Abstract
Hematologic malignancies are a heterogeneous group of conditions with an unclear etiology. We hypothesized that diabetes mellitus type 2 is associated with increased risk of developing lymphoma, leukemia, and myeloma. A literature search identified 26 studies (13 case-control and 13 cohort studies) evaluating such an association. Outcome was calculated as the odds ratio (OR) using a random effects model. Heterogeneity and publication bias were evaluated using the I2 index and the trim-and-fill analysis, respectively. Quality was assessed using the Newcastle-Ottawa scale. The OR for non-Hodgkin lymphoma was increased at 1.22 (95% confidence interval [CI], 1.07-1.39; P < .01) but the OR for Hodgkin lymphoma was not. There was an increased OR for peripheral T-cell lymphoma (OR = 2.42, 95% CI, 1.24-4.72; P = .009) but not for other non-Hodgkin lymphoma subtypes. The OR for leukemia was 1.22 (95% CI, 1.03-1.44; P = .02) and the OR for myeloma was 1.22 (95% CI, 0.98-1.53; P = .08). Although diabetes mellitus type 2 seems to increase the risk of developing lymphoma, leukemia, and myeloma, future studies should focus on evaluating other potential confounders such as obesity, dietary habits, physical activity, and/or antidiabetic therapy.
Introduction
Hematologic malignancies are a heterogeneous group of diseases characterized by the malignant uncontrolled growth of hematopoietic cells. According to Surveillance Epidemiology and End Results data, approximately 75 000, 45 000, and 20 500 persons were diagnosed with lymphoma, leukemia, and myeloma, respectively, in 2011 in the United States alone.1 The development of hematologic malignancies has been associated with different causes, such as infectious processes (eg, HTLV-1 and adult T-cell leukemia/lymphoma), autoimmune disorders (eg, rheumatoid arthritis, Sjogren syndrome, and systemic lupus erythematosus) or a positive family history. However, despite recent advances in the understanding of their pathophysiology, the etiology of these conditions remains largely unexplained.
Diabetes affects approximately 25.8 million people in the United States.2 It is estimated that diabetes mellitus type 2 (DM2) accounts for 90%-95% of all diabetes cases. DM2 has been studied as a potential risk factor for the development of hematologic malignancies; however, studies evaluating such an epidemiologic association have rendered conflicting results. In a previous meta-analysis evaluating the association between diabetes and incidence of lymphoma,3 we found a stronger association for DM2 and non-Hodgkin lymphoma (NHL). However, NHL subtype analyses were not performed given the paucity of the data available at the time.
The primary objective of the present study was to evaluate the potential association between DM2 and the incidence of lymphoma, leukemia, and myeloma. Secondary objectives were to evaluate the association between DM2 and specific subtypes of lymphoma and leukemia.
Methods
Literature search
Two authors performed a literature search independently using PubMed/MEDLINE and the Cochrane Database of Systematic Reviews through December 31, 2011. The key terms used in the search were: “diabetes AND (leukemia or lymphoma or myeloma).” The titles and abstracts were reviewed and full-text articles were selected based on our inclusion criteria. The reference list of each selected study was reviewed to search for additional studies.
Inclusion and exclusion criteria
An article was considered relevant if it contained original data from epidemiologic observational studies (either prospective cohort or case-control) reporting on the association between DM2 and the incidence of lymphoma, leukemia, and myeloma in adults regardless of the language in which it was published, with a minimum follow-up of 3 years, and reporting or providing sufficient information to allow the calculation of odds ratio (OR). Cross-sectional studies were excluded. Any discrepancies on inclusion or exclusion of a study were resolved through consensus in all cases. If there were multiple publications from the same study, only the most recent was selected, using the older publications only to clarify methodology or characteristics of the population.
Data extraction
The data extraction was performed independently by 2 authors and included author, year of publication, country of origin, sample size, inclusion and exclusion criteria, and methods of ascertainment of DM2, lymphoma, leukemia, and myeloma. For cohort studies, we extracted the source of the cohort, years of follow-up, the source of the expected incidence, the outcome measured, and the variables used for adjustment. Any discrepancies were addressed by a joint reevaluation of the original article with another author. For missing information, attempts were made to contact the authors of the original studies. The characteristics and quality of the studies included in this meta-analysis and their outcomes will be presented in accordance with the checklist proposed by the Meta-analysis Of Observational Studies group.4
Quality assessment
The quality of the selected studies was assessed independently by 2 authors using the Newcastle-Ottawa Scale (NOS).5 The NOS uses 2 different tools for case-control and cohort studies and consists of 3 parameters of quality: selection, comparability, and exposure/outcome assessment. The NOS assigns a maximum of 4 points for selection, 2 points for comparability, and 3 points for exposure or outcome. We assigned NOS scores of 1-3, 4-6, and 7-9 for low, intermediate, and high-quality studies, respectively. Any discrepancies were addressed by a joint reevaluation of the original article.
Data synthesis and analysis
Because the risk of lymphoma, leukemia, or myeloma in the general population is low, the relative risk obtained from prospective cohort studies numerically approximates the OR,6 permitting the combination of case-control and cohort studies. Therefore, the primary outcome measured was OR with 95% confidence interval (95% CI) of developing lymphoma, leukemia, or myeloma in patients with a diagnosis of DM2. To measure the outcome, the DerSimonian-Laird or random-effects model (REM) was used.7 The REM accounts for heterogeneity between and within studies. We assessed for heterogeneity using the I2 index8 ; I2 values of 25%, 50%, and 75% were considered a reflection of mild, moderate, and severe heterogeneity, respectively. Publication bias was addressed by the trim-and-fill method,9 which estimated and adjusted for the potential effect that nonpublished (imputed) studies might have had on the measured outcome. Meta-analyses were performed for lymphoma, leukemia, and myeloma separately. Lymphoma was separated into NHL and Hodgkin lymphoma (HL). NHL subtypes were diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), and peripheral T-cell lymphoma (PTCL). Leukemia subtypes were lymphoid and myeloid. Subset analyses were performed by study design, sex, and geographic region. All calculations and graphs were obtained with Comprehensive Meta-Analysis Version 2.2.050 software (Biostat). In the forest plots, OR values > 1 represent a direct association and < 1 an inverse association. The size of the squares is correlated with the weight of the respective study.
Results
Search results
From 2029 initial returns, 1992 articles were rejected because they were reviews, case reports, or did not pertain to our study. By reviewing the reference lists of the remaining 37 articles, 7 studies were added. From these 44 studies, 18 were rejected because they did not focus on incidence of lymphoma, leukemia, or myeloma; did not focus on DM2; or were cross-sectional. Finally, 26 studies (13 prospective cohort10-22 and 13 case-control23-35 ) were included in our analysis.
Characteristics of the studies
The main characteristics of the studies included in this analysis are provided in the supplemental Tables (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Cohort studies were published between 1982 and 2010. Eight studies originated from Europe, 3 from America, and 2 from Asia, accounting for approximately 8000 cases identified in a cohort of more than 7 million people. According to the NOS, 11 studies (85%) were of high quality and 2 (15%) of acceptable quality. The most common selection bias was that there was not a nonexposed cohort but rather an expected number of cases in 5 studies (38%). The most common outcome bias was the lack of reporting of completeness of follow-up in 9 studies (69%). Case-control studies were published between 1987 and 2010. Six studies originated from Europe, 5 from America, and 2 from Asia, including a total of 9282 cases and 155 109 controls. According to the NOS, 7 studies (54%) were of high quality and 6 (46%) of acceptable quality. Twelve studies (92%) had population-based controls. The most common selection bias was that cases were not confirmed independently in 10 studies (77%), and the most common exposure bias was that DM2 was self-reported in 9 studies (69%).
Outcome results
Complete subset analyses are shown in Table 1. Publication bias analyses did not affect our results.
Lymphoma.
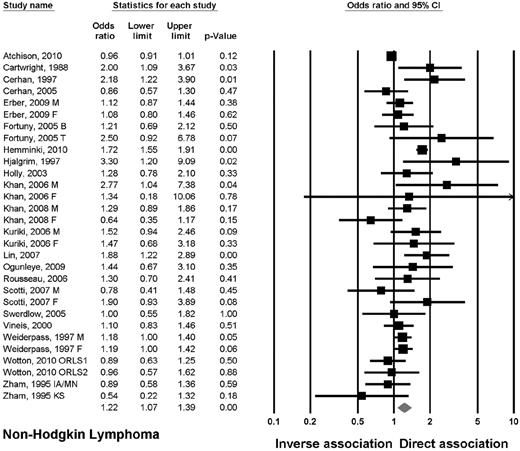
Twenty-one studies reported on the association between DM2 and incidence of lymphoma.10-14,16-21,25-35 The OR of NHL was elevated at 1.22 (95% CI, 1.07-1.39; P < .01; Figure 1), but there was no association with HL (OR = 1.02, 95% CI, 0.86-1.19; P = .86). The association with NHL remained significant when evaluating retrospective and prospective studies separately. There was a statistical trend when evaluating the odds of NHL in men as well as women. According to the region of report, there was a significant association seen in Asian and European but not in American studies.
Lymphoma subtypes.
Four studies provided data on DLBCL and FL.12,16,26,28 The OR for DLCBL was 1.16 (95% CI, 0.92-1.48; P = .22; I2 = 28%). There was an association in European studies (OR = 1.48, 95% CI, 1.10-2.01; P = .01; I2 = 0%), but not in American studies. The OR for FL was 0.91 (95% CI, 0.65-1.29; P = .60; I2 = 0%). No association was found when evaluating American or European studies separately. Three studies provided data on PTCL.16,27,30 The OR for PTCL was 2.42 (95% CI, 1.24-4.72; P = .009; I2 = 0%; supplemental Figure). There was an association seen in Asian (OR = 3.21, 95% CI, 1.14-8.44; P = .03) but not in European studies.
Leukemia.
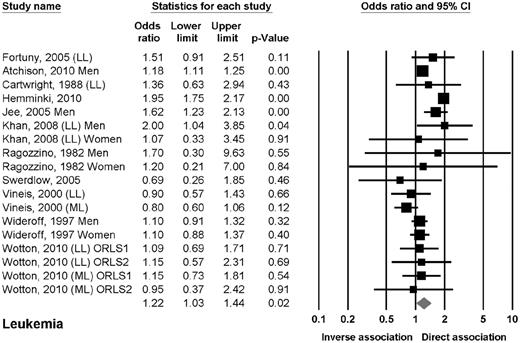
Eleven studies reported data on the association between DM2 and leukemia.10,13,15,16,19,20,22,24,27,33,34 Six studies reported data on leukemia in general,10,13,15,19,20,22 3 studies on chronic lymphocytic leukemia (CLL),16,24,27 and 2 studies on either myeloid or lymphoid leukemia.33,34 The OR of leukemia in patients with DM2 was 1.22 (95% CI, 1.03-1.44; P = .02; Figure 2). The odds of leukemia were elevated in prospective cohort studies but not in case-control studies. The odds of leukemia were also elevated in men but not in women. The odds of leukemia were elevated in Asian and American but not in European studies. No clear association was found when evaluating lymphoid or myeloid leukemia separately.
Estimates of the odds ratio of developing leukemia in patients with DM2.
Myeloma.
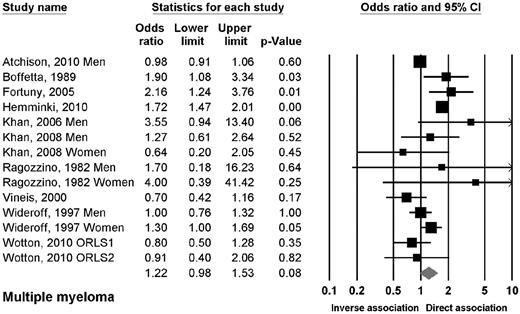
Ten studies reported data on the association between DM2 and myeloma.10,13,16,17,19,22,23,27,33,100 The OR of myeloma in patients with DM2 was 1.22 (95% CI, 0.98-1.53; P = .08; Figure 3). There were no significant associations when evaluating the studies by design, sex, or geographic region, with exception of a trend in Asian studies.
Risk estimates of the relative risk of developing myeloma in patients with DM2.
Discussion
The results of the present study revealed that: (1) patients with DM2 have mild-to-moderate increased odds of developing NHL but not H; (2) when evaluating NHL subtypes, the odds of PTCL were increased in patients with DM2, but not in those with DLBCL or FL; (3) although the odds of leukemia in general were increased, we did not identify an association with myeloid or lymphoid leukemia; (4) there was a trend toward increased odds of myeloma; and (5) the increased odds of hematologic malignancies in patients with DM2 seemed to be distinct depending on the geographic area of report.
The association between DM2 and lymphoma in general has not been elucidated completely. Based on our present results, the increased odds of lymphoma in general in patients with DM2 are largely dependent on increased odds of NHL. In a previous study, we identified a significant association between diabetes in general and NHL3 ; however, there was not a statistically significant association between DM2 and NHL (relative risk = 1.3; 95% CI, 0.9-1.9). The present study includes larger and more recent prospective datasets. In fact, the number of prospective studies has increased from 5 to 13 in the last 3 years, allowing the identification of an association likely derived from a larger number of subjects studied. The present results help to establish an epidemiologic relationship, which will need further prospective evaluation.
To our knowledge, this is the first meta-analysis evaluating the relationship between DM2 and incidence of leukemia. Interestingly, despite identifying statistically significant odds of leukemia in general in patients with DM2, our study failed to identify a subtype of leukemia driving this association. The most likely explanation for this is the lack of power, because fewer studies evaluating leukemia were included in our analysis. There are, however, other potential explanations for our findings. Many studies reported the odds of lymphoid leukemia without specifying the subtype. For example, 3 studies reported outcomes for CLL and 2 studies for lymphoid leukemia in general. Therefore, our results likely reflect the odds of CLL, the most common lymphoid leukemia in adults. However, some cases of acute lymphoblastic leukemia and other subtypes of lymphocytic leukemia could have been included. A similar situation could have occurred for myeloid leukemia. One could assume the most common myeloid leukemia in adults would be acute myelogenous leukemia; however, some cases of chronic myelogenous leukemia may have also been included. The association between DM2 and leukemia needs further study.
The association between DM2 and myeloma has not been evaluated previously using meta-analytical methodology. Our present results show a statistical trend toward significantly increased odds of myeloma in patients with DM2. In general, DM2 seems to be associated with increased odds of lymphoproliferative disorders, so it would not be surprising to establish with a larger number of patients an association between DM2 and myeloma as well. However, based on the current evidence, this remains speculative.
Our study also shows that the odds of lymphoma, leukemia, and myeloma seem to differ depending on the geographic region of the report. The odds of NHL were higher in Asia and Europe, whereas the odds of leukemia were higher in the United States and Asia. Although this is a novel finding, it is likely other confounders such as genetic predisposition, lifestyle factors, and viral exposure could have affected our results, making our associations weaker. In addition, these results were derived from small sample analyses and should be taken with caution.
There are several potential biologic explanations for a relationship between DM2 and malignancies. The American Diabetes Association and the American Cancer Society have published a joint consensus report on diabetes and cancer.36 Among the multiple points addressed in the report, possible biologic links were discussed. Hyperinsulinemia, hyperglycemia, inflammatory cytokines oversecretion, insulin-like growth factor (IGF) overproduction, and up-regulation of IGF-1 receptor are phenomena seen in patients with DM2 that would favor not only malignant transformation of cells but also progression of tumors. However, the epidemiologic association between DM2 and cancer seen in multiple studies does not formally establish DM2 as a cause of cancer. To further complicate this issue, DM2 and cancer share common risk factors, such as age, sex, overweight and obesity, waist-to-hip ratio, physical activity, dietary habits, smoking, and alcohol intake, making it difficult to discern the oncogenic effect of each specific risk factor. As an example, a recent meta-analysis has shown an increased risk of several cancers associated with obesity.37 Future prospective studies should focus on evaluating the impact that these risk factors could have on the incidence of hematologic malignancies and specific subtypes by carefully designed multivariate analyses. Similarly, other factors such as DM2 duration and severity and the use of insulin or other antidiabetic therapies should also be evaluated.
DM2 is a condition associated with immunosuppression, chronic inflammation, and B- and T-cell dysfunction,38,39 all of which have been associated with the development of lymphoproliferative disorders.40,41 For the association found herein between DM2 and PTCL, there is mounting evidence of an intrinsic T-cell dysfunction in patients with DM2 demonstrated by weaker T-cell–mediated responses to antigen exposure42 and a skewed balance favoring the activation of pro-inflammatory T-cell subsets.43,44 The fact that certain autoimmune conditions such as psoriasis or celiac disease increase the risk of PTCL further support these hypotheses.42,43
Our meta-analysis carries several weaknesses based on the quality of the included studies. First, there was a high degree of heterogeneity between studies secondary to the diversity of patients, histologic subtypes, and study designs. The effect of heterogeneity was addressed using the REM. Furthermore, when evaluating specific subtypes of lymphoma and leukemia, the heterogeneity became less evident. Second, the diagnosis of DM2 was self-reported in a substantial number of the studies, which could have introduced ascertainment bias. However, diabetes self-reporting has shown to be reliable in large epidemiologic prospective studies such as the Women's Health Initiative.45 Third, there are several potential confounders for which effects were partially addressed by the present study; 22 studies (85%) either adjusted or matched for age, 13 (50%) for sex, 12 (46%) for geographic region or race, and 4 (15%) for body mass index. Therefore, the effects of age, sex, and geographic region were likely accounted for in our study, but not other important factors such as obesity, diet, physical activity, or antidiabetic therapy. It is likely that there is interaction among these factors, cancer incidence, and DM2, which could weaken our results; however, this should be further investigated.
Our study also has several strengths. First, the number of cases included was large, rendering our study powerful enough to evaluate the epidemiologic association between DM2 and lymphoma, leukemia, and myeloma. Second, the included studies originated from different countries and included a variety of ethnic backgrounds, allowing for the generalization of our results. Third, based on the NOS, all of the studies included in this meta-analysis were of acceptable or high quality. In case-control studies, in which selection bias can be easily introduced, the large majority used age- and sex-matched, population-based controls originating from the same geographic regions as the cases, minimizing potential differences in medical care or ascertainment. Publication bias did not affect our results.
Conclusions
The present meta-analysis shows that patients with a diagnosis of DM2 have increased odds of developing NHL, leukemia, and myeloma. Regarding lymphoma subtypes, DM2 was associated with increased odds of PTCL in a small subset analysis. Additional studies are needed to elucidate the potential relationship between DM2 and hematologic malignancies.
The online version of this article contains a data supplement.
Preliminary findings from this study were presented at the 52nd Annual Meeting of the American Society of Hematology, Orlando, Florida, December 6, 2010 and at Lymphoma & Myeloma 2010: An International Congress on Hematologic Malignancies, New York, NY, October 21, 2010.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
J.M. was supported in part by the National Center for Research Resources (grant UL1RR025752), the National Center for Advancing Translational Sciences, National Institutes of Health, and The Marilyn Fishman Grant for Diabetes Research from the Endocrine Fellows Foundation.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the institutions listed above.
National Institutes of Health
Authorship
Contribution: J.J.C. designed the study; J.J.C., N.M., and J.L.R. performed the literature search and data gathering; J.J.C., N.M., J.L.R., and S.N. performed the quality assessment; J.J.C. and J.M. performed the statistical analysis and wrote the manuscript; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jorge J. Castillo, MD, 164 Summit Ave, Providence, RI 02906; e-mail: jcastillo@lifespan.org.