Abstract
Immunoglobulin light-chain (AL) amyloidosis is a rare, incurable plasma cell disorder. Its therapy has benefited immensely from the expanding drug armamentarium available for multiple myeloma. Pomalidomide in combination with weekly dexamethasone (Pom/dex) is active among patients with relapsed myeloma. In the present study, we explored the Pom/dex combination in patients with previously treated AL. Patients were eligible for this prospective phase 2 trial if they had had at least one prior regimen and if they had reasonably preserved organ function. Patients were treated with oral Pom/dex. Thirty-three patients were enrolled. The median age was 66 years. Median time from diagnosis to on-study was 37 months. Eighty-two percent had cardiac involvement. The confirmed hematologic response rate was 48%, with a median time to response of 1.9 months. Organ improvement was documented in 5 patients. The median overall and progression-free survival rates were 28 and 14 months, respectively; the 1-year overall and progression-free survival rates were 76% and 59%, respectively. There was a discordance between the hematologic response and the N-terminal pro-brain natriuretic peptide response. The most common grade 3-5 adverse events, regardless of attribution, were neutropenia and fatigue. We conclude that pomalidomide appears to be a valuable drug covering an unmet clinical need in patients with previously treated AL. The trial is registered at www.clinicaltrials.gov as NCT00558896.
Introduction
Immunoglobulin light-chain (AL) amyloidosis is a rare, incurable plasma cell disorder. Historically, the most effective therapies have been melphalan-based, either low-dose melphalan with corticosteroids or high-dose melphalan with peripheral blood stem cell support.1-4 In the past decade, thalidomide, lenalidomide, and bortezomib have also been shown to have activity in patients with AL.5-16 Single-agent pomalidomide has activity in patients with myeloma,17 but emerging data suggest that the combination with a corticosteroid is superior.18 Pomalidomide in combination with weekly dexamethasone (Pom/dex) is active among patients with relapsed multiple myeloma who have received 3 or fewer prior regimens with response rates of 63%. Responses can be achieved in 31% of lenalidomide-refractory19 and in 25%-29% of lenalidomide- and bortezomib-refractory myeloma patients.20
These observations prompted the current prospective clinical trial to test the safety and efficacy of Pom/dex in patients with AL amyloidosis. Although the addition of dexamethasone has the potential to add morbidity, the combination was chosen to maximize the likelihood of response. The primary goal of this phase 2 trial was to assess hematologic response to Pom/dex therapy among patients with previously treated AL amyloidosis. Secondary goals included determining toxicity, duration of response, progression-free survival (PFS), and overall survival (OS). Because there is a question of dose responsiveness to Pom, individual patient dose escalation was allowed.
Methods
The protocol was approved by the Mayo Clinic Institutional Review Board, and this prospective phase 2 trial was conducted according to the Declaration of Helsinki. The trial is registered at www.clinicaltrials.gov as NCT00558896. Patients were required to have previously treated, symptomatic AL amyloidosis and to have measurable hematologic disease as defined by any of the following: serum M-protein ≥ 1 g/dL, urine M-protein ≥ 200 mg/24 hours, or serum Ig free light chain (FLC) ≥ 10 mg/dL, along with an abnormal FLC ratio. Patients had to be at least 18 years of age and willing to provide written informed consent, return to Mayo Clinic for follow-up, follow RevAssist contraception and pregnancy testing guidelines, and take aspirin or alternate prophylactic anticoagulation. Patients were required to have an absolute neutrophil count ≥ 1000/μL, platelet count ≥ 75 000/μL, creatinine ≤ 2.5 mg/dL, and to have discontinued all previous chemotherapy, including investigational therapy, for at least 2 weeks before registration. Patients were excluded if they had uncontrolled infection, another active malignancy, serum troponin T greater than 0.1 ng/mL, active thromboembolism that had not been anticoagulated therapeutically, known HIV or hepatitis infection, grade 3 or 4 peripheral neuropathy, or were a New York Heart Association (NYHA) class III-IV.
Patients were treated with pomalidomide 2 mg by mouth daily for 28 days (1 cycle) along with dexamethasone 40 mg by mouth once weekly. Aspirin (325 mg daily) was used as routine thromboprophylaxis. Patients were followed monthly with blood and urine tests, toxicity evaluations, and hematologic assessment. Echocardiography was performed quarterly for those patients with baseline cardiac involvement.
Dose modifications were based on adverse events (AEs), which were graded according to the Cancer Therapy Evaluation Program Common Terminology Criteria for Adverse Events (CTCAE; Version 3.0). Patients were assessed for toxicity every 4 weeks. The daily dose of pomalidomide could be increased at the treating physician's discretion in the absence of grade 3 or higher side effects to 4 mg daily in patients who had not achieved a 25% reduction in serum or urine monoclonal protein levels after 2 cycles of therapy or at time of progression.
Dose reductions of pomalidomide and dexamethasone were made according to AE type and attribution. The starting pomalidomide dose was 2 mg/d on days 1-28, and all dose reduction levels moved to the day 1-21 every 28 day schedule, with dose levels −1, −2, −3, and −4 at 2, 1.5, 1.0, and 0.5 mg/d, respectively. The starting dexamethasone dose was 40 mg/d on days 1, 8, 15, and 22 every 28 days. Dose levels −1, −2, −3, −4, and −5 used the same schedule, but at doses of 20, 12, 8, and 4 mg/d and discontinue, respectively.
Hematologic and organ-response criteria were as described previously21 but with 2 modifications: (1) all serum FLC measurement responses were based on the difference of the involved and the uninvolved FLC, and (2) the very good partial response (VGPR) category was used, which was defined as a 90% reduction in the difference of the involved and uninvolved serum immunoglobulin FLC.
All analyses were based on the intent-to-treat principle. The primary end point for this study was the proportion of confirmed hematologic responses (complete response, VGPR, or partial response [PR]). The study used a single-stage phase 2 design based on the binomial distribution to test the hypothesis that the true confirmed response rate was at most 5% versus the alternative that it was at least 20%, with a significance level of 0.07 and 91% power. The regimen would be declared ineffective if a maximum of 3 confirmed responses were observed in the first 32 evaluable patients. Secondary end points included OS, PFS, duration of response, and AE profile. For the PFS analysis, the protocol definitions of progression events were as follows: (1) a patient with 1 cycle of unconfirmed progression who went off-study, (2) any confirmed progression, or (3) a death without documented progression. Those patients who progressed and were dose increased were not counted as progression until they progressed at the increased dose.
Exact binomial confidence intervals (CIs) were constructed for the primary end point of confirmed hematologic response. The distributions of OS (time from study entry to death) and PFS (time from study entry to disease progression or death) were estimated using the Kaplan-Meier method in which differences between groups were assessed using log-rank tests. A landmark analysis (at 3 months from registration) was performed to evaluate differences in OS between responders and nonresponders. Simple descriptive statistics were used to summarize the AE profile and baseline characteristics.
The relationships of risk factors with early drug discontinuation (within 6 months) was explored using logistic regression and Cox proportional hazard models, respectively. Potential risk factors included left ventricular septal thickness, left ventricular ejection fraction, numbers of organs involved (counting only the major organs: heart, kidney, liver, and nerves), NYHA class, troponin, N-terminal pro-brain natriuretic peptide (NT-proBNP), and Mayo cardiac stage. Optimal cutpoints for survival analyses were determined using the Contal and O'Quigley method.22
Results
Baseline characteristics
Between November 2008 and November 2010, 33 patients were enrolled in the study. The data cutoff for this manuscript was January 3, 2012. Details of baseline characteristics of the 33 patients are presented in Table 1. The median age was 66 years (range, 52-82) and 58% were male. All patients had received prior treatment; 16 had undergone high-dose chemotherapy with peripheral blood stem cell transplantation. The median time from diagnosis to on-study was 37 months. Including only cardiac, liver, renal, and nerve involvement in the organ count, the median number of organs involved was 1 (range, 0-3). In addition to these, other involved organs included the gastrointestinal tract (n = 4), skin/soft tissue (n = 12), pulmonary organs (n = 1), and muscles (n = 1). A majority (82%) of patients had cardiac involvement. Twenty-five percent had Mayo cardiac stage III disease, defined as both a troponin T of ≥ 0.035 μg/L and an NT-proBNP ≥ 332 ng/L.23,24
Response
Of the 33 patients, there were 16 confirmed responders (48%; 95% CI, 30-66; Table 2). Hematologic response was assessable by FLC in 24 patients, by serum M-protein in 7, and urine M protein in 2. The median time to hematologic response was 1.9 months (range, 0.9-11.3) and the median duration of response was 19 months (95% CI, 8.3 to not attained [NA]). Hematologic responses to pomalidomide were seen in 47% (7 of 15) of patients who had prior lenalidomide therapy and in 43% (6 of 14) of patients who had prior bortezomib. Organ improvement was documented in 5 patients, 4 of the 27 patients with cardiac involvement and 2 of the 12 patients with renal involvement. The one patient with liver involvement did not have an organ response. All patients with organ improvement also had hematologic response: 1 VGPR and 4 PRs. Of the hematologic responders, 6 remain on-study, 5 have progressed (3 organ progressions and 2 hematologic progressions), 2 have died on-study, and 3 went off treatment either because of AE or other reasons.
As a posthoc analysis, we evaluated the newly proposed consensus cardiac response criteria of a 30% reduction in NT-proBNP.25,26 The baseline cardiac characteristics of the 4 cardiac responders are shown in Table 3. None would have qualified as a cardiac response using the proposed criteria and 2 would have been considered “NT-proBNP progressions,” meaning that they had a 30% increase in baseline NT-proBNP with a minimum increase of 300 ng/L on 2 occasions. Of the 29 patients alive at 3 months from registration with NT-proBNP baseline and subsequent measures, 18 patients satisfied criteria for “NT-proBNP progression.” Nine (50%) patients with a 30% increase in NT-proBNP had a hematologic response (≥ PR) compared with 6 (55%) patients without a 30% increase.
Survival outcomes
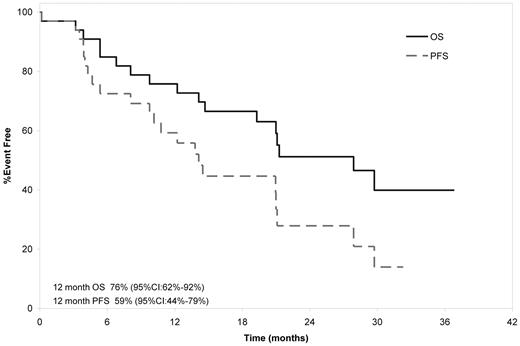
With a median follow-up of surviving patients of 28.1 months (range, 14.1-37.8), 17 patients have died, 6 of whom died on-study. The causes of death for these 6 were progressive amyloid in 4 patients, H1N1 infection in 1, and sudden death at cycle 23 in another. The median OS was 27.9 months with a 1-year OS rate of 76% (Figure 1). The median PFS was 14.1 months with a 1-year PFS of 59%.
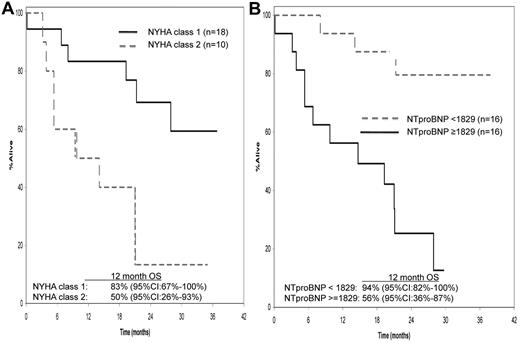
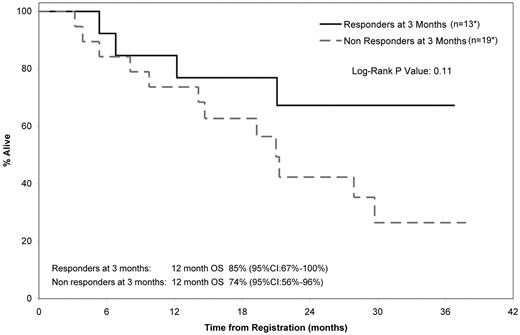
The only baseline risk factors that predicted for OS included were NYHA class (hazard ratio 1 vs 2 = 0.240; 95% CI, 0.081-0.715; P = .01; Figure 2A) and NT-ProBNP (hazard ratio ≥ 1829 vs < 1829, 8.709; 2.404-31.554; P = .001), using a Contal and O'Quigley defined cutoff point of 1829 pg/mL (Figure 2B). A landmark analysis (at 3 months from study entry) was performed to evaluate differences in OS between responders and nonresponders. Of the 32 patients who were alive at 3 months from registration, 13 achieved a confirmed hematologic response and 19 did not achieve a confirmed response. The median OS for responders and nonresponders was not attained and 21 months, respectively (Figure 3), and the 12-month OS rates were 85% (95% CI, 67%-100%) and 74% (95% CI, 56%-96%), respectively. A second landmark analysis was performed including only those patients who were assessable by FLC measurement. Twenty-seven patients were assessable for FLC (difference between κ and λ > 5 mg/dL). Thirteen of 27 patients (48%) had a 50% decrease in FLC within the first 3 months of treatment (median decrease, 62%; range, 50%-81%). For those with and without a 50% decrease, the respective median OS was not attained and 27.9 months (95% CI, 9.8-NA), respectively (log rank P = 0.64).
Factors influencing overall survival. NYHA class (A) and NT-proBNP measurement (B) predictors for OS.
Factors influencing overall survival. NYHA class (A) and NT-proBNP measurement (B) predictors for OS.
Landmark analysis (at 3 months from study entry) of OS between responders and nonresponders. *One patient died before 3 months and was therefore excluded; 3 patients responded after the 3-month cutoff, confirmed in cycles 7, 13, and 20.
Landmark analysis (at 3 months from study entry) of OS between responders and nonresponders. *One patient died before 3 months and was therefore excluded; 3 patients responded after the 3-month cutoff, confirmed in cycles 7, 13, and 20.
An additional posthoc analysis was performed assessing outcomes based on “NT-proBNP progression.”26 For patients with and without a 30% increase, the median OS was 19.3 months (95% CI, 6.8-NA) and not attained, respectively (log rank P = .04).
Toxicity/retention
Only 6 patients remain on therapy. The reasons for stopping treatment included disease progression (n = 9), death (n = 6), patient refusal (n = 5), AE, or physician's discretion (n = 3 each) and alternate treatment (n = 1). On univariate logistic regression, the baseline characteristics that predicted for withdrawal within 6 months were NYHA class (odds ratio, 0.165; 95% CI, 0.03-0.90; P = .04) and left ventricular ejection factor (odds ratio, 1.109; 95% CI, 1.015-1.211; P = .02). A documented increase in NT-proBNP in the first 3 months did not predict for trial retention with a median time on treatment for patients with and without a 30% increase of 7.3 months (range, 3.1-32.3) versus 5.4 months (range, 1.9-30.6).
Overall, the common grade 1-5 AEs regardless of attribution were fatigue (n = 32), anemia (n = 31), neutropenia (n = 21), thrombocytopenia (n = 19), cardiac arrhythmia (n = 7), pneumonia/upper respiratory infection (n = 16), sensory neuropathy (n = 29), diarrhea (n = 16), nausea (n = 13), and anorexia (n = 11; Table 4). The most common AEs at least possibly attributable to Pom/dex were fatigue (n = 27), anemia (n = 26), neutropenia (n = 18), thrombocytopenia (n = 17), cardiac arrhythmia (n = 2), infection (n = 9), sensory neuropathy (n = 10), nausea (n = 7), edema (n = 6), and dyspnea (n = 5).
Overall, the most common body systems with grade 3-5 AEs regardless of attribution were hematologic (n = 15), cardiovascular (n = 10), infectious (n = 9), metabolic/laboratory (n = 7), and constitutional (n = 6); specifically, the most common grade 3-5 AEs were neutropenia (n = 10), pneumonia/bronchitis (n = 7), and fatigue (n = 6). Two patients developed thrombosis. The most common grade 3-5 AEs at least possibly attributable to Pom/dex were neutropenia (n = 9) and fatigue (n = 5).
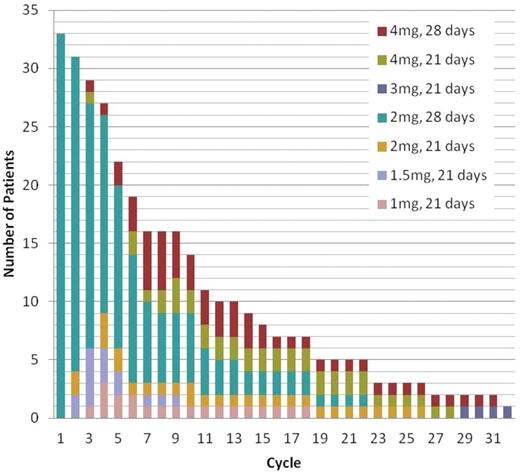
A total of 330 cycles of pomalidomide have been delivered with the median targeted dose being 100% (range, 0%-200%), Figure 4. Nine patients had protocol-directed increases in the pomalidomide dose, 8 of whom had increases to 4 mg for lack of response. Three patients had initial dose reduction, but re-escalation for lack of response, 2 of whom increased to 4 mg. Of the 9 whose doses were increased, 7 have ended treatment (because of progression in 4 patients, physician discretion in 2, and death in 1). One patient had her hematologic response upgraded from no response to PR on increasing the pomalidomide dose from 2-4 mg. Another patient had initially achieved a PR to Pom/dex by cycle 2, but had signs of progression in cycle 6. Increasing the dose to 4 mg on days 1-21 at cycle 8 resulted in a PR by cycle 12.
Sixteen patients had pomalidomide dose reductions. The most common reason for decreasing the dose was neutropenia (n = 8). A total of 314 cycles of dexamethasone have been given, with a median of 50% of targeted dose delivered. Twenty-seven patients had dose reductions, the most common reason being confusion/mood alteration (n = 12).
Discussion
The results of the present study indicate that Pom/dex is active in relapsed AL amyloidosis, with an overall hematologic response rate of 48%. This response rate compares favorably to other treatment options for patients with AL, including thalidomide-dexamethasone (48%),27 lenalidomide-dexamethasone (38%-47%),8,9,11 and cyclophosphamide-lenalidomide-dexamethasone (40%-60%).28,29 Response rates with cyclophosphamide-thalidomide-dexamethasone30 and bortezomib-dexamethasone12,31 are higher at 80%-94%, but the duration of response and long-term survival have not yet been defined with these regimens, with median follow-up times typically being less than 2 years. Hematologic response rates were also rapid, with half of responding patients doing so in within 2 months. Remarkably, hematologic responses to pomalidomide were seen in 40%-50% of patients who had prior lenalidomide or bortezomib.
Organ response rates were relatively low, with only 15% of patients having documented organ response, but this organ response rate was not dissimilar from the 0%-26% organ response rate reported with other regimens tested in relapsed AL patients.8,9,11,12,27-29,31 The low organ response rates found previously may be in part a function of short follow-up. In the present study, which had more than 80% of patients with cardiac involvement, it may be a reflection of the shortcomings of echocardiographic response measurement. Interpreting the role that changes in NT-proBNP had among patients treated with Pom/dex was challenging. There was a trend toward better outcomes among patients who did not have an increase in NT-proBNP in the first 3 months of treatment; however, 9 of these patients with 30% increases in NT-proBNP criteria had hematologic response, 4 of which were VGPR or better. This paradox raises the question of whether these patients who achieved hematologic response but NT-proBNP progression would have fared better had they been removed from the study early. This question cannot be answered by this small phase 2 trial, but raises concerns about the NT-proBNP response criteria for immunomodulatory drug–based trials.
The toxicity profile of the regimen was manageable, with the most common AEs being modest myelosuppression, fatigue, sensory neuropathy, mild gastrointestinal distress and anorexia, and upper respiratory tract infections. Thrombosis was uncommon, as was renal toxicity. The incidence of rash was less than what has been observed with lenalidomide.32 The sensory neuropathy was grade 3-4 in only 2 patients and both of them had preexisting peripheral neuropathy. Only 3 patients discontinued therapy because of AEs. Similar to what has been seen in other studies of AL patients taking immunomodulatory drugs,11,33-35 the NT-proBNP reduction did not follow the hematologic response like it does with treatment with alkylator-corticosteroid combinations or bortezomib with or without corticosteroids.14,36
Because the best dose and schedule of Pom/dex had not been established at the time of study design, physicians were allowed to increase the pomalidomide dose in patients who were either progressing or not responding. This proved to be of value in 9 (27%) of the patients so treated for a median of 9 cycles (range, 1-30). Although the study was not designed to address optimal dosing of the combination, 2 mg of pomalidomide daily appears to be a reasonable dose because 50% more patients decreased rather than increased their dose. Additional studies need to be performed to discern whether a starting dose of 4 mg daily for days 1-21 every 28 days would be a better regimen both in terms of toxicity and efficacy, as has been explored in multiple myeloma.18,37
How generalizable are our data and how does Pom/dex compare with other regimens? These are difficult questions to address in a small phase 2 study. Although the median number of organs involved was 1, cardiac involvement was overrepresented and renal involvement was underrepresented. The severity of cardiac involvement on average was not as bad as in some relapsed trials,8 as illustrated by only 25% of our patients having Mayo cardiac stage III disease.23,24 In summary, the study design goals were met for this prospective phase 2 study, demonstrating the activity of the Pom/dex combination even among lenalidomide and bortezomib failures. The results of this study indicate that pomalidomide will be a significant drug, potentially covering an unmet clinical need in patients with previously treated AL.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported in part by Celgene, the Robert A. Kyle Hematologic Malignancies Program, the JABBS Foundation, the Predolin Foundation, and the National Institutes of Health (grant P30 CA 15083).
National Institutes of Health
Authorship
Contribution: A.D., S.V.R., and M.Q.L. designed the study, collected and analyzed the data, and wrote the manuscript; A.D., F.B., S.R.H., S.K.K., D.D., S.R.Z., J.R.M., R.H., S.V.R., C.R., R.F., P.L.B., A.K.S., V.R., T.E.W., J.A.L., S.J.R., M.A.G., and M.Q.L. provided study patients and critically reviewed and edited the manuscript; and K.L. and B.L. collected and analyzed the statistical data and critically reviewed and edited the manuscript.
Conflict-of-interest disclosure: A.D. receives research funding from Celgene and Millennium. M.Q.L. receives research funding from Celgene. M.A.G. receives honoraria from Celgene and Millennium and is a member of an entity's board of directors or advisory committees for Millennium. A.D. receives honoraria and research funding from Celgene and honoraria from Binding Site. S.K.K. is a consultant for and receives research funding from Celgene, Merck, and Genzyme and receives research funding from Millennium, Novartis, and Cephalon. R.F. has received a patent for the prognostication of MM based on genetic categorization of the disease; has received consulting fees from Medtronic, Otsuka, Celgene, Genzyme, BMS, Lilly, Millennium, and AMGEN; and has sponsored research from Cylene and Onyx. P.L.B. is a consultant for Celgene, Centocor, Genentech, Amgen, and Novartis. A.K.S. receives honoraria from Celgene. The remaining authors declare no competing financial interests.
Correspondence: Angela Dispenzieri, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: dispenzieri.angela@mayo.edu.