Abstract
Human cytomegalovirus (HCMV) encodes four 7-transmembrane-spanning (7TM) proteins, US28, US27, UL33, and UL78, which present important sequence homology with human chemokine receptors. Whereas US28 binds a large range of chemokines and disturbs host cell signaling at different levels, the others are orphans with largely unknown functions. Assembly of 2 different 7TM proteins into hetero-oligomeric complexes may profoundly change their respective functional properties. We show that HCMV-encoded UL33 and UL78 form heteromers with CCR5 and CXCR4 chemokine receptors in transfected human embryonic kidney 293T cells and monocytic THP-1 cells. Expression of UL33 and UL78 had pleiotropic, predominantly negative, effects on CCR5 and CXCR4 cell surface expression, ligand-induced internalization, signal transduction, and migration without modifying the chemokine binding properties of CCR5 and CXCR4. Importantly, the coreceptor activity of CCR5 and CXCR4 for HIV was largely impaired in the presence of UL33 and UL78 without affecting expression of the primary HIV entry receptor CD4 and its interaction with CCR5 and CXCR4. Collectively, we identified the first molecular function for the HCMV-encoded orphan UL33 and UL78 7TM proteins, namely the regulation of cellular chemokine receptors through receptor heteromerization.
Introduction
Human cytomegalovirus (HCMV), which belongs to the Herpesviridae family and more specifically to the β-herpes virus subfamily, can establish a life-long infection within its human host. HCMV is widely present among the general population, with up to 90% of the patients harboring a latent infection. HCMV infection is asymptomatic in immune-competent patients but can be life threatening for immune-compromised patients, such as HIV-infected persons, organ transplant recipients, or newborn infants.1 HCMV infection has been correlated to several pathologic conditions, such as atherosclerosis,2 acquired immunodeficiency syndrome progression,3 and cancer.4
HCMV encodes 4 proteins, US27, US28, UL33, and UL78, which exhibit important sequence homology with chemokine receptors of 7-transmembrane-spanning (7TM), G protein–coupled receptor (GPCR) family. These virally encoded 7TM (v7TM) proteins have been proposed to mimic cellular chemokine receptors thus hijacking the cellular signaling machinery to promote HCMV replication and dissemination.1 Among the 4 v7TM, US28 is the best-characterized and plays a crucial role in the viral life cycle by promoting viral spread5 and by activating the immediate early HCMV promoter, which is necessary for the transactivation of other viral genes.6 US28, which is constitutively active, can also bind to a wide range of chemokines, possibly acting as a “chemokine sink” to reduce the immune responses at the site of the infection.7 Alternatively, the constitutive or chemokine-induced signaling activities of US28 may modulate intracellular signaling pathways consequently promoting virus replication. Moreover, the interaction of US28 with human chemokines seems to participate in efficient HCMV dissemination throughout the body via migration of US28-expressing cells along multiple chemokine gradients (for review see Vischer et al8 ). In addition, US28 was reported to act as a HIV coreceptor in certain cell types.9 The 3 other HCMV-encoded v7TM proteins are orphan receptors without known ligands. Some constitutive signaling activity has been reported for UL33.10,11 Apart from a putative role for UL78 in viral replication,12 the functions of UL78 and US27 remain largely unknown.
In recent years, it has been shown that 7TM proteins can form dimers or higher-order oligomers13,14 including heteromeric complexes with other receptors of the same family. GPCR heteromerization impacts receptor function and seems to be implicated in certain pathologies, as exemplified by the role of dopamine D1/D2 heteromers in depression.15 Homo- and heteromerization was reported for human chemokine receptors, such as CCR5 or CXCR4.16-19 Evidence for CCR5 heteromerization with CXCR4 or CCR2 was provided by pharmacologic studies showing functional cross-inhibition, because of negative binding cooperativity between the various ligand binding sites.18 In addition to their role in leukocyte chemotaxis and in inflammation, CCR5 and CXCR4 are coreceptors for HIV entry.20,21
In an attempt to decipher the function of UL33 and UL78, we explored their possible heteromerization with human CCR5 and CXCR4 and the functional consequences thereof in the THP-1 monocytic cell line. We show here that UL33 and UL78 heteromerize with CCR5 and CXCR4 and negatively modulate their functional properties including their HIV coreceptor activity.
Methods
Cell culture and transfection
THP-1 cells were a kind gift from Dr Stefano Marullo (Institut Cochin, Paris, France) and were cultured in RPMI 1640 medium supplemented with 10% FBS, 100 U/mL penicillin, and 0.1 mg/mL streptomycin (Invitrogen). Human embryonic kidney (HEK) 293T cells were grown in complete medium (Dulbecco modified Eagle medium supplemented with 10% FBS, 4.5 g/L glucose, 100 U/mL penicillin, 0.1 mg/mL streptomycin, and 1mM glutamine; Invitrogen). Transient transfections were performed using JetPEI (Polyplus Transfection) or Amaxa Nucleofector technology (Amaxa Biosystems), according to the manufacturer's instructions.
Immunoprecipitation
For coimmunoprecipitation assays, HEK 293T or THP-1 cells were transfected with 4 μg or 2 μg of the indicated plasmids, respectively. Forty-eight hours after transfection, cells were washed 2 times in PBS, lysed, and then solubilized before immunoprecipitation assay as previously described.22 Immunoprecipitated proteins were eluted with Laemmli buffer 2X and immunoblotted using the indicated primary antibodies (mouse anti-GFP [Roche], rabbit anti-Flag [Sigma-Aldrich], rabbit anti-CXCR4 [Abcam], and mouse anti-CD4 [R&D Systems]). Immunoreactivity was revealed using secondary antibodies coupled to 680 or 800 nm fluorophores using the Odyssey LI-COR infrared fluorescent scanner (ScienceTec).
Fluorescent ligand-binding assay
Chemokine receptor binding assay was performed using a Tag-lite assay kit (Cisbio International) according to the manufacturer's instructions. Briefly, HEK 293T cells were transiently transfected with the Halo-tag fused chemokine receptors, Halo-Myc-CXCR4 or Halo-Myc-CCR5 (5-20 ng), alone or with Flag-UL33-YFP or Flag-UL78-YFP (150 ng) in 96-well plates. Forty-eight hours after transfection, cells were labeled with the Halo-tag substrate, Halo-tag-Lumi4-Tb, for 1 hour at 4°C, washed 4 times, and then incubated with the corresponding chemokine receptor red-dyed ligand (CXCL12-red for CXCR4 and CCL3-red for CCR5) in increasing concentrations. The nonspecific binding was determined using 1μM of unlabeled CXCL12 for CXCR4 and 10μM of unlabeled maraviroc for CCR5. Receptor expression was assessed using the anti–myc-Lumi4-Tb or anti–flag-Lumi4-Tb antibody. After an overnight incubation at 4°C, plates were read on a PHERAstar microplate reader (BMG Labtech). Specific binding was determined by subtracting the nonspecific HTRF ratio from the total HTRF ratio. Kd values of the fluorescent ligands were obtained from saturation curves of the specific binding.23
BRET measurement
For bioluminescence resonance energy transfer (BRET) donor saturation curves, HEK 293T cells seeded in 12-well plates were transiently transfected with 200 ng of HA-UL33-Rluc or 100 ng of HA-UL78-Rluc and 100 to 1800 ng of yellow fluorescent protein (YFP) plasmids. Twenty-four hours after transfection, cells were transferred into a 96-well white Optiplate (Perkin Elmer Life Sciences), precoated with 10 mg/mL poly-L-lysine (Sigma-Aldrich), and incubated for another 24 hours before BRET measurements as previously described.24
Flow cytometric analysis
A Cytomycs FC500 FACS analyzer (Beckman Coulter) was used to analyze CCR5, CXCR4, CD4, UL33, and UL78 surface expression in THP-1 cells. The proportion of surface CCR5 or CXCR4 receptors was determined by analyzing in parallel, intact and permeabilized cells. Cells (106) were fixed in 1% paraformaldehyde for 10 minutes, washed with PBS, and permeabilized or not for 1 hour at 4°C in PBS containing 2% FBS, and 0.1% saponin. Cells were then incubated on ice for 1 hour in 100 μL PBS, 2% FBS with the 1/85a Alexa Fluor 647–conjugated anti–human CCR5 antibody (1:50; Biolegend) or the phycoerythrin (PE)–conjugated anti–human CXCR4 antibody (1:50; BD Biosciences), and control isotypes at the same dilutions. After washing with PBS, cells were fixed in 2% paraformaldehyde and analyzed by FACS. Similarly, surface expression of exogenous UL33 or UL78 in THP-1 cells was assessed using the primary anti-Flag antibody (Sigma-Aldrich), followed by the Cy5-conjugated anti–rabbit IgG (1:100) and CD4 surface expression was evaluated using PE-conjugated mouse anti–human CD4 (Beckman Coulter). Fluorescence profiles (10 000 events) were recorded, and then analyzed using the Cytomics RXP Version 2000 software.
Chemotaxis assay
Cell migration was assessed using THP-1 cells transfected with mock, Flag-UL33-YFP, Flag-UL78-YFP, or cotransfected with the same plasmids and HA-CCR5. Forty-eight hours after transfection, approximately 106 cells were resuspended in 0.1 mL of RPMI 1640 medium supplemented with 20mM HEPES (N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid) and 0.5% BSA were loaded in the upper wells of a transwell chamber (5 μm pore size; Costar). CCL5 and CXCL12 were added to the lower wells in a volume of 0.6 mL. After 4 hours at 37°C, cells in the lower chamber were collected and counted. Values are expressed as the percentage of input cells that migrated through the filter.
Intracellular calcium mobilization
THP-1 cells were cotransfected with HA-CCR5 and mock, Flag-UL33, or Flag-UL78 or with mock, Flag-UL33 or Flag-UL78. Forty-eight hours after transfection, cells were washed with calcium buffer (10mM HEPES [p.H 7.4], 137.5mM NaCl, 1.25mM CaCl2, 1.25mM MgCl2, 0.4mM NaH2PO4, 6mM KCl, and 10mM glucose). Then 2 × 106 cells were incubated for 1 hour at 37°C in the dark in 1 mL of calcium buffer containing 0.1% pluronic acid and 5μM Fluo-4 AM (Molecular Probes). Cells were washed and resuspended in calcium buffer and 100nM CCL5 or 10nM CXCL12 were injected to 2 × 105 cells/well and fluorescence was monitored using the lumino/fluorometer Mithras (Berthold).
IP1 production studies
Inositol-1-phosphate (IP1) levels in UL33 or UL78 expressing THP-1 cells after CCL5 and CXCL12 stimulation were determined by homogenous time-resolved fluorescence (HTRF) using the Cisbio IP-One Tb kit according to the manufacturer's instructions (Cisbio Bioassay).
Virus stock preparation
A stock of HIV-1 JR-CSF (clade B, R5 tropic) and of HIV-1 LAI (clade B, X4 tropic) molecular clones were produced by transfection of HEK 293T cells with proviral DNA (NIH) as previously described.25 The cell culture supernatant was concentrated, separated into single use aliquots and stored at −80°C. Virus concentration was quantified by the p24 Innotest HIV-1 enzyme-linked immunosorbent assay (ELISA; Innotest HIV-1 Antigen monoclonal antibody; Innogentetics).
Single-cycle infection assay
THP-1 cells transfected with the indicated plasmids were inoculated with either R5-tropic strain JR-CSF or X4-tropic strain LAI (30 ng p24/mL). After 16 hours, fresh medium was added (100 μL) and the infection was continued for 24 hours. The percentage of infected v7TM-expressing cells was monitored by p24 intracellular staining of YFP-positive cells as previously described.26
Statistical analysis
Results were analyzed by Prism Version 5 software (GraphPad). Data are expressed as mean ± SEM. Student t test was applied for statistical analysis.
Results
UL33 and UL78 physically interact with CCR5 and CXCR4
First, we carried out coimmunoprecipitation (co-IP) experiments to assess the possible interaction between UL33 and UL78 with CCR5 and CXCR4. These experiments revealed that UL33 forms homomers and interacts with CCR5 and CXCR4 but not with the vasopressin V2 receptor in HEK 293T cells (Figure 1A). UL33 C-terminus was fused to Renilla luciferase (Rluc) and the luciferase activity of the corresponding fusion protein was measured to assess fusion protein expression. CCR5, CXCR4, and UL33 were fused to the YFP at their respective C-termini and protein levels were monitored by Western blotting. Similar to UL33, UL78 formed homomers and heteromers with CCR5 and CXCR4 (Figure 1B). No interaction was detected when lysates of cells expressing receptors separately were mixed for co-IP experiments (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). To further validate these results in intact cells, BRET assays were performed. UL33-Rluc and UL78-Rluc fusion proteins acted as energy donors, whereas CCR5-YFP and CXCR4-YFP fusion proteins were used as energy acceptors. Donor saturation curves were performed by cotransfecting a fixed amount of UL33-Rluc (Figure 1C) or UL78-Rluc expression plasmids (Figure 1D) in the presence of increasing amounts of either CCR5-YFP or CXCR4-YFP expression plasmids. The expected hyperbolic donor saturation curve reaching an asymptote with increasing YFP/Rluc ratios was observed for all receptor combinations, reflecting a specific interaction between BRET donor and acceptor pairs. The V2-YFP fusion protein was used as a negative control. For this construct, a quasi-linear increase in BRET was observed with increasing YFP/Rluc ratios reflecting a nonspecific interaction, because of random collision. The specificity of BRET signals between the 2 v7TM proteins and the 2 chemokine receptors was further confirmed in BRET competition experiments (supplemental Figure 2). Coexpression of v7TM proteins decreased also the BRET between CCR5-Rluc and CXCR4-YFP indicating that v7TM proteins may also interfere with CCR5/CXCR4 heteromer formation. Heteromers probably form already during biosynthesis in the endoplasmatic reticulum (ER), as similar BRET signals were obtained in cells pretreated with BFA, a drug that induces Golgi apparatus disassembly and redistribution of Golgi-derived vesicles to the ER (supplemental Figure 3). This was further confirmed with a natural mutant of CCR5, lacking its C-terminal intracellular tail, that is completely retained in the ER27 for which unmodified BRET signals were measured with the 2 v7TM proteins (supplemental Figure 3). Overall, co-IP and BRET results show that UL33 and UL78 can form homomers as well as heteromers with CCR5 and CXCR4 as early as in the ER in HEK 293T cells.
Evidence for UL33 and UL78 heteromerization with CCR5 and CXCR4. (A-B) Detection of v7TM/chemokine receptor heteromers using co-IP. Human embryonic kidney (HEK) 293T cells were transfected with HA-UL33-Rluc (A) or HA-UL78-Rluc (B) in presence or absence of CCR5-YFP, CXCR4-YFP, V2-YFP, or Flag-UL33-YFP (A) or Flag-UL78-YFP (B). Lysates were immunoprecipitated with rat anti-HA, resolved by SDS-PAGE and immunoblotted with mouse anti-GFP antibodies (top blot). Expression levels of YFP fusion proteins were assessed by immunoblotting with mouse anti-GFP antibodies (middle blot) and expression of Rluc fusion proteins by monitoring Renilla luciferase activity (bottom graph). (C-D) BRET donor saturation curves were performed by cotransfecting a fixed amount of HA-UL33-Rluc (C) or HA-UL78-Rluc (D) in presence of increasing amounts of CCR5-YFP, CXCR4-YFP, V2-YFP, or Flag-UL33-YFP (C) or Flag-UL78-YFP (D) in HEK 293T cells. The saturation curves are obtained from 3 independent experiments. Vertical lines have been inserted to indicate repositioned gel lanes.
Evidence for UL33 and UL78 heteromerization with CCR5 and CXCR4. (A-B) Detection of v7TM/chemokine receptor heteromers using co-IP. Human embryonic kidney (HEK) 293T cells were transfected with HA-UL33-Rluc (A) or HA-UL78-Rluc (B) in presence or absence of CCR5-YFP, CXCR4-YFP, V2-YFP, or Flag-UL33-YFP (A) or Flag-UL78-YFP (B). Lysates were immunoprecipitated with rat anti-HA, resolved by SDS-PAGE and immunoblotted with mouse anti-GFP antibodies (top blot). Expression levels of YFP fusion proteins were assessed by immunoblotting with mouse anti-GFP antibodies (middle blot) and expression of Rluc fusion proteins by monitoring Renilla luciferase activity (bottom graph). (C-D) BRET donor saturation curves were performed by cotransfecting a fixed amount of HA-UL33-Rluc (C) or HA-UL78-Rluc (D) in presence of increasing amounts of CCR5-YFP, CXCR4-YFP, V2-YFP, or Flag-UL33-YFP (C) or Flag-UL78-YFP (D) in HEK 293T cells. The saturation curves are obtained from 3 independent experiments. Vertical lines have been inserted to indicate repositioned gel lanes.
UL33 and UL78 modulate CCR5 and CXCR4 surface expression and internalization through heteromerization in THP-1 cells
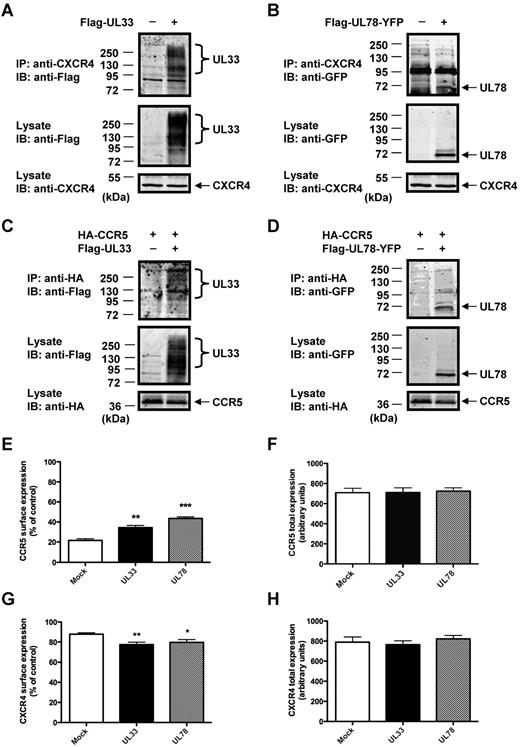
We set out to evaluate the functional consequences of receptor heteromerization in a more physiologic context using THP-1 cells. This cell line expresses endogenous CCR5 and CXCR4 receptors and represents an established model of monocytic cells, one of the major target cells of HCMV.1 We first validated the formation of heteromers between v7TM proteins and CCR5 and CXCR4 in this cellular system by co-IP assays. The interaction between endogenous CXCR4 and the 2 v7TM was shown in cells transfected with either Flag-UL33 (Figure 2A) or Flag-UL78-YFP (Figure 2B). The interaction of CCR5 with the 2 v7TM proteins was confirmed in cells cotransfected with Flag-UL33 (Figure 2C) or Flag-UL78-YFP (Figure 2D) and HA-CCR5 (endogenous CCR5 cannot be detected because of the absence of anti-CCR5 antibodies validated for IP and Western blot experiments). Immunofluorescence microscopy studies confirmed the spatial proximity between v7TM proteins and CCR5 and CXCR4 chemokine receptors in THP-1 cells (supplemental Figure 4).
UL33 and UL78 interact with and modulate the surface expressions of CCR5 and CXCR4 in THP-1 cells. (A-D) Detection of heteromers in THP-1 cells by co-IP. THP-1 cells were transfected with (A) Flag-UL33, (B) Flag-UL78-YFP, (C) HA-CCR5 with or without Flag-UL33, or (D) HA-CCR5 with or without Flag-UL78-YFP. Lysates were immunoprecipitated with mouse anti-CXCR4 or rat anti-HA, resolved by SDS-PAGE and immunoblotted with the indicated antibodies. (E-H) Flow cytometry analysis of cell-surface (E-G) and total (F-H) CCR5 (E-F) or CXCR4 (G-H) expression using 1/85a Alexa Fluor 647–conjugated anti–human CCR5 (E-F) or PE-conjugated anti–human CXCR4 (G-H) in mock, Flag-UL33-YFP or Flag-UL78-YFP expressing THP-1 cells. The proportion of surface versus total receptors was calculated by comparing signals from nonpermeabilized and permeabilized cells in 3 independent experiments. Mock = pcDNA3 expression vector. Statistical differences compared with mock nucleofected cells were assessed using the Student t test (*P < .05; **P < .01; ***P < .001).
UL33 and UL78 interact with and modulate the surface expressions of CCR5 and CXCR4 in THP-1 cells. (A-D) Detection of heteromers in THP-1 cells by co-IP. THP-1 cells were transfected with (A) Flag-UL33, (B) Flag-UL78-YFP, (C) HA-CCR5 with or without Flag-UL33, or (D) HA-CCR5 with or without Flag-UL78-YFP. Lysates were immunoprecipitated with mouse anti-CXCR4 or rat anti-HA, resolved by SDS-PAGE and immunoblotted with the indicated antibodies. (E-H) Flow cytometry analysis of cell-surface (E-G) and total (F-H) CCR5 (E-F) or CXCR4 (G-H) expression using 1/85a Alexa Fluor 647–conjugated anti–human CCR5 (E-F) or PE-conjugated anti–human CXCR4 (G-H) in mock, Flag-UL33-YFP or Flag-UL78-YFP expressing THP-1 cells. The proportion of surface versus total receptors was calculated by comparing signals from nonpermeabilized and permeabilized cells in 3 independent experiments. Mock = pcDNA3 expression vector. Statistical differences compared with mock nucleofected cells were assessed using the Student t test (*P < .05; **P < .01; ***P < .001).
Next, using flow cytometry analysis, we assessed THP-1 surface expression of endogenous CCR5 (Figure 2E, supplemental Figure 5A) and CXCR4 (Figure 2G, supplemental Figure 5B) in the absence and presence of UL33 and UL78. In agreement with previous reports, only 20% of total CCR5 was expressed at the cell surface in mock-transfected cells.28 In cells expressing UL33 or UL78 a significant increase in CCR5 surface expression of 1.5- to 2-fold was observed (Figure 2E) without any change in total CCR5 expression (Figure 2F). In contrast, the majority of CXCR4 receptors (90%) were expressed at the cell surface of mock-transfected THP-1 cells (Figure 2G). In cells expressing UL33 or UL78 a decreased CXCR4 surface expression of 20% was observed without any change in total CXCR4 expression (Figure 2H). These results show that UL33 and UL78 modulate the subcellular distribution of CXCR4 and in particular that of CCR5.
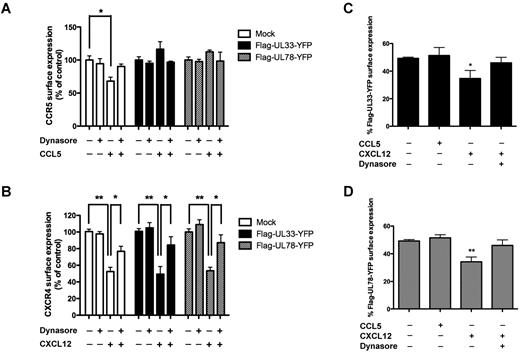
To better understand the effect of UL33 and UL78 on steady-state CCR5 and CXCR4 surface expression, we blocked dynamin-dependent internalization pathways by treating cells with dynasore, a dynamin inhibitor (Figure 3).29 CCR5 and CXCR4 surface expression was not altered under these conditions, neither in the absence nor in the presence of UL33 or UL78 (Figure 3A-B). Modified cell surface expression of CCR5 and CXCR4 in cells coexpressing either UL33 or UL78 might be the consequence of a heteromer formation with these 2 v7TM proteins. To provide further support for this hypothesis, we studied agonist-induced internalization of CCR5 and CXCR4, in the presence and absence of UL33 and UL78. Stimulation of mock-transfected THP-1 cells with the CCR5 agonist CCL5 resulted in the internalization of 30% of CCR5 within 15 minutes through a dynamin-dependent pathway (blocked by dynasore; Figure 3A). In contrast, CCL5-promoted internalization of CCR5 was completely inhibited in the presence of either UL33 or UL78 (Figure 3A), suggesting that CCR5 becomes refractory to ligand-induced internalization in CCR5/UL33 and CCR5/UL78 heteromers. Consistently, the fraction of UL33 and UL78 at the cell surface remained unchanged under these conditions (Figure 3C-D).
Effect of dynasore on CCL5- and CXCL12-induced CCR5 and CXCR4 internalization, respectively. (A-B) Flow cytometry analysis of cell-surface CCR5 (A) or CXCR4 (B) using 1/85a Alexa Fluor 647–conjugated anti–human CCR5 (A) or PE-conjugated anti–human CXCR4 (B) in THP-1 cells expressing mock, Flag-UL33-YFP or Flag-UL78-YFP and pretreated or not with dynasore (100μM) for 1 hour before 15 minutes CCL5 (100nM) or CXCL12 (10nM) stimulation. The proportion of surface CCR5 (A) and CXCR4 (B) was calculated by comparing the signal in nonpermeabilized and permeabilized cells in 3 independent experiments. Values were normalized to the levels seen in each group in the absence of ligand and dynasore. (C-D) Flow cytometry analysis of cell-surface UL33 and UL78 using rabbit anti-Flag as primary antibody and rabbit-Cy5 as secondary antibody in THP-1 cells expressing mock, Flag-UL33-YFP (C) or Flag-UL78-YFP (D) after CCL5 (100nM) or CXCL12 (10nM) stimulation for 15 minutes pretreated or not with dynasore (100μM) for 1 hour. The proportion of surface UL33 or UL78 was calculated by comparing the signal in nonpermeabilized and permeabilized cells in 3 independent experiments. Mock = pcDNA3 expression vector. Statistical differences compared with unstimulated cells were assessed using the Student t test (*P < .05; **P < .01).
Effect of dynasore on CCL5- and CXCL12-induced CCR5 and CXCR4 internalization, respectively. (A-B) Flow cytometry analysis of cell-surface CCR5 (A) or CXCR4 (B) using 1/85a Alexa Fluor 647–conjugated anti–human CCR5 (A) or PE-conjugated anti–human CXCR4 (B) in THP-1 cells expressing mock, Flag-UL33-YFP or Flag-UL78-YFP and pretreated or not with dynasore (100μM) for 1 hour before 15 minutes CCL5 (100nM) or CXCL12 (10nM) stimulation. The proportion of surface CCR5 (A) and CXCR4 (B) was calculated by comparing the signal in nonpermeabilized and permeabilized cells in 3 independent experiments. Values were normalized to the levels seen in each group in the absence of ligand and dynasore. (C-D) Flow cytometry analysis of cell-surface UL33 and UL78 using rabbit anti-Flag as primary antibody and rabbit-Cy5 as secondary antibody in THP-1 cells expressing mock, Flag-UL33-YFP (C) or Flag-UL78-YFP (D) after CCL5 (100nM) or CXCL12 (10nM) stimulation for 15 minutes pretreated or not with dynasore (100μM) for 1 hour. The proportion of surface UL33 or UL78 was calculated by comparing the signal in nonpermeabilized and permeabilized cells in 3 independent experiments. Mock = pcDNA3 expression vector. Statistical differences compared with unstimulated cells were assessed using the Student t test (*P < .05; **P < .01).
Treatment of mock-transfected THP-1 cells with the CXCR4 agonist CXCL12 resulted in the internalization of approximately 50% of CXCR4 receptors within 15 minutes (Figure 3B). CXCR4 internalization was not modified in cells expressing either UL33 or UL78. CXCL12-induced CXCR4 internalization was partially sensitive to dynasore pretreatment in the absence and presence of UL33 or UL78. Importantly, CXCL12 treatment also induced a 30%-40% dynasore-sensitive reduction of UL33 and UL78 surface expression consistent with the cointernalization of CXCR4 with either UL33 or UL78 within the respective heteromers (Figure 3D). Of note, neither UL33 nor UL78 show any binding affinity for CXCL12.1,30 Collectively, improved surface expression of CCR5 and the absence of ligand-induced CCR5 internalization in the presence of UL33 and UL78 as well as cointernalization of UL33 and UL78 with CXCL12-activated CXCR4 provide strong support for heteromer formation of CCR5 and CXCR4 with UL33 and UL78 at the surface of THP-1 cells.
Effect of UL33 and UL78 on CCR5 and CXCR4 ligand binding and signaling properties
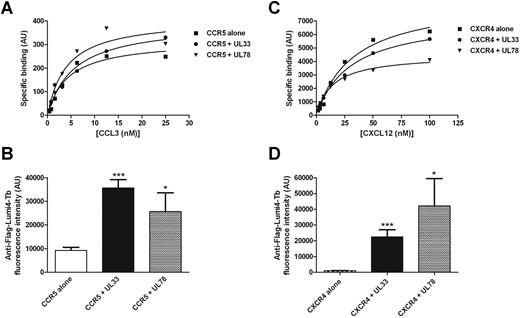
The influence of UL33 and UL78 on ligand binding properties of CCR5 and CXCR4 was determined with the recently developed CCL3 and CXCL12 binding assays for CCR5 and CXCR4, respectively.23 Similar dissociation constants (Kd) for fluorescently labeled CCL3 of 4.1 ± 0.1nM, 5.9 ± 0.2nM, and 7.8 ± 3.7nM were obtained in cells expressing CCR5 alone or together with UL33 or UL78, respectively (Figure 4A). Similarly, no modification of Kd values for fluorescently labeled CXCL12 were observed in cells expressing CXCR4 alone (Kd = 37.8 ± 3.9nM) or together with UL33 (43.1 ± 9.7nM) or UL78 (28.3 ± 10.1nM; Figure 4B). We next determined the impact of UL33 and UL78 on signaling pathways downstream of CCR5 or CXCR4. Because of the very low expression level of endogenous CCR5 receptors, THP-1 cells were complemented with HA-tagged CCR5 to obtain reliable signaling responses. This approach almost doubled the number of CCR5 receptors at the cell surface as revealed by flow cytometry (data not shown). Stimulation of THP-1 cells with 50nM CCL5 or CXCL12 increased the phosphorylation of ERK1/2 and AKT in a time-dependent manner with a peak at 5 to 10 minutes as expected (supplemental Figure 6). The expression of UL33 or UL78 did neither significantly alter the kinetics nor the amplitude of CCL5 and CXCL12 responses (supplemental Figure 6). These results indicate signaling through the ERK1/2 and AKT pathway are not modified by the heteromerization with UL33 and UL78. Furthermore, these data show that CCR5 remains fully responsive to CCL5, thus excluding the possibility that the impaired CCL5-promoted internalization of CCR5 in CCR5/UL33 and CCR5/UL78 heteromers is because of impaired agonist binding.
Effect of UL33 and UL78 expression on CCR5 and CXCR4 ligand binding properties. Saturation isotherms of fluorescence labeled CCL3 (A) and CXCL12 (C) binding in HEK293 cells expressing Halo-Myc-CXCR4 (C, closed squares) or Halo-Myc-CCR5 (A, closed squares) receptors alone or with Flag-UL33-YFP (closed circles) or Flag-UL78-YFP (closed triangles). Specific binding is shown; nonspecific binding was determined in the presence of 1μM CXCL12 for CXCR4 and 10μM of Maraviroc for CCR5. (B-D) Flag-UL33-YFP or Flag-UL78-YFP expression was assessed using Lumi4-Tb labeled anti-Flag antibodies. Data shown are representative of 3 experiments. Statistical differences compared with mock nucleofected cells were assessed using the Student t test (*P < .05; ***P < .001).
Effect of UL33 and UL78 expression on CCR5 and CXCR4 ligand binding properties. Saturation isotherms of fluorescence labeled CCL3 (A) and CXCL12 (C) binding in HEK293 cells expressing Halo-Myc-CXCR4 (C, closed squares) or Halo-Myc-CCR5 (A, closed squares) receptors alone or with Flag-UL33-YFP (closed circles) or Flag-UL78-YFP (closed triangles). Specific binding is shown; nonspecific binding was determined in the presence of 1μM CXCL12 for CXCR4 and 10μM of Maraviroc for CCR5. (B-D) Flag-UL33-YFP or Flag-UL78-YFP expression was assessed using Lumi4-Tb labeled anti-Flag antibodies. Data shown are representative of 3 experiments. Statistical differences compared with mock nucleofected cells were assessed using the Student t test (*P < .05; ***P < .001).
We next studied the effect of UL33 and UL78 expression on the Gαq/phospholipase C (PLC) pathway, which is known to be activated by CCR5 and CXCR4.31,32 Therefore, THP-1 cells were stimulated with CCL5 and intracellular inositol phosphate (IP) levels were determined. CCL5 dose-dependently increased IP levels with an EC50 value of 0.30 ± 0.04nM (Figure 5A). Maximal IP levels increased 3-fold in the presence of UL78 and were almost completely abolished in the presence of UL33. The differential effect of the 2 v7TMs on CCR5 signaling was further confirmed by measuring intracellular Ca2+ concentrations on CCL5 treatment. Indeed, a significant increase in peak Ca2+ concentration was observed in the presence of UL78, whereas a 2-fold reduction was observed in cells expressing UL33 compared with control cells (Figure 5B), probably because of reduced responsiveness of THP-1 cells expressing constitutively active UL33 (data not shown). Stimulation of THP-1 cells with CXCL12 dose-dependently increased IP levels with an EC50 value of 0.9 ± 0.6nM (Figure 5C). In cells expressing UL33 and UL78, maximal CXCL12-induced IP production was decreased by more than 50% as well as CXCR4 potency in the presence of UL33 (EC50 = 4.6 ± 0.6nM) and UL78 (EC50 = 28 ± 9nM). Similarly, maximal intracellular Ca2+ concentrations were decreased (Figure 5D).
UL33 and UL78 modulate calcium mobilization in response to CCL5 and decrease CXCL12-induced IP production in THP-1 cells. (A-C) IP production after stimulation with increasing concentrations of CCL5 (A) or CXCL12 (C) was assessed in THP-1 cells expressing mock (closed squares), Flag-UL33-YFP (closed circles), or Flag-UL78-YFP (closed triangles). (B-D) Average peak Ca2+ concentrations triggered by 100nM CCL5 in HA-CCR5 transfected THP-1 cells (B) or by 10nM CXCL12 in THP-1 cells (D) coexpressing mock, Flag-UL33 or Flag-UL78. Mock = pcDNA3 expression vector. Statistical differences of calcium concentrations or maximal IP1 production compared with mock nucleofected cells were assessed using the Student t test (*P < .05; **P < .01; ***P < .001).
UL33 and UL78 modulate calcium mobilization in response to CCL5 and decrease CXCL12-induced IP production in THP-1 cells. (A-C) IP production after stimulation with increasing concentrations of CCL5 (A) or CXCL12 (C) was assessed in THP-1 cells expressing mock (closed squares), Flag-UL33-YFP (closed circles), or Flag-UL78-YFP (closed triangles). (B-D) Average peak Ca2+ concentrations triggered by 100nM CCL5 in HA-CCR5 transfected THP-1 cells (B) or by 10nM CXCL12 in THP-1 cells (D) coexpressing mock, Flag-UL33 or Flag-UL78. Mock = pcDNA3 expression vector. Statistical differences of calcium concentrations or maximal IP1 production compared with mock nucleofected cells were assessed using the Student t test (*P < .05; **P < .01; ***P < .001).
Taken together, these results suggest that UL33 and UL78 have pleiotropic effects on CCR5 and CXCR4 signaling, which are predominantly negative.
UL33 and UL78 modify the migration of THP-1 cells
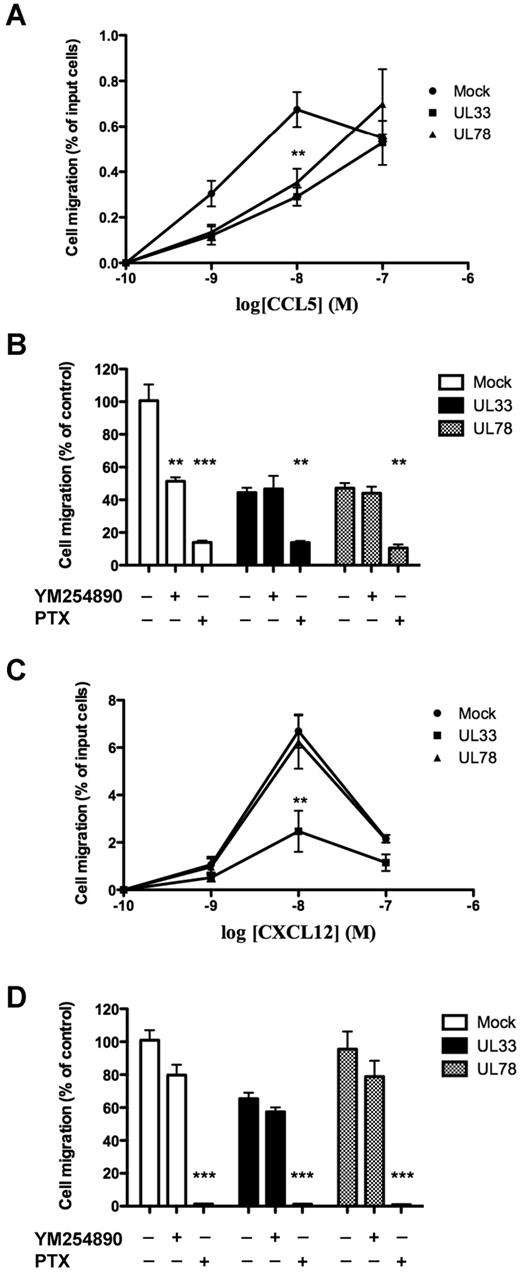
Cell migration is the primary cellular event initiated by chemokine receptor activation.31 We therefore assessed the migration of THP-1 cells expressing either UL33 or UL78 in response to CCL5 and CXCL12. To increase the reproducibility of THP-1 cell migration after CCL5 stimulation, we increased the number of CCR5 2-fold by transfecting THP-1 cells with a HA-tagged CCR5 expression plasmid as mentioned in the previous section. Stimulation with CCL5 induced the typical bell-shaped dose-response migration curve with a peak at 10nM CCL5 (Figure 6A). The presence of either UL33 or UL78 shifted the migration curve to the right with a 50% reduction at 10nM CCL5 and a dose-dependent increase up to the highest CCL5 concentration (100nM) applied. In the migrated UL33-YFP or UL78-YFP positive cells a reduction of 75% was observed compared with control cells expressing soluble YFP (0.28 ± 0.01 versus 0.07 ± 0.008 and 0.07 ± 0.006% for YFP versus UL33-YFP and UL78-YFP expressing cells, respectively). The Gαi protein inhibitor pertussis toxin (PTX) completely abolished cell migration under all conditions (Figure 6B). Interestingly, migration was also partially sensitive to pretreatment with the Gαq protein inhibitor YM254890 only in the absence of v7TM proteins (Figure 6B). Furthermore, CXCL12 induced the typical bell-shaped dose-response migration curve with a peak at 10nM CXCL12 (Figure 6C). A similar curve was observed in cells expressing UL78. However, THP-1 cells expressing UL33 were completely unresponsive to CXCL12 treatment (Figure 6C). CXCL12-promoted migration was sensitive to PTX and insensitive to YM254890 treatment for all conditions (Figure 6D). These results show that CCL5 and CXCL12-promoted migration in the presence of UL33 and UL78 are entirely dependent on Gαi protein-coupled signaling pathways. Both, UL33 and UL78 have profound effects on THP-1 migration in response to cellular chemokine receptors, with a rightward shift of the dose-response curve of CCL5 and a dramatic loss of CXCL12 responsiveness in UL33 expressing cells.
Chemotactic response to CCL5 and CXCL12 in THP-1 cells expressing mock, UL33 or UL78. THP-1 cells coexpressing HA-CCR5 (A-B) or not (C-D) and mock (closed circles), Flag-UL33-YFP (closed squares) or Flag-UL78-YFP (closed triangles) were pretreated or not as indicated with pertussis toxin (PTX, 100 ng/mL, overnight) or YM254890 (0.1μM, 15 minutes) and then stimulated with 10nM (B-D) or increasing (A-C) doses of CCL5 (A-B) or CXCL12 (C-D) in a 24-well chemotaxis chamber. The assay was done in triplicates, and the number of migrated cells was counted per well. Each point represents the mean percentage of migrated cells ± SEM in 3 separate experiments. Mock = pcDNA3 expression vector. Statistical differences in the percentage of migrated cells compared with mock nucleofected cells were assessed using the Student t test (**P < .01; ***P < .001).
Chemotactic response to CCL5 and CXCL12 in THP-1 cells expressing mock, UL33 or UL78. THP-1 cells coexpressing HA-CCR5 (A-B) or not (C-D) and mock (closed circles), Flag-UL33-YFP (closed squares) or Flag-UL78-YFP (closed triangles) were pretreated or not as indicated with pertussis toxin (PTX, 100 ng/mL, overnight) or YM254890 (0.1μM, 15 minutes) and then stimulated with 10nM (B-D) or increasing (A-C) doses of CCL5 (A-B) or CXCL12 (C-D) in a 24-well chemotaxis chamber. The assay was done in triplicates, and the number of migrated cells was counted per well. Each point represents the mean percentage of migrated cells ± SEM in 3 separate experiments. Mock = pcDNA3 expression vector. Statistical differences in the percentage of migrated cells compared with mock nucleofected cells were assessed using the Student t test (**P < .01; ***P < .001).
UL33 and UL78 block HIV infection of THP-1 cells
CCR5 and CXCR4 are crucial coreceptors for HIV entry in CD4+ target cells.20,21 To assess the effects of UL33 and UL78 on the capacity of CCR5 or CXCR4 to promote HIV entry, we infected THP-1 cells expressing UL33 or UL78 either with the R5-tropic JR-CSF strain or the X4-tropic LAI strain for 40 hours. The percentage of HIV-infected cells was evaluated by flow cytometric detection of YFP-positive cells (expressing UL33-YFP or UL78-YFP) and of the intracellular p24-Ag (HIV-infected cells).26 Interestingly, the percentage of HIV-infected cells was dramatically decreased on expression of UL33 or UL78 for both, R5 (Figure 7A, supplemental Figure 7A), and X4-tropic strain (Figure 7B, supplemental Figure 7B) compared with mock-transfected cells. Importantly, CD4 surface expression, the main HIV attachment receptor33 (Figure 7C, supplemental Figure 7C), and its interaction with CCR5 and CXCR4 (Figure 7D-E) was not affected by UL33 or UL78 expression. These results show that UL33 and UL78 diminish the HIV infection of THP-1 cells by modulating the HIV coreceptor activity of CCR5 and CXCR4, probably by engaging them into heteromeric complexes.
UL33 and UL78 block HIV infection in THP-1 cells. (A-B) THP-1 cells expressing YFP, Flag-UL33-YFP, or Flag-UL78-YFP were infected 48 hours after transfection with the CCR5-tropic JR-CSF strain (A) or the CXCR4-tropic LAI strain (B). The percentage of HIV-infected cells in YFP, Flag-UL33-YFP or Flag-UL78-YFP positive and negative cell populations was assessed by flow cytometry using anti-HIV p24 antibodies. (C) Flow cytometry analysis of cell-surface CD4 by surface staining with anti-CD4 in THP-1 cells expressing mock, Flag-UL33-YFP or Flag-UL78-YFP. (D-E) Lysates prepared from THP-1 cells expressing the indicated proteins were immunoprecipitated with either anti-HA or anti-CXCR4 antibodies, resolved by SDS-PAGE and immunoblotted with anti-CD4 antibodies. Expression controls of transfected proteins are shown in Figure 2A through D (same lysates have been used for both experiments). Experiments were at least repeated twice with similar results. Mock = pcDNA3 expression vector. Statistical differences compared with mock nucleofected cells were assessed using the Student t test (***P < .001).
UL33 and UL78 block HIV infection in THP-1 cells. (A-B) THP-1 cells expressing YFP, Flag-UL33-YFP, or Flag-UL78-YFP were infected 48 hours after transfection with the CCR5-tropic JR-CSF strain (A) or the CXCR4-tropic LAI strain (B). The percentage of HIV-infected cells in YFP, Flag-UL33-YFP or Flag-UL78-YFP positive and negative cell populations was assessed by flow cytometry using anti-HIV p24 antibodies. (C) Flow cytometry analysis of cell-surface CD4 by surface staining with anti-CD4 in THP-1 cells expressing mock, Flag-UL33-YFP or Flag-UL78-YFP. (D-E) Lysates prepared from THP-1 cells expressing the indicated proteins were immunoprecipitated with either anti-HA or anti-CXCR4 antibodies, resolved by SDS-PAGE and immunoblotted with anti-CD4 antibodies. Expression controls of transfected proteins are shown in Figure 2A through D (same lysates have been used for both experiments). Experiments were at least repeated twice with similar results. Mock = pcDNA3 expression vector. Statistical differences compared with mock nucleofected cells were assessed using the Student t test (***P < .001).
Discussion
In this study, we report for the first time that the orphan HCMV-encoded UL33 and UL78 v7TM proteins heteromerize with CCR5 and CXCR4 consequently modulating their function. Interestingly, both UL33 and UL78 exhibit an inhibitory effect on THP-1 cell infection by R5 and X4-tropic HIV strains, as well as on CCL5-promoted migration. In addition, UL33 and UL78 modulate CXCL12-promoted migration and signaling pathways. The observed modified functions of CCR5 and CXCR4 probably represent new functional properties as a consequence of their heteromerization with UL33 and UL78.
Several lines of evidence support the formation of heteromers between the 2 v7TM proteins and host CCR5 and CXCR4. Physical interactions between these proteins have been identified by co-IP in HEK 293T and THP-1 cells and by BRET experiments in HEK 293T cells. UL33 and UL78 expression modified the cellular distribution of CCR5 and CXCR4 at the basal and agonist-activated state (without affecting the overall expression levels of these receptors). Although both UL33 and UL78 increased the surface expression of CCR5 by approximately 2-fold, a decrease of 20% in CXCR4 surface expression was observed. Importantly, CCL5-induced internalization of CCR5 was inhibited in the presence of either UL33 or UL78 demonstrating that these 2 v7TM proteins have a dominant negative effect on CCR5 internalization. The absence of CCL5-induced internalization was not because of the lack of ligand binding to CCR5 in the presence of UL33 or UL78 as shown in ligand binding experiments and the activation of CCR5-dependent signaling pathways under these conditions. Consistently, the 2 v7TMs did not internalize on CCL5 stimulation. In contrast, both v7TM proteins were readily internalized in cells stimulated with the CXCR4-selective CXCL12 ligand. The fact that both v7TM proteins do not bind to CXCL121,30 together with the observation that CXCR4 receptors were internalized in these same cells to a similar extent, strongly suggest that UL33 and UL78 cointernalize with CXCL12-activated CXCR4 in the respective heteromers. Collectively, these data provide strong evidence for the formation of functional heteromers of CCR5 and CXCR4 with UL33 and UL78 at the surface of THP-1 cells.
Overall, the effect of UL33 and UL78 on the surface expression and signaling of chemokine receptor through the Gαq/PLC pathway appear to correlate. Increased CCR5 surface expression in the presence of UL78 might explain the increased signaling of CCR5 through the Gαq/PLC pathway. Similarly, diminished CXCR4 surface expression in the presence of UL33 and UL78 might explain the reduced activation of the Gαq/PLC pathway. In contrast, in cells expressing UL33, a largely diminished CCR5 response is observed, probably because of the down-regulation of this pathway because of the constitutive activity of UL33.
Our data provide a first hint of the functional role of the orphan UL33 and UL78 v7TM in HCMV-infected host cells and support the recently proposed hypothesis that orphan 7TM proteins might have important ligand-independent functions.34 Physical interaction between orphan and nonorphan GPCRs results in the allosteric modulation of the signaling pathways activated by the ligand-bound receptor as exemplified by the heteromer between the melatonin MT1 receptor and the orphan GPR50.35 In the case of virally encoded GPCRs, BILF1, a constitutively active GPCR encoded by the Epstein-Barr virus (EBV), was reported to heteromerize with CXCR4 resulting in a negative cross-regulation at the level of Gαi proteins by sequestering these G proteins away from CXCR4.36
UL33 and UL78 as well as CCR5 and CXCR4 have been reported to heteromerize with each other and with other GPCRs. Both, UL33 and UL78 were recently shown to heteromerize with the HCMV-encoded US28 and to inhibit the constitutive activation of the transcription factor NF-κB by US28 but not that of the Gαq/PLC pathway.22 CCR5 and CXCR4 were shown to form heteromers that are involved in T-lymphocyte responses,17 and both physically and functionally interact with CCR2 in T lymphocytes.18 Our results on UL33 and UL78 provide further insight in the chemokine receptors heteromerization network in HCMV infected cells.
Modulation of the number of cell surface exposed receptors is considered an important regulatory mechanism for receptor function.37 CCR5 and CXCR4 show differential subcellular localizations in THP-1 cells. Whereas the majority of CXCR4 is located at the cell surface, only a sub-fraction of CCR5, approximately 20%, reaches the cell surface. Proteins, such as CD4, have been shown to promote CCR5 surface expression by forming a stable complex with CCR5 during biosynthesis in THP-1 cells.28 Our data extend these observations, as UL33 and UL78 are also able to promote CCR5 surface expression. However, in contrast to CD4, which appears to have no direct impact on CCR5 function, UL33 and UL78 modify CCR5 function indicating that their effect goes far beyond the simple facilitation of CCR5 cell surface expression at steady-state conditions consistent with the idea that these heteromers represent new functional entities.
Cell migration is an important functional output of chemokine receptors that is involved in the immune response and cancer development. We observed that UL33 and UL78 have differential effects on CCL5 and CXCL12-induced migration of THP-1 cells. The dose-response curve of CCR5-induced migration was rightward-shifted in the presence of UL33 and UL78, reducing THP-1 migration by one-half at the peak concentration of CCL5 (10nM) in the absence of v7TM proteins. Maximal migration in the presence of v7TM proteins was only observed at 10-times higher CCL5 concentrations indicating a delayed response in the presence of UL33 and UL78. Different effects were observed for CXCL12-induced THP-1 migration. Whereas UL78 had no effect, UL33 dramatically reduced the responsiveness to CXCL12. THP-1 migration was Gαi protein-dependent in all cases. These results show that heteromerization per se is not sufficient to modify the migration pattern as exemplified by the UL78/CXCR4 heteromer and that UL33 and UL78 have nonredundant functions that are dependent on the host chemokine receptor. These results probably reflect the distinct functional profile of the different v7TM/CCR5(CXCR4) couples.
Based on their differential expression pattern, UL33 and UL78 have been proposed to play different roles during the HCMV life cycle. UL78 seems to have a potential role in viral replication and its expression starts at early phases of HCMV infection and continues through late stages.12 In contrast, UL33 seems to be important for viral dissemination and its expression is restricted to late stages of HCMV infection. Indeed, mouse (M33) and rat homologues of UL33 were reported to play a role in viral dissemination within the host,38,39 a function that could be complemented by the human UL33 in a viral strain lacking M33.40 Our study indicates that UL33 and UL78 not only regulate the function of the HCMV-encoded US28 through heteromerization, but also that of the host chemokine receptors, CCR5 and CXCR4. Our results clearly suggest that these 2 orphan v7TM proteins may play a central and integrative role during the different phases of HCMV infection. It will be therefore interesting to determine in future studies the effect of UL33 and UL78 on host chemokine receptors in HCMV infected cells expressing all the other virally encoded proteins.
The inhibitory effect of UL33 and UL78 on HIV entry in THP-1 cells points to a potential interplay between HCMV and HIV in coinfected monocytes. Because CCR5 and CXCR4 are crucial coreceptors of HIV infection, their heteromerization with UL33 and UL78 are probably at the origin of this effect. Indeed, the potential relationship between the oligomeric state of CCR5 and CXCR4 and their role as coreceptors of HIV infection has been proposed more than 10 years ago.16 First, a specific anti-CCR5 antibody was suggested to prevent HIV-1 infection by promoting CCR5 dimerization.41 Then, the natural Δ32 CCR5 mutant was documented to confer resistance to HIV-1 infection in heterozygous people42 and suggested to trap the wild-type CCR5 in heteromers located in intracellular compartments.43 Moreover, delayed AIDS progression44 of carriers of a natural mutation of CCR2 (CCR2V64I) was suggested to be associated with cross-inhibition of CCR5 function within the corresponding heteromers. However, some of these results could not be replicated or might be explained by mechanisms other than GPCR heteromerization leaving the importance of chemokine receptor oligomerization on HIV entry an open question. The association of CCR5 and CXCR4 with UL33 or UL78 provides new support for the role of receptor oligomerization in HIV infection.
Interestingly, we have shown that the percentage of infection by the R5-tropic JR-CSF strain or the X4-tropic LAI strain was dramatically decreased in THP-1 cells expressing UL33 or UL78. This was not because of a reduction in cell surface expression of CCR5, CXCR4, or CD4, the primary HIV entry receptor, and its interaction with CCR5 and CXCR4 are not affected on expression of UL33 and UL78. These results suggest that UL33 and UL78 block the HIV infection in THP-1 cells via the modulation of CCR5 and CXCR4 coreceptor activity through heteromerization. The precise mechanism of the inhibitory effect of UL33 and UL78 on the coreceptor function of CCR5 or CXCR4 remains to be established. Possible options are: (1) The modification of the CCR5 or CXCR4 structure in the heteromer that is less prone to interact with the virus envelope. (2) The shielding of CCR5 or CXCR4 epitopes important for virus entry. (3) The inhibition of intracellular signaling events, such as the modulation of cytoskeleton proteins, during the HIV entry process.45
In the 1980s, circumstantial evidence implicated HCMV infection as a cofactor for the progression to AIDS in humans.46 In addition, HCMV infection of peripheral blood monocyte-derived macrophages (MDMs) was reported to increase HIV-1 production.47 Our results do not corroborate these studies but rather suggest less efficient HIV infection of HCMV-infected cells because of the inhibitory effect of UL33 and UL78 on CCR5 and CXCR4 coreceptor activity. However, a more recent study, conducted in differentiated THP-1 cells provides a reasonable explanation for this apparent contradiction.48 Whereas HCMV-infected cells were refractory to infection with HIV-1, HCMV-uninfected bystander cells within the same population were more susceptible to HIV-1 infection. Taken together with our results, it is thus probable that the engagement of CCR5 and CXCR4 in heteromeric complexes with UL33 or UL78 in HCMV-infected cells inhibit the coreceptor activity of CCR5 and CXCR4, therefore preventing HIV infection. At the same time these HCMV-infected cells would secret a yet to be indentified soluble factor favoring HIV infection of neighboring HCMV-uninfected cells thus explaining the overall promoting effect of HCMV on HIV infection.
In summary, our results suggest that the HCMV-encoded, orphan 7TM proteins UL33 and UL78 can form heteromers with CCR5 and CXCR4, thus modulating their cellular functions and inhibiting their HIV cofactor activity in HCMV-infected cells. Promoting the engagement of CCR5 and CXCR4 into heteromers with impaired HIV coreceptor activity may become part of a novel approach to limit HIV infection.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Stefano Marullo (Institut Cochin, Paris), Dr Angélique Levoye (Inserm U698, Paris), and Drs Fabrice Maurin and Eric Trinquet (Cisbio Bioassay, Bagnols-sur-Cèze) for reagents and expert advice.
This work was supported by grants from the Association pour la Recherche sur le Cancer (ARC, no. 5051, to R.J.), Inserm, Center National de la Recherche Scientifique, the Austrian Science Fund (P18723), and the Jubiläumsfonds of the Austrian National Bank (to M.W.); the Lanyar Stiftung Graz, Austria (to M.W. and P.T.); the Molecular Medicine PhD program and a research fellowship of the Medical University of Graz (to P.T.); and the research fellowship of the Université Paris Descartes (to K.T.).
Authorship
Contribution: K.T., D.T., and F.G. performed research and analyzed data; P.T. helped in certain experiments and plasmid cloning; M.W. and M.B. helped design research and data analysis; and R.J. and M.K. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for M.W. is Hagedorn Research Institute, Novo Nordisk A/S, Gentofte, DK.
Correspondence: Ralf Jockers, Institut Cochin, 22 rue Méchain, Paris, France 75014; e-mail: ralf.jockers@inserm.fr.