Abstract
We hypothesized that immune dysregulation, as represented by abnormal immunoglobulin (Ig) levels, may increase immune thrombocytopenia (ITP) severity. A cross-sectional analysis was performed encompassing patients with ITP seen at the New York Presbyterian Platelet Disorder Center in the past 10 years. The subjects' Ig levels were measured, and subjects were analyzed for differences in treatment response. Subjects with an IgA level greater than median had a significantly increased chance of failing to respond to standard treatment (steroids, intravenous Ig, and intravenous anti-D) than did subjects with an IgA level lower than median (37 of 271, 14%; vs 22 of 281, 8%; P = .03) and an increased risk for bleeding (36 of 378, 10%; vs 19 of 386, 5%; P = .02). Subjects with an IgM less than 56 (lower limit of normal) failed to respond to standard treatment more often than patients with a normal IgM (12 of 67, 18%; vs 44 of 467, 9%; P = .05) with a trend toward worsened response to splenectomy (3 of 18, 17%; vs 36 of 86, 42%; P = .06). These observations suggest that immune dysregulation, as represented by elevations in IgA or decreased levels of IgM, are associated with ITP that is more resistant to treatment.
Introduction
Immune thrombocytopenia (ITP) is a disorder of antibody-mediated destruction and inhibition of production of platelets.1 In general, this is an idiopathic disease, and it is unclear what immune factors relate to disease predisposition, severity, and especially response to treatment.2 We hypothesize that immune dysregulation, as represented by elevated or decreased serum immunoglobulin (Ig) levels, may increase disease severity as represented by failure to respond to treatment. These alterations in Ig levels may represent an inflammatory or activated immune state that makes the disease more difficult to control with specific treatments.
Certain patient populations with known immunologic disorders have an increased incidence of ITP, supporting the concept that immune dysregulation may contribute to the development of ITP. Patients with common variable immunodeficiency (CVID) with low IgG, IgA, and/or IgM levels have a 22% incidence of autoimmune diseases, such as ITP,3 supporting the association of abnormal Ig levels with ITP. In addition, current guidelines recommend the evaluation of baseline Ig levels in patients with ITP given the possible association with CVID.4 IgA deficiency is also related to ITP in perhaps 1% to 2% of cases of ITP, but this may be primarily in those cases with concomitant IgG subclass deficiency.5,6
The role of circulating IgA in systemic immunity and its relationship to mucosal inflammation is uncertain. Elevations in IgA may represent aberrations in mucosal immunity, leading to systemic autoimmune effects. The clear relation between Helicobacter pylori infection and immune thrombocytopenia7 suggests a possible association between mucosal inflammation and immune-mediated destruction of platelets.
This study explored the database of the Platelet Disorders Center at Weill Medical College of Cornell University and looked at patients who had Ig levels measured at presentation to the Center. These levels were then related to clinical outcomes, including response to treatment as reported at the initial visit.
Methods
A cross-sectional analysis of the Platelet Disorder Center patient list was performed, encompassing patients with ITP seen at the New York Presbyterian Platelet Disorder Center in the past 10 years. The data include demographics at presentation to the center, history of use of and response to treatments, and certain selected laboratory values, including levels of Ig and platelet count at first presentation to the Center. Institutional review board approval was obtained from the Weill Cornell Medical Center for the study, and informed consent was obtained from patients to allow their information to be used in this study in accordance with the Declaration of Helsinki.
The subject's IgA, IgM, and IgG levels and platelet count were measured, and subjects were analyzed for differences in history of response to standard treatment and history of bleeding. Standard treatment was defined as treatment with steroids, intravenous Ig (IVIG), and intravenous anti-D. Response to treatment was defined as an improvement in platelet count of at least 50. Subjects who had not responded to any of those agents or a combination of these agents were defined as failing standard treatment.
Subjects were categorized as below the lower limit of normal, within normal limits, or above the upper limit of normal or as above or the below the median for IgA, IgM, and IgG level, and the frequency of failure to respond to treatment, the frequency of major bleeding events, and platelet count were analyzed. Comparisons of frequencies among groups were made using the Kruskal-Wallis test for ordinal variables, the Fisher exact test for categorical variables. The Wilcoxon rank-sum test was used for quantitative variables. For all analyses, a P value less than .05 was considered statistically significant. The analysis was performed using SAS Version 9.2 and STATA Version 10.1 statistical software.
Results
Baseline demographics
In total, data were available for 946 subjects with ITP of which 775 had baseline IgA, IgM, and IgG levels. The population was predominantly female (62%; data were missing in 1 case) with a median age of 30.0 years (range, 1-94 years; data were missing in 5 cases).
The IgA median was 166 mg/dL (range, 6.67-1535 mg/dL). A total of 107 subjects (13.8%) had an elevated IgA and 114 subjects (14.7%) had a low IgA (normal range, 70-312 mg/dL). Median ages increased from 13 years (range, 1-82 years) to 29 years (range, 1-94 years) and to 50 years (range, 4-86 years) as IgA levels increased (below the normal range, within the normal range, and above the normal range, respectively; P < .0001).
The IgM median was 114 mg/dL (range, 7-1550 mg/dL). A total of 29 subjects (3.7%) had an elevated IgM and 85 subjects (11.0%) had a low IgM (normal range, 56-352 mg/dL).
The IgG median was 1120 mg/dL (range, 128-6710 mg/dL). A total of 245 subjects (31.6%) had an elevated IgG and 66 subjects (8.5%) had a low IgG (normal range, 639-1349 mg/dL).
Subjects were treated with steroids, IVIG, or intravenous anti–D or had received combinations of these treatments (Table 1). A total of 223 (29%) patients did not receive any of the 3 drugs, so for the analyses related to response to standard treatment our sample size was reduced to 552 patients. The information on major bleeds was missing in 11 (1%) patients.
Higher IgA levels associate with treatment failure and with increased major bleeding but not platelet count
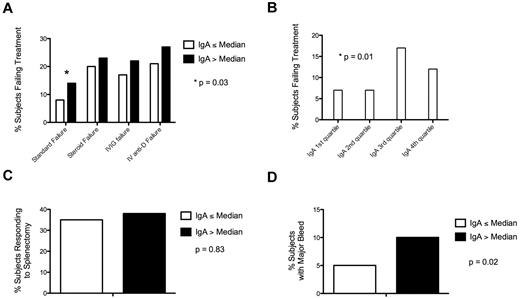
Subjects with an IgA level greater than the median had a significantly increased chance of failing to respond to standard treatment (steroids, IVIG, and intravenous anti-D) than patients with an IgA level equal to or lower than median (37 of 271, 14%; vs 22 of 281, 8%; P = .03) and a nonsignificant tendency toward failure to respond to individual treatments (steroids: 49 of 210, 23%; vs 39 of 196, 20%; P = .47; IVIG: 42 of 192, 22%; vs 35 of 207, 17%; P = .25; intravenous anti-D: 36 of 131, 27%; vs 30 of 146, 21%; P = .20; Figure 1A).
Elevated IgA is associated with treatment failure and bleeding. (A) Subjects with an IgA greater than median had a significantly increased chance of failing to respond to standard treatment relative to subjects with an IgA less than median. (B) Subjects with an IgA in the third quartile were most likely to fail to respond to standard therapy. (C) Subjects with an IgA greater than median had no difference in response to splenectomy than subjects with an IgA less than median. (D) Subjects with an IgA greater than median had a higher proportion of major bleeding than subjects with an IgA less than median.
Elevated IgA is associated with treatment failure and bleeding. (A) Subjects with an IgA greater than median had a significantly increased chance of failing to respond to standard treatment relative to subjects with an IgA less than median. (B) Subjects with an IgA in the third quartile were most likely to fail to respond to standard therapy. (C) Subjects with an IgA greater than median had no difference in response to splenectomy than subjects with an IgA less than median. (D) Subjects with an IgA greater than median had a higher proportion of major bleeding than subjects with an IgA less than median.
When IgA and response were analyzed by quartiles, the third quartile had the highest proportion of subjects not responding to standard treatment (first quartile: 9 of 137, 7%; second quartile: 10 of 139, 7%; third quartile: 23 of 133, 17%; fourth quartile: 17 of 143, 12%; P = .01; Figure 1B).
Among those subjects with splenectomy, for whom splenectomy response data were available (39 of 104, 38% subjects responded to splenectomy), there was no difference in response between subjects with an IgA level greater than median relative to subjects with an IgA less than median (28 of 73, 38%; vs 11 of 31, 35%; P = .83; Figure 1C).
Subjects with an IgA greater than median also had an increased risk of having a major bleed (36 of 378, 10%; vs 19 of 386, 5%; P = .02; Figure 1D). The median platelet count was not statistically different in subjects with an IgA above median relative to subjects with an IgA below the median (61 K/μL vs 64 K/μL; P = .16). There was also no difference in platelet count for subjects with an IgA below the normal range, within the normal range, or above the normal range (60 K/μL vs 63 K/μL vs 61 K/μL; P = .99).
Given the concern that higher IgA levels may associate with older age and confound the results, we measured the failure to respond by IgA in subjects 65 years of age or older and subjects younger than 65 years. Subjects older than 65 years had a nonsignificant increase in risk of failure to respond to standard treatment if IgA was greater than median relative to subjects with an IgA less than or equal to the median (13 of 49, 27%; vs 7 of 42, 17%; P = .31). This trend was similar in subjects younger than 65 years (22 of 222, 10%; vs 15 of 239, 6%; P = .09).
Low IgM levels associate with treatment failure and platelet count but not major bleeding
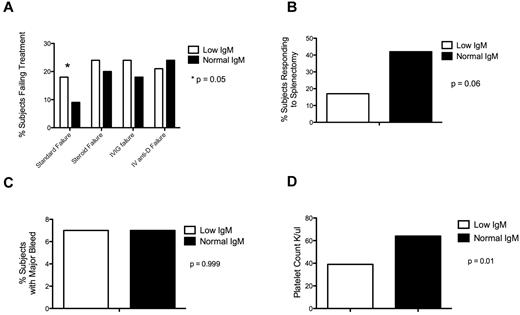
Subjects with an IgM less than 56 (lower limit of normal) were more likely to fail to respond to standard treatment than patients with a normal IgM (12 of 67, 18%; vs 44 of 467, 9%; P = .05; Figure 2A) but no significant difference in failure to respond to any particular single treatment (steroids: 12 of 49, 24%; vs 71 of 346, 20%; P = .57; IVIG: 13 of 53, 25%; vs 60 of 330, 18%; P = .26; intravenous anti-D: 7 of 34, 21%; vs 55 of 232, 24%; P = .83).
Low IgM is associated with treatment failure and low platelet count. (A) Subjects with a low IgM were more likely to fail to respond to standard treatment. (B) Subjects with a low IgM had a trend toward decreased response to splenectomy. (C) There was no difference in frequency of major bleed based on IgM level. (D) Subjects with low IgM had a lower median platelet count than subjects with a normal IgM.
Low IgM is associated with treatment failure and low platelet count. (A) Subjects with a low IgM were more likely to fail to respond to standard treatment. (B) Subjects with a low IgM had a trend toward decreased response to splenectomy. (C) There was no difference in frequency of major bleed based on IgM level. (D) Subjects with low IgM had a lower median platelet count than subjects with a normal IgM.
Among those subjects with splenectomy, for whom splenectomy response data were available (37 of 99, 38% subjects responded to splenectomy), subjects with a low IgM had no difference in response to splenectomy relative to subjects with normal IgM, although there appeared to be a trend toward decreased response in subjects with low IgM (3 of 18, 17%; vs 34 of 81, 42%; P = .06; Figure 2B).
There was no difference in rates of major bleed in subjects with low IgM relative to subjects with normal IgM (6 of 83, 7%; vs 47 of 652, 7%; P = .999; Figure 2C), despite a significant increase in median platelet count between subjects with low and normal IgM levels (39 K/μL vs 64 K/μL; P = .01; Figure 2D).
To account for the possible confounder of decreased IgM in subjects who had previously undergone splenectomy, treatment failure at low IgM was measured in the subset of patients who had not received a splenectomy. Subjects who still had their spleen had a nonsignificant trend toward more likely to fail to respond to treatment if IgM was low than patients with a normal IgM (7 of 43, 16%; vs 25 of 349, 7%; P = .07). For subjects that had undergone splenectomy, there was a similar nonsignificant trend toward more failure for subjects with a low IgM relative to subjects with a normal IgM (5 of 24, 21%; vs 19 of 117, 16%; P = .56).
IgG levels associate with platelet count but not with major bleeding or treatment failure
A significant difference in median platelet count was seen in subjects with IgG levels outside the normal range (IgG low: 49 K/μL; IgG normal: 69 K/μL; IgG high: 54 K/μL; P = .01). However, a significant difference in major bleeding was not seen between subjects with low and normal IgG levels (4 of 66, 6%; vs 30 of 458, 7%; P = .999) or between subject with high and normal IgG levels (21 of 240, 9%; vs 30 of 457, 7%; P = .29)
Subjects with an IgG below the normal range did not have a significantly increased rate of treatment failure relative to subjects with normal IgG (8 of 46, 17%; vs 31 of 314, 10%; P = .13). Subjects with an IgG above the normal range also did not have a significantly increased rate of treatment failure relative to subjects with an IgG within the normal range (20 of 192, 10%; vs 31 of 314, 10%; P = .999).
High IgA levels and low IgM levels associate together
For subjects with high IgA, there is a greater proportion of subjects with low IgM relative to subjects with elevated IgM (10 of 107, 9%; vs 5 of 107, 5%; P = .0001).
Treatment failure and abnormalities in Ig but not platelet count or bleeding are more frequent in subjects 65 years of age or older
Subjects 65 year of age and older were more likely to fail to respond to standard treatment than patients younger than 65 years (20 of 91, 22%; vs 39 of 458, 9%; P = .004). Subjects older than 65 years were also more likely to have a failure to respond intravenous anti-D (15 of 35, 43%; vs 51 of 240, 21%; P = .005) and steroids (23 of 72, 32%; vs 65 of 332, 20%; P = .02), but not IVIG (15 of 65, 23%; vs 62 of 333, 19%; P = .41).
A higher proportion of subjects 65 years of age and older had IgA levels above the normal range relative to subjects less than 65 (24 of 120, 20%; vs 82 of 650, 12%; P = .0001). Subjects 65 years of age and older were also found to have a higher median IgA (183 mg/dL vs 161 mg/dL; P = .001).
Although there was no significant difference between median IgM in patients 65 years of age or older versus younger patients (96 mg/dL vs 115 mg/dL; P = .09), subjects older than 65 years were more likely to have elevated IgM (11 of 120, 9%; vs 18 of 650, 3%; P = .001) and low IgM relative to subjects younger than 65 years (21 of 120, 18%; vs 64 of 650, 10%; P = .001).
Interestingly, in subjects 65 years of age or older compared with subjects younger than 65 years, there was no significant difference in median platelet count (54 vs 64; P = .20) or history of major bleeding (6 of 118, 5%; vs 48 of 641, 7%; P = .44).
Patients with IBD but not H pylori had higher IgA levels than the general population
Four subjects had known inflammatory bowel disease (IBD). For those patients, all had IgA levels higher than the median of the general population. For IBD patients, the median IgA level was 241.5 (range, 193-395). Three patients with IBD responded to standard treatment. The other one was not treated.
However, of the 30 patients treated for H pylori, the median IgA level was 150 (range, 8-498). Two (7%) of the patients infected with H pylori failed to respond to standard treatment, 24 (80%) responded to standard treatment, and 4 (13%) patients were not treated.
Discussion
In this cross-sectional analysis of a single center ITP database, 2 populations of patients were identified that appear to have ITP that is more treatment resistant. The first population of patients had a low level elevation in IgA and were more resistant to standard medical treatment but with no difference in response to splenectomy. The second population had a low IgM and was more likely to fail standard treatment and had a trend to worse response to splenectomy.
Although serum IgA has previously been demonstrated to be elevated in patients with ITP,8 the role of IgA in patients with ITP has never been addressed beyond the finding that approximately 1% of patients with ITP have IgA deficiency and another 1% have CVID. In this study, more than 10% of patients with ITP were found to have an elevated serum IgA. Although there was not a dramatic association of increased IgA with clinical findings, several potentially important trends emerged linking increased IgA levels to more resistant ITP. Subjects with IgA above median are less likely to respond to standard treatment and are more likely to have a major bleed than patients with an IgA below median. However, platelet counts were not found to be significantly different in subjects with low, normal, or high IgA or when analyzed in patients with an IgA level above or below the median, suggesting an alternative mechanism for bleeding beyond platelet count.
As previously described,9 an association was found between age and IgA levels. A nonsignificant trend toward more resistant disease in patients with an IgA greater than median was seen in the subset of elderly patients, the trend was also seen in the subset of patients younger than 65 years, although the numbers were not great enough to reach significance in either subset. One possible connection may be that elevations in serum IgA are linked to abnormalities in the gastrointestinal-associated mucosal immune system (possibly with concomitant inflammation) that are not clinically apparent. Perhaps increased intestinal immune dysfunction in the elderly population puts them at increased risk for resistant ITP. This role of the gastrointestinal-associated mucosal immune system in ITP may be supported by the observation that increases in serum IgA were observed in all subjects known to have the autoimmune disease IBD, although not in subjects with H pylori infections; however, the absolute number of patients with these comorbid diagnoses was low, making it difficult to draw a clear conclusion.
Surprisingly, although there appears to be an association between modest elevations in IgA and resistant disease, those patients with the highest levels of IgA did not have a significantly increased risk of failing to respond to standard treatment relative to the general population. Indeed, it was patients who had an IgA level in the third quartile that had the highest proportion of nonresponders. We think that minor elevations in IgA represent an inflammatory state that may be more difficult to address with standard treatments for ITP. Further investigation into the role of IgA in the pathology of refractory ITP appears warranted and needs to be linked to genetic analysis.
The population of subjects with low IgM also appeared to have ITP more resistant to standard treatment and possibly to splenectomy. The low IgM may represent a low-level, subclinical immune dysregulation similar in phenotype to CVID. Neither IgG subclasses nor specific antibody titers were performed in these patients. As with elevations in IgA, low levels of IgM were more common in subjects older than 65 years. Interestingly, lower platelet counts were observed in patients with low IgM but no significant increase in bleeding, further supporting alternative causes for bleeding beyond absolute platelet count.
Unfortunately, the failure to identify a similar association in response to ITP therapy with IgG levels may be the result of IVIG treatment confounding IgG levels. It was not possible to eliminate these patients with certainty because many patients in the series were one-time referrals in whom the past history was imprecise. Of note, an association was noted between IgG levels and platelet count. Patients with either low or high IgG levels were noted to have significantly lower platelet counts than patients with normal IgG levels. This difference in platelet count did not correspond to an increased risk of bleeding.
Taken together, these findings suggest that immune dysregulation, as represented by minor elevations in IgA or decreased levels of IgM, are associated with ITP that is more resistant to treatment. These abnormalities were more commonly seen in subjects older than 65 years and may be associated with the different pathology of ITP in elderly patients relative to younger patients. Given the increased risk of major bleeding in subjects with IgA greater than the median but lack of difference in platelet count, an alternative mechanism for bleeding in this population may exist. Given the limitations of a cross-sectional analysis, further investigation into possible mechanisms by which these immune abnormalities influence the disease course, response to treatment and bleeding could contribute to a deeper understanding of the pathophysiology of ITP and lead to the development of more rational immune therapy for ITP.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: J.E.A. designed the research, analyzed the data, and wrote the manuscript; J.B.B. generated the database, analyzed data, and wrote the manuscript; and F.C. and D.N. performed statistical analysis.
Conflict-of-interest disclosure: J.B.B. currently receives clinical research support from the following companies: Amgen, Cangene, GlaxoSmithKline, Genzyme, IgG of America, Immunomedics, Ligand, Eisai Inc, Shionogi, and Sysmex. His family owns stock in Amgen and GlaxoSmithKline. He has participated in Advisory Boards for Amgen, GlaxoSmithKline, Ligand, Shionogi, and Eisai Inc. He also had a 1-day consult with Portola. The remaining authors declare no competing financial interests.
Correspondence: Jon E. Arnason, Hematologic Malignancy and Bone Marrow Transplantation Program, Beth Israel Deaconess Medical Center, 330 Brookline Ave, Boston, MA, 02215; e-mail: jarnason@bidmc.harvard.edu.