elevated as well. What, then, mediates the hyperresponsiveness of endothelial cells to VEGF in the absence of NHERF-2? Stimulation of downstream pathways may provide an answer. Levels of the proliferative oncogene C-myc are up-regulated in the absence of NHERF-2 and are further potentiated by exogenous VEGF. Finally, the cell-cycle inhibitor p27 is down-regulated by NHERF-2 knockdown. Significantly, down-regulation of NHERF-2 in the bend3 model of hemangioma leads to augmented growth in vivo. Thus, NHERF-2 loss leads to activation of a C-myc–p27 pathway, with the functional consequence of augmenting endothelial proliferation in vitro and in vivo.
Informatics on the oncogenes and tumor suppressors downstream of NHERF-2 loss reveals that beta-catenin can stimulate C-myc transcription through a TCF-dependent mechanism.2 In a tumor setting, enhanced C-myc signaling is associated with Skp2-mediated degradation of p27. NHERF-2 was found to be a binding partner, and possibly a negative regulator, of beta-catenin. The question of whether NHERF-2 prevents nuclear localization of beta-catenin is worthy of further study, given that nuclear localized beta-catenin is common in many solid tumors.
The physiologic function of NHERF-2 is not completely understood. NHERF-2 has been demonstrated to be a regulator of G protein receptor signaling, involving the parathyroid receptor, lysophosphatidic acid receptors, and the cystic fibrosis transporter (CFTR).3 NHERF-2 has been knocked out, giving viable and fertile animals with a subtle phenotype in the gut. Thus, none of these findings can fully explain the angioproliferative phenotype of NHERF-2 loss. Intriguingly, the closely related NHERF-1 protein binds the tumor suppressor PTEN, resulting in down-regulation of Akt.4 Loss of PTEN is associated with the genetic abnormalities Cowden disease and Bannayan-Zonanna syndrome, which are associated with hemangiomas and vascular malformations.5 Thus, it is possible that loss of NHERF-2 might cause activation of Akt. The lack of other signaling events seen with NHERF-2 knockdown (no effect on VEGFR2 receptor phosphorylation or MAPK), make Akt activation a very plausible and easily testable hypothesis, especially the presence of NHERF-2 overexpressing endothelial cells. One would predict that increased NHERF-2 would result in decreased Akt expression.
Hemangiomas are the original members of a class of tumors known as the reactive oxygen– driven tumor.6 These neoplasms are characterized by elevated Akt, which prevents apoptosis because of reactive oxygen, and at the same time have high levels of superoxide, which oxidizes wild-type p53, IkB, and PTEN. The functional consequences of this are wild-type inactive p53, activation of NFkB, and phosphorylation of Akt. These tumors can be treated by inhibitors of superoxide and hydrogen peroxide, which as single agents have a major impact on these pathways. NADPH oxidase 1 and 2, which produces superoxide, and NADPH oxidase 4, which produces hydrogen peroxide, have been implicated in hemangioma growth, as inhibitors of these NADPH oxidases have been highly efficacious against murine hemangioma models as well as human hemangiomas.7 A final question is whether NHERF-2 is functionally equivalent to inhibition of NADPH oxidases.
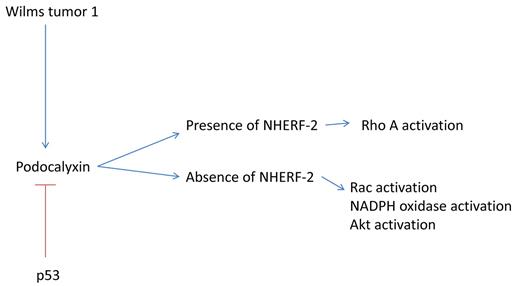
NHERF-2 is also prominent as a partner of the polarity-related protein podocalyxin. Podocalyxin, a transcriptional target of the Wilms tumor 1 gene (WT-1), was initially described as a protein involved in renal podosomes, but has been more recently implicated in tumor migration. High-level expression of podocalyxin has been associated with increased metastatic ability and poor outcome in renal cell carcinoma.8 The high-level expression of podocalyxin is associated with elevated rac activation.8 Given that rac activation is required for generation of superoxide through NADPH oxidases, it may be that the presence of NHERF-2 represents a molecular switch, in that the presence of NHERF-2 causes a decrease in rac activation and an increase in rhoA activation9 (see figure). The loss of NHERF-2 may lead to the reverse phenotype, rac activation, increased podocalyxin, and increased reactive oxygen, thus leading to increased actin polymerization and increased motility of both endothelial and tumor cells.10 Interestingly, WT-1, a major activator of podocalyxin, is highly expressed in hemangioma cells, as well as highly invasive tumors (ie, glioblastoma).11 Thus, NHERF-2 represents a novel target for pharmacologic therapy, and drugs that act to reduce reactive oxygen could potentially phenocopy NHERF-2.
NHERF-2 is a molecular switch that controls endothelial and tumor migration. In the presence of NHERF-2, rho A activation predominates, while in the absence of NHERF-2, a WT-1/podocalyxin/rac/reactive oxygen signaling pathway is activated.
NHERF-2 is a molecular switch that controls endothelial and tumor migration. In the presence of NHERF-2, rho A activation predominates, while in the absence of NHERF-2, a WT-1/podocalyxin/rac/reactive oxygen signaling pathway is activated.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
REFERENCES
National Institutes of Health