To the editor:
The specific function of Langerhans cells (LCs) in the skin immune system is controversial and a matter of intense debate.1 As epidermal LCs are potential targets for cancer immunotherapy, the topic is of primary importance.2 Recently, van de Ven et al showed that LCs are phenotypically mature,3 but, surprisingly, functionally defective in melanoma negative sentinel lymph nodes (SLNs).3 Complementing that study, we additionally analyzed LCs in melanoma positive as well as negative SLNs and, in light of the obtained data, we believe that LC immunophenotype in melanoma patients deserves additional, critical discussion.
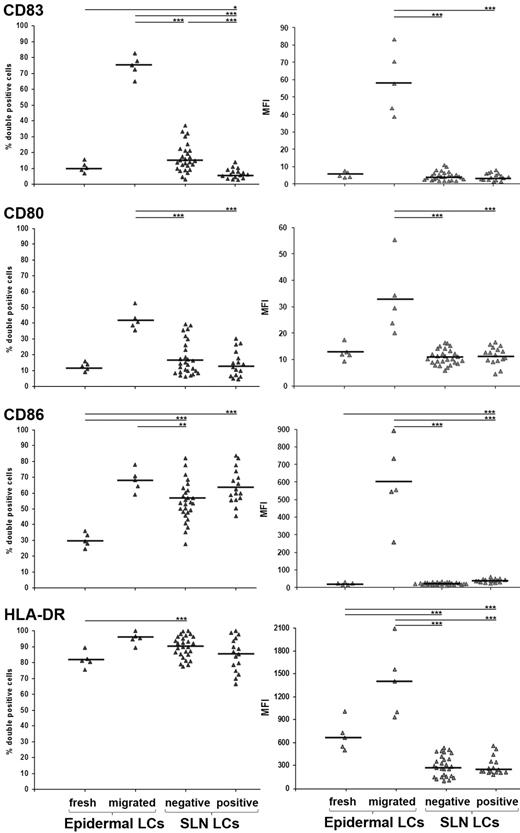
Building on our previous study showing that LCs are the most represented DC subset in melanoma SLNs,4 we now analyzed their immunophenotype and compared it with both freshly isolated and epidermal explant–migrated LCs as prototypes of immature and mature LCs, respectively. Melanoma patients undergoing SLN biopsy were enrolled after informed written consent and local institutional ethics committee approval. We investigated 16 melanoma positive SLNs (from 11 patients; stage III; 4 women, 7 men; mean age 62 years, range 25-84 years; average Breslow thickness 3.21 mm, range 0.45-10.4 mm; mean interval from melanoma excision 10 days, range 0-39 days), and 27 melanoma negative SLNs (from 16 patients; stage I/II; 4 women, 12 men; mean age 57 years, range 19-86 years; average Breslow thickness 2.43 mm, range 0.58-14 mm; mean interval from melanoma excision 16 days, range 0-56 days). By means of combinational analysis of percentage of positive cells and mean fluorescence intensity, we found that CD83, CD80, CD86, and HLA-DR expression in LCs from both negative and positive SLNs was lower than that found in LCs migrated from epidermal explants and, oddly, similar to that observed in freshly isolated epidermal LCs (Figure 1). Importantly, while the expression of co-stimulatory molecules CD80 and CD86 in LCs from negative SLNs was similar to that reported by van de Ven et al,3 the percentage of LCs expressing the prototypical DC maturation marker CD83 was considerably lower (16.68 ± 7.73% vs 45-50%; see Figure 3A in van de Ven et al).3 We reason that this remarkable difference depends on the fact that we immediately performed cell analysis on SLN biopsy, to avoid DC activation,5 at variance with van de Ven et al.3 Alternatively, the immature LC immunophenotype can be ascribed to the short mean interval from primary melanoma excision and SLN biopsy we conducted (16 days) in comparison to van de Ven et al (44 days3 ), a condition that might preserve the melanoma-induced suppressive effects on SLNs. Consistently, the percentage of LCs expressing CD83 in positive SLNs was significantly lower than that found in negative SLNs (6.51 ± 2.26% and 16.68 ± 7.73%, respectively), whereas that of LCs expressing CD80, CD86, and HLA-DR did not differ significantly (Figure 1).
Flow cytometry analyses of LCs in SLN from melanoma patients. SLN single cell suspension was obtained as described3 with modifications.4 Cells were collected in PBS, without enzymatic treatments or culturing, and immediately processed for flow cytometry analysis. Five-color cell staining was performed after cell membrane permeabilization with antibodies against Langerin PE (Immunotech), CD80 FITC and CD86 Pe-Cy5 (BD Pharmingen), CD83 APC and HLA-DR Pe-Cy7 (BioLegend). Cells were analyzed using FACS Canto with FACSDiva Version 6.0 software (BD Immunocytometry Systems). Isotype-matched Abs were used as negative controls. Results are expressed as percentages of double positive cells (left panels) and mean fluorescence intensity (right panels) among total gated Langerin+ cells, from freshly isolated and in vitro-migrated epidermal LCs (n = 5; from normal skin dermatomes of informed patients undergoing plastic surgery) and negative and positive SLNs LCs (n = 27 and n = 16, respectively). Bars represent mean value and asterisks indicate P value by 2-side Student t test statistical analysis (*P < .05; **P < .01; ***P < .001, respectively).
Flow cytometry analyses of LCs in SLN from melanoma patients. SLN single cell suspension was obtained as described3 with modifications.4 Cells were collected in PBS, without enzymatic treatments or culturing, and immediately processed for flow cytometry analysis. Five-color cell staining was performed after cell membrane permeabilization with antibodies against Langerin PE (Immunotech), CD80 FITC and CD86 Pe-Cy5 (BD Pharmingen), CD83 APC and HLA-DR Pe-Cy7 (BioLegend). Cells were analyzed using FACS Canto with FACSDiva Version 6.0 software (BD Immunocytometry Systems). Isotype-matched Abs were used as negative controls. Results are expressed as percentages of double positive cells (left panels) and mean fluorescence intensity (right panels) among total gated Langerin+ cells, from freshly isolated and in vitro-migrated epidermal LCs (n = 5; from normal skin dermatomes of informed patients undergoing plastic surgery) and negative and positive SLNs LCs (n = 27 and n = 16, respectively). Bars represent mean value and asterisks indicate P value by 2-side Student t test statistical analysis (*P < .05; **P < .01; ***P < .001, respectively).
The present findings are in line with previous reports showing phenotypic and functional defects of DCs in cancer patient SLNs, which harbor a highly immunosuppressive microenvironment.6,7 In conclusion, LCs in melanoma patients migrate from the skin to SLNs with an immature phenotype, which is crucial to tolerate melanoma associated antigens.8 Although the presence of immature LCs might indicate a steady-state condition,9 the obtained data strongly suggest a melanoma-related effect.
Authorship
Acknowledgments: This work was supported by Ente Cassa di Risparmio di Firenze, Florence, Italy (grant number 2010.1392).
Contribution: G.G. and P.D.G. designed and performed the research, analyzed data, and co-wrote the manuscript; G.M. and R.C. performed the research; A.C. co-wrote the manuscript; C.U. supplied the clinical research material; and N.P. and L.B. treated patients and supplied the clinical research material.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gianni Gerlini, MD, Plastic Surgery Unit, Regional Melanoma Referral Centre, ITT, Santa Maria Annunziata Hospital, Via Antella, 58. I-50012, Bagno a Ripoli, Florence, Italy; e-mail: gianni.gerlini@asf.toscana.it.
References
Author notes
G.G. and P.D.G. contributed equally to this work