Abstract
Dendritic cell (DC) homeostasis in peripheral tissues reflect a balance between DC generation, migration, and death. The current model of DC ontogeny indicates that pre-cDCs are committed to become terminal conventional DCs (cDCs). Here, we report the unexpected finding that proliferating immunostimulatory CD11c+ MHC class II+ cDCs derived from pre-cDCs can lose their DC identity and generate progeny that exhibit morphologic, phenotypic, and functional characteristics of regulatory macrophages. DC-derived–macrophages (DC-d-Ms) potently suppress T-cell responses through the production of immunosuppressive molecules including nitric oxide, arginase, and IL-10. Relative deficiency of granulocyte-macrophage colony stimulating factor (GM-CSF) provided a permissive signal for DC-d-M generation. Using a transgenic mouse model that allows tracking of CD11c+ cells in vivo, we found that DC-d-M development occurs commonly in cancer, but not in lymphoid or nonlymphoid tissues under steady-state conditions. We propose that this developmental pathway serves as an alternative mechanism of regulating DC homeostasis during inflammatory processes.
Introduction
Dendritic cells (DCs) play a central role in initiating and regulating immune responses to foreign and self-antigens.1 Under steady-state conditions, lymphoid and nonlymphoid tissues contain stable numbers of DCs, which is achieved through a dynamic interplay between the influx of new precursors, DC emigration, and death. Bone marrow (BM)–derived pre-cDCs, the immediate precursor of conventional DCs (cDCs), migrate via blood and enter peripheral lymphoid and nonlymphoid tissues, where they differentiate into cDCs that proliferate for several generations.2-4 Monocytes also participate in the generation of cDCs in some tissues.5-7 Nonlymphoid tissue cDCs emigrate continuously via lymphatics into draining lymph nodes8 ; similar to the resident populations of lymphoid tissue DCs, most migrant cDCs die in lymphoid tissue and do not reenter the blood circulation.1 Whether all peripheral tissue cDCs home to lymph nodes or some are destined to die in their local milieu is unclear. Dysregulation or loss-of-function of various cell types and molecules can alter DC homeostasis.9,10 Chief among them is the cytokine Flt3 ligand, which regulates the number of early progenitors in BM and the rate of DC proliferation in peripheral tissues.11,12
During acute inflammatory processes, DC numbers fluctuate markedly. Strong inflammatory stimuli can cause an abrupt decrease in the number of lymphoid cDCs, a consequence of maturation-induced apoptosis and migration.13,14 DC numbers usually rebound within 2 to 3 days, then expand through recruitment of new precursors, augmentation of cDCs proliferation in situ, and differentiation of monocytes into “inflammatory” DCs.3,15-18 Increased expression of granulocyte/macrophage colony stimulating factor (GM-CSF) is considered key to this response.19 Inflammation also causes egress of tissue DCs into lymph nodes by modulating chemokine receptor expression and altering the structure of regional lymphatics and lymph nodes.20-22 With resolution of inflammation, DC numbers normalize through apoptosis and cytotoxic T-cell mediated killing.23,24 In chronic inflammatory processes, such as in cancer, DC fate remains poorly understood.
The current model of DC ontogeny indicates that the monocyte/macrophage and DC lineages diverge from a common monocyte-DC progenitor (MDP) in BM.25 The subsequent defined progenitors in the DC lineage, common DC precursors (CDP or pro-DC) followed by pre-cDCs, are considered irreversibly committed to become terminal DCs. The general assumption of this model is that death completes the life cycle of all DCs. In this study, we report a new termination pathway for cDCs. We found that immunostimulatory CD11c+ MHC class II+ cDCs retain the capacity to evolve into CD11c− MHC class II− macrophage-like cells with potent immunosuppressive activity. We show the critical importance of GM-CSF in maintaining DC identity. By tracking genetically tagged CD11c+ cells in vivo, we found that tumors induce a high proportion of cDCs to develop into macrophages. We propose that this previously unrecognized developmental pathway provides a mechanism to regulate cDCs numbers and function during inflammatory and immunosuppressive conditions.
Methods
Mice
Male C57BL/6 and BALB/c mice were purchased from Charles River. C57BL/6.SJL congenic mice, OT-I, CD11c-Cre, ROSA26-enhanced green fluorescent protein (EGFP), and C57BL/6-Tg (ACTbEGFP)OPsb/J transgenic mice were originally purchased from The Jackson Laboratory or Taconic Farms, and bred in our animal facility. Mice were maintained in pathogen-free conditions in accordance with institutional guidelines, and used at 2 to 3 months of age. The Animal Research Committee of University Health Network reviewed and approved the studies.
Antibodies and reagents
Anti-CD11c (clone HL3), I-Ab (KH74, 25-9-17), I-Ad (AMS-32.1), CD3 (17A2), CD19 (1D3), CD49b (pan-NK, DX5), Gr-1 (RB6-8C5), CD11b (M1/70), B220 (RA3 6B2), CD45 (30-F11), CD45.2,(104) CD45.1 (A20), CD40 (3/23), CD80 (16-10A1), CD86 (GL1), CD25 (PC61), CD69 (H1.2F3), CD16/32 (2.4G2), IL-4R (MILD-4R-M1), CTLA-4 (UC-4F10-11), and PD-L2 (TY-25) were purchased from BD Pharmingen. Anti-F4/80 (A3-1) was purchased from Serotec. Anti-CD115 (AFS98), IL-10R (JES5-16E3), anti-GITR (DTA-1), and anti–PD-L1 were purchased from eBioscience. These antibodies were unlabeled or conjugated to fluorescein isothiocyanate (FITC), phycoerythrin (PE), allophycocyanin (APC), Pe-PC7, or biotin as indicated. Biotinylated antibodies were revealed with FITC, PE, APC, Tex-red, PC5, or PC7. Murine GM-CSF and FLT3L were purchased from BD Pharmingen. N6-(1-iminoethyl)-lysine, hydrochloride (L-NIL) and N-hydroxy-L-arginine (NOHA) were purchased from Calbiochem.
Flow cytometry and cell sorting
Flow cytometry was performed as previously described.26 In all experiments, control isotype-matched monoclonal antibodies (mAbs) were included to determine the level of background staining. Dead cells were excluded by propidium iodide (PI) staining. Lin− CD11c+ MHC II− Flt3+CD172-α− BM pre-cDCs, Lin− CD11c+ MHC II+ spleen cDCs, and Lin−CD11b+CD115+ BM monocytes were sorted on a MoFlo High-speed Cell Sorter using Summit Version 4.5 acquisition and analysis software (DakoCytomation), as previously described.2,27,28 The purity of the cell populations was ≥ 99% based on reanalyzed samples.
Tumor models
B16-F10 melanoma (B16) and Lewis lung carcinoma (LLC) cell lines were purchased from ATCC. GM-CSF producing B16 and LLC cells were generated with a retrovirus encoding murine GM-CSF as described.27 Supernatants from 24-hour cultured B16-GM-CSF cells (0.5 × 106 in 4 mL medium) contained ∼ 3000 pg/mL of GM-CSF. To establish tumors in mice, 0.5 to 1 × 106 tumor cells in 50 μL PBS were injected subcutaneously into the flank, and recovered at 10 to 12 days for analysis.
Cell culture
In coculture experiments, sorted BM pre-cDCs (1 × 105) were labeled with carboxyfluorescein diacetate, succinimidyl ester (CFSE), and incubated on a confluent monolayer of irradiated (25 Gy) neonatal skin fibroblasts or S17 cells (a kind gift from K. Dorshkind, David Geffen School of Medicine at UCLA, Los Angeles, CA) with or without added cytokines, as described.2 At the end of culture, the monolayer was disrupted with 0.25% trypsin and 10mM ethylenediaminetetraacetic acid (EDTA), and the cells were recovered and stained with fluorochrome-conjugated antibodies for flow cytometric analysis. In other studies, sorted cells were cultured in triplicate in 96-well U-bottom culture plates with 200 μL RPMI 1640 containing 10% FBS, 50μM 2-mercaptoethanol, 1mM sodium pyruvate, 10mM nonessential amino acids, 50 units/mL penicillin, and 50 μg/mL streptomycin with or without added cytokines.
Conditioned media
Supernatant collected from stromal fibroblasts and LLC tumor cells cultured in Dulbecco modified Eagle medium (DMEM) for 24 hours was filtered through a 0.22 μm filter, stored at −80°C, and thawed immediately before use.
Measurement of cytokines, nitric oxide, and arginase activity
IL-10 concentration was measured culture supernatants by enzyme-linked immunosorbent assay (ELISA). Nitric oxide concentration was calculated with the Griess Reagent Kit (Molecular Probes). Arginase activity was measured as described.29
Limiting dilution assay
Sorted GFP+pre-cDCs were cultured by limiting dilution on S17 monolayers, as described.2 Cell growth was monitored daily under an inverse fluorescence microscope. Wells containing colonies ≥ 6 cells on day 8 were counted as positive. Clonal efficiency was calculated by Poisson statistics.
Immunohistochemical staining
GFP+-pre-cDCs cocultured on S17 cells for 8 days in 96-well plates were fixed with 4% paraformaldehyde, washed, and stained with allex-647–conjugated anti–I-Ab or anti-CD11c antibodies, as described.2 Cells were visualized and photographed with Olympus FluoView 1000 laser scanning confocal microscope (lens: 20×, UplanSApo, NA 0.75, zoom factor 2) and analyzed by FV10-ASW Version 3.0 software.
Adoptive transfer studies
CFSE-labeled CD45.2 pre-cDCs (5-10 × 105) were injected intravenously into congenic CD45.1 tumor-bearing recipients, as described.2 Spleens and tumors were removed from recipients 3 to 4 days later; mononuclear cells were isolated by Lympholyte-M (Cedarlane) density gradient centrifugation, stained with fluorochrome-conjugated antibodies, and analyzed by flow cytometry.
Allogeneic mixed leukocyte reactions
Graded numbers of stimulator cells were seeded in triplicate with responder BALB/c spleen cells (1 × 105/well), and cultured for 3 days in a humidified atmosphere of 5% CO2 in air at 37°C. Cells were pulsed with 1 μCi of [3H]thymidine (Amersham) 16 hours before harvest, and collected onto glass fiber filters (Millipore); [3H]thymidine incorporation was quantified using a Beckman scintillation counter.
T-cell suppression assays
OT-I T cells (1 × 105 /well) were incubated in triplicate for 3 days with sorted ovalbumin (OVA)–pulsed spleen cDCs (1 × 104) and graded numbers of DC-derived-macrophages or nonpeptide pulsed control DC. T-cell proliferation was assessed by [3H]thymidine incorporation.
Statistics
Statistical significance (P < .05) of data were determined by the Student t test. Data were analyzed with GraphPad Prism 5 software.
Results
Proliferating cDCs generate CD11c−MHCII− progeny
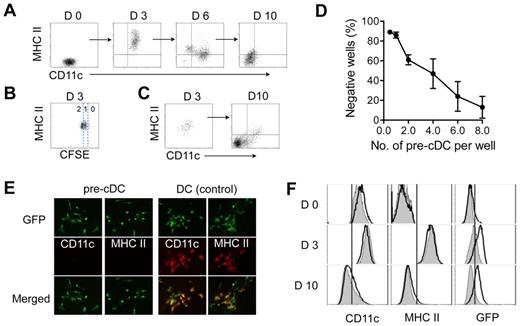
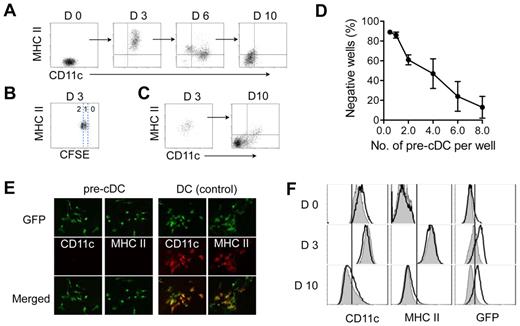
Pre-cDCs interact with stromal cells after extravasating from blood into lymphoid and nonlymphoid tissues. To investigate pre-cDCs differentiation in a stroma microenvironment, we placed sorted BM pre-cDCs (Lin−CD11c+MHC class II−Flt3+CD172α−) on a stromal monolayer derived from newborn mouse skin fibroblasts. As expected, pre-cDCs generated CD11c+ MHC class II+ progeny after 2 to 3 days of culture (Figure 1A). These cells displayed typical features of cDCs including morphology and the capacity to mature and stimulate lymphocyte proliferation (data not shown). CFSE labeling studies revealed that most of these cells had completed 1 to 2 rounds of cell division and expressed MHC class II, indicating that pre-cDCs differentiated into proliferating cDCs (Figure 1B). The cDCs progeny continued to divide during the next 10 to 12 days, expanding 16.4 ± 1.9-fold in number, and eventually stopped dividing at 12 to 16 days. Surprisingly, the progeny at 10 to 12 days expressed neither CD11c nor MHC class II, the classic hallmarks of murine cDCs. A time-course analysis showed that a variable proportion of cells at 6 days, and most cells at 10 days had lost expression of these surface markers (Figure 1A); loss of MHC class II expression usually preceded loss of CD11c. Once lost, the cells failed to reexpress these molecules even after exposure to high concentrations of DC growth factors (GM-CSF and Flt3L) or to various DC maturation stimuli including lipopolysaccharide (LPS), tumor necrosis factor (TNF), anti-CD40 antibodies, and CpG (data not shown). Spleen and tumor pre-cDCs also proliferated on stroma, and their progeny behaved similarly to those derived from BM pre-cDCs (data not shown).
Proliferating cDCs generate CD11c− MHC class II− progeny. Purified BM pre-cDCs were labeled with CFSE and cultured on irradiated stroma derived from neonatal fibroblasts; their progeny were recovered at 3, 6, and 10 days for analysis of CD11c and MHC class II expression (A) and number of cell divisions as determined by CFSE dilution (B). (C) CD11c+MHCII+ progeny from day 3 cultures in panel A were sorted and seeded on new stroma. Left and right dot-plots show CD11c and MHC class II expression on sorted day 3 cells before culture and their progeny 7 days later. (D) Limiting dilution clonal analysis of purified GFP+ pre-cDCs cultured on S17 stromal monolayers for 10 days. Wells with clones containing ≥ 6 cells were deemed positive. Each data point is the mean ± SD of 4 independent experiments. (E) Fluorescence microscopy of clones derived from a single GFP+ pre-cDC at 10 days and stained with anti-CD11c or anti-MHC class II. GFP+ cDC clones were used as positive controls for CD11c and MHC class II expression. Original magnification, ×20. (F) Pre-cDCs from CD11c-Cre+Rosa26-EGFP transgenic mice (white histograms) and their CD11c-Cre− littermate controls (gray histograms) were cultured on stroma. Histograms show CD11c, MHC class II, and GFP expression on the indicated days. Data are representative of 3 independent experiments.
Proliferating cDCs generate CD11c− MHC class II− progeny. Purified BM pre-cDCs were labeled with CFSE and cultured on irradiated stroma derived from neonatal fibroblasts; their progeny were recovered at 3, 6, and 10 days for analysis of CD11c and MHC class II expression (A) and number of cell divisions as determined by CFSE dilution (B). (C) CD11c+MHCII+ progeny from day 3 cultures in panel A were sorted and seeded on new stroma. Left and right dot-plots show CD11c and MHC class II expression on sorted day 3 cells before culture and their progeny 7 days later. (D) Limiting dilution clonal analysis of purified GFP+ pre-cDCs cultured on S17 stromal monolayers for 10 days. Wells with clones containing ≥ 6 cells were deemed positive. Each data point is the mean ± SD of 4 independent experiments. (E) Fluorescence microscopy of clones derived from a single GFP+ pre-cDC at 10 days and stained with anti-CD11c or anti-MHC class II. GFP+ cDC clones were used as positive controls for CD11c and MHC class II expression. Original magnification, ×20. (F) Pre-cDCs from CD11c-Cre+Rosa26-EGFP transgenic mice (white histograms) and their CD11c-Cre− littermate controls (gray histograms) were cultured on stroma. Histograms show CD11c, MHC class II, and GFP expression on the indicated days. Data are representative of 3 independent experiments.
We also obtained similar results when pre-cDCs were cultured on stroma derived from NIH 3T3, S17, and OP9 cell lines, as well as primary cell lines (γ-irradiated or nonirradiated) derived from mouse spleen, thymus, lung, and liver (data not shown). Thus, generation of CD11c−MHCII− progeny from proliferating CD11c+MHCII+ cDCs was not limited to a particular type of stroma.
Monocytes possess the bipotential capacity to differentiate into macrophages or DCs depending on growth conditions.30 Direct differentiation of pre-cDCs into CD11c−MHC class II− cells seemed unlikely, however, because all the cells at 3 days expressed both CD11c and MHC class II. To verify this conclusion, day 3 CD11c+MHC class II+ cells were sorted from the cultures and placed on a new stromal monolayer. These day 3 cells proliferated vigorously and their progeny lost CD11c and MHC class II expression 7 days later (Figure 1C). We compared differentiation of pre-cDCs and monocytes (lin− CD11b+ CD115+) cultured in parallel (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Monocytes generated few CD11c+ MHC class II+ cells at 3 days or at later time points; addition of high concentrations of exogenous GM-CSF to the medium had no effect on cell phenotype (supplemental Figure 1B). These findings were consistent with a previous report showing that fibroblasts inhibit monocyte differentiation into DCs.31 We also monitored the behavior of single GFP+–pre-cDCs and monocytes on stroma (supplemental Figure 1C). During a 10 day culture period, 50% of monocytes failed to divide, and the remaining cells generated 2 to 6 progeny, whereas > 90% of pre-cDCs produced clones of 10 to 80 cells. Thus, pre-cDCs and monocytes have distinct developmental properties on stroma.
Early hematopoietic progenitors and monocytes can generate progeny that resemble day 10 CD11c−MHC class II− cells. To address the possibility that purified pre-cDCs included stem cell or monocyte contaminants, we performed limiting-dilution clonal assays on pre-cDCs. The clonal efficiency of pre-cDCs, as determined by Poisson statistics, was 23.4 ± 6.2% (n = 3; Figure 1D). Immuno-fluorescence microscopy of 100 pre-cDCs–derived clones at 8 days revealed that all cells were CD11c− and MHC class II− (Figure 1E), whereas DC controls were positive for these markers. These findings provided clonal evidence that pre-cDCs can generate MHC class II− cells. The high clonal frequency of pre-cDCs also excluded the possibility that these cells arose from a stem cell contaminant, which constitute < 1% of the starting pre-cDC population.
To further establish that CD11c−MHC class II− progeny arose from proliferating CD11c+MHC class II+ cDCs, we used pre-cDCs from CD11c-Cre+Rosa26-EGFP transgenic mice to trace the life history of CD11c− cells back to CD11c+ DC.32 Cre recombinase, driven by the CD11c promoter, deletes the stop codon for ROSA-EGFP; cells that express CD11c are permanently tagged by EGFP irrespective of subsequent CD11c expression levels in the progeny. Before culture, EGFP could be detected in approximately 10 to 20% of pre-cDCs from CD11c-Cre+Rosa26-EGFP transgenic mice (Figure 1F), consistent with previous studies.32 CD11c-Cre+pre-cDCs differentiated into MHC class II+ cDCs at 3 days concurrent with the up-regulation of EGFP expression. At 10 days, when the Cre+ cells had lost CD11c and MHC class II expression, EGFP expression persisted. Pre-cDCs from CD11c-Cre− littermate controls behaved similarly apart from not expressing EGFP. Collectively, these data indicate that proliferating CD11c+MHC class II+ cDCs derived from pre-cDCs can produce CD11c− MHC class II− progeny.
cDCs-derived CD11c−MHCII− cells exhibit macrophage features
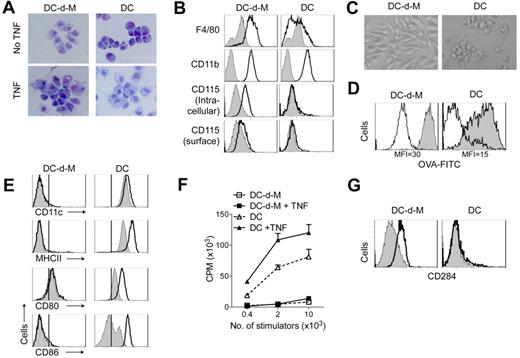
Compared with spleen CD11c+MHC class II+ cDCs or cDCs generated from pre-cDCs in vitro, cDCs-derived CD11c− MHC class II− cells exhibited features typical of macrophage-like cells (DC-d-Ms): they had short dendrites and large phagosomal vacuoles; expressed high levels of CD11b, F4/80, and intracellular CD115; and adhered firmly to plastic when replated (Figure 2A-C). DC-d-M also had a higher capacity to endocytose soluble ovalbumin (Figure 2D) and latex beads (data not shown) than cDCs. In contrast with cDCs, maturation stimuli (TNF, LPS) had little effect on their morphology, surface MHC class II, CD80 and CD86 expression, or lymphocyte stimulatory capacity (Figure 2A,E-F). This poor response to LPS could not be attributed to lack of Toll-like receptor (TLR) expression because DC-d-Ms expressed higher levels of CD284 (TLR4) than cDCs (Figure 2G). Further, DC-d-Ms up-regulated IL-10 and inducible nitric oxide synthase (iNOS) expression after exposure to LPS (Figure 3C-3D). These results indicated that loss of CD11c expression coincides with the conversion of cDCs to macrophage-like cells that have an altered response to inflammatory stimuli.
Characteristics of DC-d-Ms. Purified pre-cDCs were cocultured on irradiated stroma and recovered 10 to 12 days later for analysis. In some wells, TNF was added for the final 24 hours of culture. Splenic cDCs or pre-cDCs–derived cDCs were used as controls. (A) Giemsa-staining of cytospin preparations (original magnification 40×). (B) Expression of indicated antigens (white histogram) and isotype control (gray histogram). (C) Morphology of sorted DC-d-Ms and cDCs after overnight incubation in GM-CSF (original magnification 40×). (D) Phagocytosis of OVA conjugated with FITC (gray histogram). MFI indicates mean fluorescence intensity. (E) Expression of indicated surface antigens after 16-hour exposure to LPS (white histogram) or control (gray histogram). Vertical black lines indicates staining with isotype controls. (F) Stimulation capacity in mixed allogeneic lymphocyte reactions. Results are expressed as mean cpm × 103 ± SD. (G) Expression of CD284 (white histogram) and isotype control (gray histogram). Data are representative of > 3 independent experiments.
Characteristics of DC-d-Ms. Purified pre-cDCs were cocultured on irradiated stroma and recovered 10 to 12 days later for analysis. In some wells, TNF was added for the final 24 hours of culture. Splenic cDCs or pre-cDCs–derived cDCs were used as controls. (A) Giemsa-staining of cytospin preparations (original magnification 40×). (B) Expression of indicated antigens (white histogram) and isotype control (gray histogram). (C) Morphology of sorted DC-d-Ms and cDCs after overnight incubation in GM-CSF (original magnification 40×). (D) Phagocytosis of OVA conjugated with FITC (gray histogram). MFI indicates mean fluorescence intensity. (E) Expression of indicated surface antigens after 16-hour exposure to LPS (white histogram) or control (gray histogram). Vertical black lines indicates staining with isotype controls. (F) Stimulation capacity in mixed allogeneic lymphocyte reactions. Results are expressed as mean cpm × 103 ± SD. (G) Expression of CD284 (white histogram) and isotype control (gray histogram). Data are representative of > 3 independent experiments.
cDCs-derived macrophages suppress T-cell proliferation. (A) Serial 2-fold diluted DC-d-Ms or cDCs were added into the wells containing 1 × 105 OT-I T cells and 1 × 104 OVA-peptide–pulsed cDCs. The cells were incubated for 60 hours. Tritiated thymidine was added to the wells for last 12 hours. Results are expressed as mean cpm × 103 ± SD. Data are representative of > 3 independent experiments. (B) Expression of indicated antigens on DC-d-Ms. (C-E) Production of IL-10, nitric oxide, and arginase by DCs and DC-d-Ms stimulated with or without LPS for 16 hours. Data are representative of 3 independent experiments with similar results. (F) Proliferation of OT-I T cells cultured for 60 hours as in panel A in the presence or absence of anti–IL-10R mAb (10 μg/mL), anti–PD-L1 mAb (10 μg/mL), Nor-NOHA (500μM), or L-NIL (200μM). Data are representative of > 3 independent experiments. NS indicates not significant (P > .05); *P < .05, **P < .005, ***P < .001.
cDCs-derived macrophages suppress T-cell proliferation. (A) Serial 2-fold diluted DC-d-Ms or cDCs were added into the wells containing 1 × 105 OT-I T cells and 1 × 104 OVA-peptide–pulsed cDCs. The cells were incubated for 60 hours. Tritiated thymidine was added to the wells for last 12 hours. Results are expressed as mean cpm × 103 ± SD. Data are representative of > 3 independent experiments. (B) Expression of indicated antigens on DC-d-Ms. (C-E) Production of IL-10, nitric oxide, and arginase by DCs and DC-d-Ms stimulated with or without LPS for 16 hours. Data are representative of 3 independent experiments with similar results. (F) Proliferation of OT-I T cells cultured for 60 hours as in panel A in the presence or absence of anti–IL-10R mAb (10 μg/mL), anti–PD-L1 mAb (10 μg/mL), Nor-NOHA (500μM), or L-NIL (200μM). Data are representative of > 3 independent experiments. NS indicates not significant (P > .05); *P < .05, **P < .005, ***P < .001.
DC-d-Ms are immunosuppressive
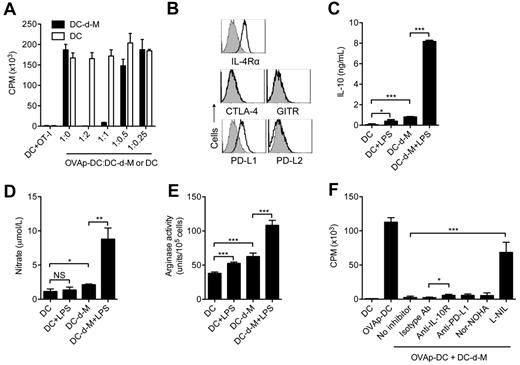
The inability of DC-d-Ms to induce lymphocyte proliferation might arise from poor stimulatory capacity or active suppression. To test whether DC-d-Ms have suppressive properties, we added graded numbers (0.25-2 × 104) of DC-d-Ms or nonpeptide-pulsed spleen cDCs (control) into cultures containing 1 × 105 CD8+ OT-I T cells and 1 × 104 OVA peptide-pulsed spleen cDCs (OVAp-DC). OVAp-cDCs induced vigorous proliferation of OT-I T cells (Figure 3A). Addition of DC-d-Ms, but not control splenic cDCs, inhibited OT-I T cell proliferation in a dose-dependent manner (Figure 3A). Almost complete suppression of OT-I and OT-II T cell proliferation was achieved with a low ratio of 1 DC-d-M:10 T cells. This potent inhibitory effect also extended to allogeneic T cells and antigen-specific CD4+ OT-II T cells (data not shown).
To explore possible mechanisms of DC-d-M–mediated suppression, we examined the expression and activity of various molecules identified previously in suppressor cell populations. DC-d-Ms expressed high levels of IL-4 receptor alpha, which is involved in the development, but not the suppressive function of myeloid-derived suppressor cells (MDSCs; Figure 3B).33 DC-d-Ms did not express CTLA-4 or GITR. DC-d-M expressed PD-L1, although the level was similar to that on splenic cDCs (data not shown), and expressed little or no PD-L2 (Figure 3B). DC-d-Ms produced significantly more IL-10, arginase, and nitric oxide after activation with LPS than DCs (Figure 3C-E). To determine the functional relevance of these molecules, inhibitors of arginase (Nor-NOHA), and iNOS (L-Nil) and neutralizing antibodies against PD-L1 and IL-10R were added to cocultures containing OVAp-DC, DC-d-M, and OT-I T cells. Compared with control, blockade of IL-10, or arginase reversed 5%-10% of the suppressed proliferation of OT-I T cells, whereas blockade of iNos activity reversed > 60% of the suppression (Figure 3F). PD-L1 blockade gave inconsistent results, and sometimes increased suppression. Thus, DC-d-Ms acquire multiple mechanisms to suppress T-cell proliferation with increased nitric oxide production being dominant.
Role of GM-CSF in DC-d-M development
The development of DC-d-Ms from DCs in the pre-cDCs/stroma cocultures suggested that stromal cell–derived cytokines might drive this process. To test this possibility, pre-cDCs were placed in culture medium containing 50% stromal cell supernatant. Most pre-cDCs survived and generated floating CD11c+MHC class II+ cDCs during the first 3 days. At 6 days, some cells had adhered to the culture plate, and over time the number of adherent cells increased via proliferation of adherent cells and continuous generation from floating cDCs; at 10 to 12 days, all cells were adherent and none expressed CD11c or MHC class II. These results indicated that stroma-derived soluble factors supported expansion and generation of DC-d-Ms from cDCs. Reverse transcription-polymerase chain reaction (RT-PCR) analysis of stromal cell mRNA revealed expression of TGF-β, IL-10, M-CSF, and IL-6 (data not shown), which are known to inhibit generation of immuno-stimulatory DCs.31,34,35 Adding neutralizing antibodies against these cytokines failed to block DC-d-M development; however, simultaneous blockade of IL-6 and M-CSF showed partial inhibition in the stroma coculture system (supplemental Figure 2).
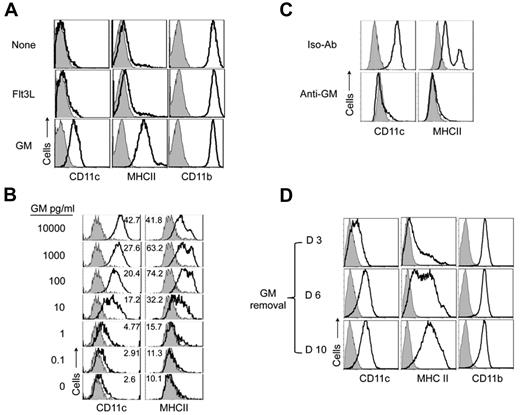
We next considered whether insufficient DC supportive signals might contribute to the generation of DC-d-Ms. Stromal cells express mRNA for Flt3L and GM-CSF; however, neither was detectable in stroma supernatants by ELISA (data not shown). Addition of exogenous Flt3L to the culture medium failed to block DC-d-M development (Figure 4A) and did not increase cell yield (supplemental Figure 3). By contrast, addition of GM-CSF completely blocked the generation of DC-d-Ms. GM-CSF also augmented the number of cell divisions and cell yield (supplemental Figure 3). We found that CD11c and MHC expression levels varied directly with GM-CSF concentration; and blockade of DC-d-M development occurred reliably with a concentration of 100 pg/mL (∼ 5pM; Figure 4B). CD11c expression levels increased progressively as the GM-CSF concentration was titrated above 100 pg/mL, whereas MHC class II expression levels declined.
GM-CSF regulates the fate of proliferating cDCs. (A) Expression of CD11c, MHC class II and CD11b by 10 day CD45+ progeny of BM pre-cDCs cocultured on irradiated stroma in the presence or absence of exogenous GM-CSF (10 ng/mL) and Flt3L (100 ng/mL). (B) Expression of CD11c and MHC class II by day 10 progeny of pre-cDCs (1 × 104) cultured in 50% stromal cell conditioned medium with or without the indicated concentrations of GM-CSF. One-half of the medium was replaced every second day with fresh conditioned medium and GM-CSF. Numbers in histograms indicate MFI. Data are representative of > 3 independent experiments. (C) Expression of CD11c and MHC class II by the day 10 progeny of pre-cDCs (1 × 104) cultured in 50% stromal cell conditioned medium and GM-CSF (1 ng/mL) with anti–GM-CSF or isotype control antibodies (10 μg/mL). Data are representative of 2 independent experiments. (D) Pre-cDCs were cocultured on stroma with GM-CSF (10 ng/mL) for the first 3 days, and then GM-CSF deprived on the indicated days. Expression of CD11c, MHC class II, and CD11b 7 days after removing GM-CSF supplements is shown. Data are representative of 3 independent experiments.
GM-CSF regulates the fate of proliferating cDCs. (A) Expression of CD11c, MHC class II and CD11b by 10 day CD45+ progeny of BM pre-cDCs cocultured on irradiated stroma in the presence or absence of exogenous GM-CSF (10 ng/mL) and Flt3L (100 ng/mL). (B) Expression of CD11c and MHC class II by day 10 progeny of pre-cDCs (1 × 104) cultured in 50% stromal cell conditioned medium with or without the indicated concentrations of GM-CSF. One-half of the medium was replaced every second day with fresh conditioned medium and GM-CSF. Numbers in histograms indicate MFI. Data are representative of > 3 independent experiments. (C) Expression of CD11c and MHC class II by the day 10 progeny of pre-cDCs (1 × 104) cultured in 50% stromal cell conditioned medium and GM-CSF (1 ng/mL) with anti–GM-CSF or isotype control antibodies (10 μg/mL). Data are representative of 2 independent experiments. (D) Pre-cDCs were cocultured on stroma with GM-CSF (10 ng/mL) for the first 3 days, and then GM-CSF deprived on the indicated days. Expression of CD11c, MHC class II, and CD11b 7 days after removing GM-CSF supplements is shown. Data are representative of 3 independent experiments.
Recombinant GM-CSF contains negligible amounts of endotoxin (≤ 100 pg per μg of GM-CSF; ≤ 1 pg/mL in our cultures). To verify further the importance of GM-CSF and to exclude a role for endotoxin in cDCs differentiation, we cultured pre-cDCs in 50% stroma supernatant with exogenous GM-CSF and added an anti–GM-CSF neutralizing antibody or isotype control antibody. Cells cultured with the control antibody remained CD11c+ MHC class II+, whereas blockade of GM-CSF resulted in the development of DC-d-Ms (Figure 4C).
GM-CSF levels fluctuate during the course of most in vivo immune responses.19 To determine whether a drop in the concentration of GM-CSF would permit DC-d-M development, we cultured pre-cDCs on stroma with GM-CSF for 3 days, then removed GM-CSF supplements at 3, 6, and 10 days; the cells were analyzed 7 days after removing GM-CSF. Withdrawal of GM-CSF at 3 days resulted in the generation of DC-d-Ms whereas most cells remained as DCs when it was withdrawn at 6 or 10 days. (Figure 4D). In addition, sorted day 10 CD11c+MHCII+ cDCs that were seeded on new stroma could not generate DC-d-Ms, and most died within 2 to 3 days. These findings indicated that the early generations of DCs require GM-CSF to maintain their DC identity.
To determine whether DC maturity influences dependence on GM-CSF, mature cDCs generated from LPS or TNF-treated pre-cDCs were placed on stroma without GM-CSF supplements. These cells failed to divide, most died within 1 to 2 days, and none generated DC-d-Ms (data not shown). Thus, mature cDCs lose the capacity to generate DC-d-Ms irrespective of GM-CSF levels.
DC-d-Ms are undetectable in mice under steady-state conditions
Maintenance of DC homeostasis in lymphoid tissues requires continuous apoptosis of DCs. Our findings suggested that DC-d-M generation might be an alternative fate. To investigate whether this pathway exists in vivo, CD11c-Cre+ Rosa26-EGFP mice were used to track cDC development. We reasoned that if cDCs could generate macrophages, then CD11c− cells expressing EGFP would be detectable by flow cytometry. Auto-fluorescent cells, CD45− cells, and lin (CD3, CD19, B220, CD49b)+ cells were excluded from the gate to increase the accuracy and sensitivity of this analysis. Spleen and lymph nodes contained few EGFP+CD45+lin− CD11c− cells (supplemental Figure 4A). A large proportion of CD45+Lin− EGFP+ CD11c− cells were evident in thymus; however, these cells did not express CD11b (supplemental Figure 4B). Peyers's patches also had a small proportion of CD45+Lin− EGFP+ CD11c− cells: some expressed MHC class II and most were CD11b−. The nature of these CD45+Lin− EGFP+ CD11c− CD11b− cells remains to be determined. Few CD45+Lin− EGFP+ CD11c− cells were detected in BM, lung and liver. Collectively, these findings suggested that DC-d-Ms were not a significant destination for cDCs under steady-state conditions.
Tumors induce generation of DC-d-M
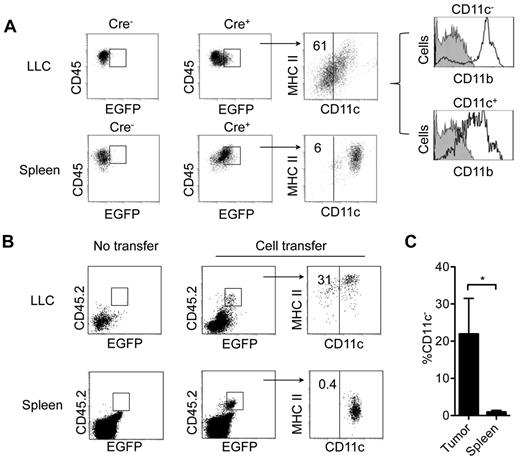
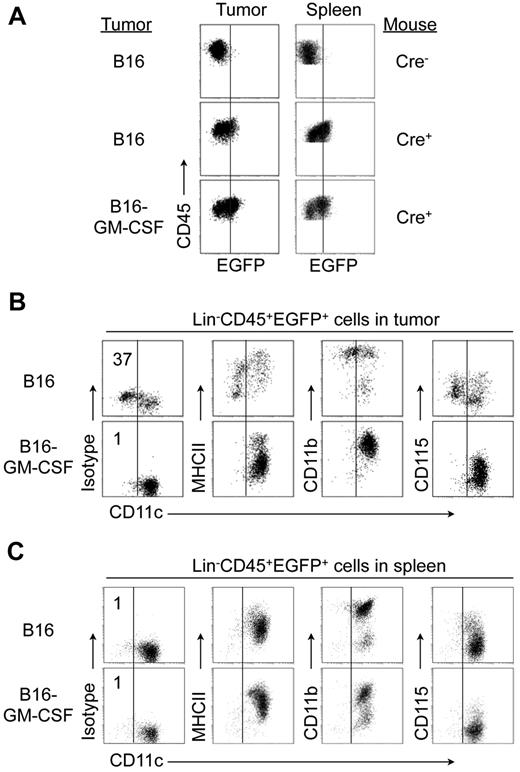
We hypothesized that the fate of cDCs might differ in pathologic microenvironments with altered levels of DC-suppressive and supportive cytokines. To test this hypothesis, we analyzed EGFP+ CD45+ Lin– cells in subcutaneous LLC and B16 tumors grown in CD11c-Cre+ Rosa26-EGFP mice. CD11c− cells constituted a high proportion of these cells in LLC (61 ± 13.3%; Figure 5A) and B16 tumors (47 ± 11.6%) (Figure 6B), but not in the spleens of these mice. Most CD45+Lin− EGFP+ CD11c− cells expressed CD11b and low levels of CD115, and expressed little or no surface MHC class II (Figures 5A and 6B). Most of these cells also expressed high levels of F4/80 and Ly6, and low levels of Gr-1 (supplemental Figure 5). Sorted tumor CD45+Lin−EGFP+ CD11c− cells displayed other key features of in vitro generated DC-d-Ms including firm adherence to plastic, high endocytosis capacity, and the inability to up-regulate MHC class II and costimulatory molecule expression (data not shown).
Detection of cDCs-derived macrophages in CD11c-Cre Rosa26-EGFP mice. (A) Expression of CD11c, MHC class II, and CD11b by Lin−CD45+ EGFP+ cells in LLC tumors and spleens from CD11c-Cre+ Rosa26-EGFP mice and their CD11c-Cre− littermate controls bearing LLC tumors (0.3-0.5 cm diameter). Vertical black line in far right dot-plots indicates staining with isotype control for CD11c. (B) Expression of CD11c and MHC class II on pre-cDC–derived CD45.2 EGFP+ cells. BM pre-cDCs (5-10 × 105) from CD45.2 CD11c-Cre+ Rosa26-EGFP mice were injected intravenously into nonirradiated CD45.1 congenic B6 mice bearing subcutaneous LLC tumors transduced with a retrovirus encoding CCL3. At 3 days, spleens and tumors were recovered for analysis. (C) Percentage of Lin−CD45+ EGFP+ cells that do not express CD11c (mean ± SD; *P = .03). Data are representative of 3 independent experiments with 3 mice per experiment.
Detection of cDCs-derived macrophages in CD11c-Cre Rosa26-EGFP mice. (A) Expression of CD11c, MHC class II, and CD11b by Lin−CD45+ EGFP+ cells in LLC tumors and spleens from CD11c-Cre+ Rosa26-EGFP mice and their CD11c-Cre− littermate controls bearing LLC tumors (0.3-0.5 cm diameter). Vertical black line in far right dot-plots indicates staining with isotype control for CD11c. (B) Expression of CD11c and MHC class II on pre-cDC–derived CD45.2 EGFP+ cells. BM pre-cDCs (5-10 × 105) from CD45.2 CD11c-Cre+ Rosa26-EGFP mice were injected intravenously into nonirradiated CD45.1 congenic B6 mice bearing subcutaneous LLC tumors transduced with a retrovirus encoding CCL3. At 3 days, spleens and tumors were recovered for analysis. (C) Percentage of Lin−CD45+ EGFP+ cells that do not express CD11c (mean ± SD; *P = .03). Data are representative of 3 independent experiments with 3 mice per experiment.
Overexpression of GM-CSF in tumors prevents generation of DC-derived macrophages. B16 tumor cells stably transduced with a retroviral vector encoding murine GM-CSF or wild-type B16 tumor cells were injected into CD11c-Cre+Rosa26-EGFP mice or CD11c-Cre− littermate controls. (A) Expression of EGFP on CD45+ cells in tumor and spleen. Vertical black lines indicate background EGFP expression by Lin−CD45+ from control mice. (B-C) Expression of indicated markers by Lin−CD45+ EGFP+ cells in tumors (B) and spleen (C). Vertical black lines indicate staining with isotype control for CD11c. Data are representative of 2 independent experiments with 8 mice per group.
Overexpression of GM-CSF in tumors prevents generation of DC-derived macrophages. B16 tumor cells stably transduced with a retroviral vector encoding murine GM-CSF or wild-type B16 tumor cells were injected into CD11c-Cre+Rosa26-EGFP mice or CD11c-Cre− littermate controls. (A) Expression of EGFP on CD45+ cells in tumor and spleen. Vertical black lines indicate background EGFP expression by Lin−CD45+ from control mice. (B-C) Expression of indicated markers by Lin−CD45+ EGFP+ cells in tumors (B) and spleen (C). Vertical black lines indicate staining with isotype control for CD11c. Data are representative of 2 independent experiments with 8 mice per group.
To verify that CD11c+ cDCs were precursors of tumor CD45+Lin− EGFP+ CD11c− CD11b+ cells, we intravenously transferred Lin−CD11c+MHC class II−/low BM pre-cDCs and immature cDCs from CD11c-Cre+ Rosa26-EGFP CD45.2+ mice into CD45.1+ congenic mice bearing LLC tumors that expressed CCL3 (to increase tumor infiltration by the transferred cells). At 3 to 4 days, ∼ 20% of CD45.2+EGFP+ cells in tumors had lost CD11c expression, which coincided with down-regulation of MHC class II expression, whereas all CD45.2+GFP+ cells in spleen expressed CD11c and MHC class II (Figure 5B-C). Notably, the CD45.2 marker helped exclude the possibility that GFP+ CD11c− cells in tumors represented host macrophages with engulfed fragments of GFP+ cDCs. Thus, tumors induced generation of DC-d-Ms.
To further examine the ability of tumor cDCs to generate DC-d-M, Lin−CD11c+MHC class II+ cDCs were sorted from LLC tumors and cultured on stroma for 10 days with or without exogenous GM-CSF. In the absence of GM-CSF, ∼ 50% of the cells lost CD11c expression and ∼ 25% lost CD11c and MHC class II expression; expression of both markers was maintained in the presence of GM-CSF (supplemental Figure 6).
Although GM-CSF levels are higher in B16 and LLC tumors than in normal tissues,36 other regulatory molecules in the tumor microenvironment may attenuate responses to GM-CSF. We therefore hypothesized that a further increase in the concentration of GM-CSF in tumors would block DC-d-M development. To test this, we transduced LLC and B16 tumor cells with a retroviral vector encoding murine GM-CSF or a control vector; the growth rate of these tumors cell lines in vitro and in vivo was similar to untransduced cells (data not shown). Overexpression of GM-CSF completely blocked the appearance of CD45+Lin−EGFP+ CD11c− cells in B16 tumors (Figure 6 A-C) and LLC tumors (data not shown). All CD11c+ cells in B16-GM-CSF tumors also expressed MHC class II, although the expression level was lower than in cDCs from wild-type tumors. As expected, no CD45+Lin−EGFP+ CD11c− CD11b+ cells were detected in spleen of mice bearing control-vector or GM-CSF–expressing B16 tumors (Figure 6C). In an in vitro system, we confirmed that pre-cDCs generated CD11c− MHC class II− cells when cultured in B16 or LLC conditioned medium, which was blocked by adding GM-CSF (data not shown). We also studied Flt3L-expressing tumors, and found that Flt3L did not inhibit cDC de-differentiation (data not shown). Collectively, these data show that the concentration of GM-CSF in tumors influences the developmental fate of tumor cDCs.
Discussion
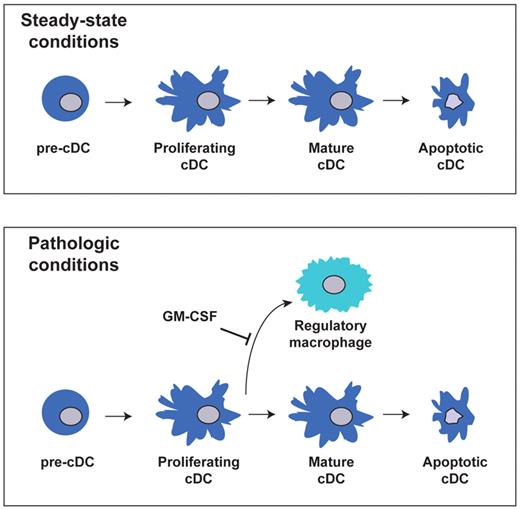
In this study, we found that proliferating immunostimulatory cDCs retain the potential to generate macrophages with potent regulatory activity. Although this developmental pathway occurred rarely in lymphoid and nonlymphoid tissues under steady-state conditions, it represented a significant route for cDCs in tumors. GM-CSF appeared to serve as a master regulator of cDC fate, as high concentrations of this cytokine completely prevented DC-d-M development, even in tumors. We propose that this pathway provides a novel mechanism for controlling the number and function of cDCs during inflammatory conditions, and may foster immunosuppression in situ (Figure 7).
Model of cDCs development from pre-cDCs under steady-state and pathologic conditions.
Model of cDCs development from pre-cDCs under steady-state and pathologic conditions.
Our finding that pre-cDCs can lead to the generation of macrophages does not conflict with the current view that pre-cDCs are committed DC precursors and distinct from monocytes. The generation of macrophages from pre-cDCs involved a multi-step process: pre-cDC (CD11c+MHCII−CD11b−/+) → cDCs (CD11c+MHCII+CD11b+) → DC-d-M (CD11c−MHCII−CD11b+). Notably, proliferating cDCs, and not pre-cDCs, were the actual progenitors of macrophages. By contrast, monocytes generate macrophages directly. The behavior of pre-cDCs and monocytes on stroma highlighted different cytokine requirements: whereas the initial generation of cDCs from pre-cDCs proceeded without exogenous growth factors, the DC potential of monocytes was blocked despite high concentrations of GM-CSF. It is also notable that DC-d-M production was limited to the early generations of cDC progeny that were actively dividing. Later generations of cDCs and mature DCs, which had stopped dividing, maintained their DC phenotype until death. A previous report showed that BM-derived mature DCs could differentiate into macrophage-like inhibitory cells in vitro, and indicated that this process occurred in spleen under steady-state conditions37 ; however, it was suggested that their findings might stem from using monocyte-derived DCs.38 In an earlier study, we found that sorted spleen cDCs, unlike tumor cDCs, died rapidly when placed on stroma.2 Pre-cDCs from BM, spleen, and tumors all possess the capacity to generate DC-d-Ms through a proliferating CD11c+ MHC class II+ cDCs intermediate. Whether lymphoid tissue cDC precursors committed to specific cDC lineages (eg, CD24+cDCs → CD8α+cDCs) can undergo this process merits further investigation.
DCs display enormous heterogeneity in phenotype, function, and response to pathogens.39-41 We consider the conversion of cDCs into DC-d-Ms to be beyond the usual scope of DC diversity, however. DC-d-Ms lose the morphologic, phenotypic, and functional features of cDCs. In addition, they express CD115 and high levels of F4/80, possess a high endocytosis capacity, and adhere tightly to plastic, features that typically define macrophages.42 Indeed, it is impossible to distinguish DC-d-Ms from macrophages in tumors without the ability to trace their origin in CD11c-Cre Rosa26-EGFP mice. Functionally, DC-d-Ms strongly suppressed T-cell responses through well-defined molecular mechanisms, and in this respect, resembled regulatory macrophages and MDSCs that occur in tumors and various chronic inflammatory conditions. It remains unclear whether differences exist between regulatory macrophages that arise from monocytes versus cDCs. In addition, their relative contribution to the immunosuppressive tumor microenvironment needs to be defined.
Inflammation recruits pre-cDCs into peripheral and tumor tissues.27 Recently, we reported that tumor pre-cDCs rapidly differentiate into proliferating cDCs, and under the influence of the tumor milieu, some of these cells generate a Gr-1+ cDCs subpopulation that induces T-cell anergy.36 In contrast with DC-d-Ms, Gr-1+ cDCs express CD11c and MHC class II and cannot directly suppress proliferation of T cells stimulated by third-party antigen-presenting cells. Most DC-d-Ms in tumors also express Gr-1, and could be classified as Gr-1+ MDSCs. Whether Gr-1+ cDCs represent an intermediate between proliferating Gr-1− cDCs and DC-d-Ms or an independent population remains unclear. An attempt to clarify their relationship in vitro proved futile because the Gr-1 epitope is unstable on cultured cells. However, high concentrations of GM-CSF in tumors blocked the development of both Gr-1+ cDCs and DC-d-Ms, suggesting that these populations are linked.
GM-CSF is a proinflammatory cytokine that promotes survival, proliferation, differentiation, and activation of myeloid cells; and induces generation of inflammatory DCs from hematopoietic stem cells and monocytes. The finding that GM-CSF prevents DC-d-M development is a novel aspect of this cytokine. Recent studies have shown that GM-CSF interacts with the GM-CSF receptor (GM-CSFR) to create a dodecamer complex, which activates Janus kinase 2 and downstream signaling cascades including signal transducer and activator of transcription (STAT), mitogen-activated protein kinase, and phosphoinositide-3 kinase.43,44 The diverse biologic functions of GM-CSF are regulated, at least partly, through concentration-dependent assembly of the receptor complex and phosphorylation of the common β-chain subunit that is shared by GM-CSF, IL-3, and IL-5 receptors.45 The concentration of GM-CSF required to prevent DC-d-M development in vitro in our study (5-50pM) was similar to the range reported to promote myeloid cell survival and proliferation (> 10pM).46 However our study suggests that the signal threshold for cDC proliferation is lower than that required to prevent DC-d-M differentiation. This relationship probably varies depending on the presence of other regulatory molecules, which may explain why DC-d-M develop in wild-type tumors despite the presence of relatively high GM-CSF levels. Our study also indicates that in vitro GM-CSF concentrations > 500pM decreases surface MHC class II expression, consistent with findings in a recent report.47 Although it is difficult to measure accurately GM-CSF concentrations in our tumor models, excessive GM-CSF signaling may have contributed to the lower MHC class II expression levels detected on cDCs from B16-GM-CSF tumors compared with those from wild-type tumors. Thus, the optimal GM-CSF concentration that prevents DC-d-M differentiation while maintaining high MHC class II expression levels is in the relatively narrow range of 50 to 500pM.
Restoration of DC homeostasis after an inflammatory process is obligatory to reduce tissue damage.48 The standard mechanisms that decrease DC number and function are cytotoxic T lymphocyte (CTL)–mediated killing and apoptosis induced by cytokine starvation.23,46 The finding that tumor cDCs differentiate into DC-d-Ms raises the intriguing possibility that tumors are coopting a normal regulatory process, which dampens immune responses. Preliminary data from our laboratory shows that DC-d-Ms are present in virus-infected lung tissues at late, but not early, stages of infection. It is tempting to speculate that during the resolution phase of inflammation, decreased production of GM-CSF permits proliferating cDCs in nonlymphoid tissues to differentiate into immunosuppressive DC-d-Ms. Notably, this process would probably occur with GM-CSF levels that exceed the threshold for apoptosis. Conversely, maintenance of high GM-CSF levels might thwart this regulatory process and amplify inflammation. In this regard, recent studies implicate GM-CSF released from Th17 cells as the key causative factor for perpetuating Th17-mediated encephalomyelitis.49,50 The inability to detect DC-d-Ms in normal mice suggests that it is not a major homeostatic mechanism for DCs during steady-state conditions. However, deficient GM-CSFR signaling may permit DC-d-M differentiation in noninflammatory conditions. In fact, GM-CSF and GM-CSFR knock-out mice have reduced DC numbers in peripheral tissues despite normal development of early DC progenitors in BM.7,12
Our finding that cDCs possess the capacity to generate regulatory macrophages highlights a new and challenging perspective of the current model of DC ontogeny. This unexpected flexibility of the DC lineage also provides a basis for novel approaches to manipulate immune responses in cancer, infectious disease, and autoimmunity.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank R. Gorczynski and N. Iscove for helpful comments and advice.
This work was supported by the Canadian Cancer Society, Heart and Stroke Foundation of Canada, Canadian Institutes for Health Research, Astellas Inc, and the Toronto General Hospital Transplant Program. A.M. and M.T. were recipients of Canadian Institutes of Health Research training awards.
Authorship
Contribution: J.D. designed and performed research, and drafted the paper; a.m. designed and performed research, M.T., H.G., and J.Z. performed experiments; and M.S.C. designed and supervised research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mark S. Cattral, Toronto General Hospital, Robert McEwen Building, 11c-1232, 585 University Avenue, Toronto, ON, Canada M5G 2N2; e-mail: mark.cattral@uhn.on.ca.