Abstract
CD4+FoxP3+ regulatory T cells (Tregs) have been shown to suppress T cell–mediated host immune responses against self- and nonself-antigens; however, the impact of CD4+ Tregs on human antitumor immune responses and their influence on cancer treatment are unknown. In the present study, we explored the factors that influence CD4+ Treg reconstitution in patients receiving adoptive immunotherapy following conditioning regimens designed to enhance T-cell function and evaluated potential associations between CD4+ Treg levels and clinical responses to therapy. The analysis of 4 trials employing nonmyeloablative chemotherapy with or without total body irradiation (TBI) before adoptive T-cell transfer revealed that the percentage and number of reconstituting CD4+FoxP3+ Tregs observed in the peripheral blood was higher in nonresponders than in responders. The addition of TBI resulted in a further depletion of CD4+ Tregs, and the degree of depletion was dependent on the TBI dose. The number of administered doses of IL-2 was found to be positively associated with peripheral Treg reconstitution. These observations provide strong evidence that endogenous CD4+ Tregs have a negative impact on cancer therapy, and suggest that strategies reducing Treg levels may provide clinical benefit to cancer patients. All 5 clinical trials are registered at www.clinicaltrials.gov as NCT00001832, NCT00096382, NCT00335127, NCT00509496, and NCT00513604.
Introduction
Treatment with chemotherapy, irradiation, or immune modulators rarely lead to durable responses in patients with solid tumors; however, adoptive cell therapy (ACT) can result in long-term, complete tumor regression in patients with metastatic melanoma.1,2 Objective response (OR) rates of approximately 50% were observed in ACT patients who received autologous tumor-infiltrating lymphocytes (TILs) plus IL-2 after nonmyeloablative (NMA) chemotherapy.3 In patients who received either 2 or 12 Gy of total body irradiation (TBI) + NMA chemotherapy before ACT, there was a statistically significant association between increased doses of irradiation and complete responses rate.4 In recent clinical trials, patients treated with unselected bulk “young” TILs that were minimally cultured in vitro demonstrated an OR rate of 50%,5 and an OR of 58% was observed in patients receiving young TILs that were enriched for CD8+ T cells.6 Autologous PBMCs that were engineered to express tumor-reactive TCR genes recognizing tumor antigens have also been shown to mediate tumor regression in patients with metastatic melanoma.7-9 Analysis of these trials revealed that the in vivo persistence of adoptively transferred T cells, as well as the telomere lengths of the infused T cells were associated with clinical response to therapy.10-12
One of the factors that may influence response to therapy is the reconstitution of endogenous lymphocyte subpopulations that follows treatment with conditioning regimens that result in transient lymphopenia. A population of CD4+ T cells called regulatory T cells (Tregs) has been shown to suppress T cell–mediated host immune responses against self- and nonself-antigens.13-15 The cytokine IL-2 is needed for the development, homeostasis, and function of CD4+ Tregs, which also express high levels of the IL-2 receptor α chain CD25, as well as the inhibitory molecule CTLA-4.16 Expression of FoxP3 plays an important role in the development and maintenance of CD4+ Treg function,15 and in recent studies, demethylation within the first intron of the FOXP3 gene locus was associated with a stable Treg phenotype.17,18
Inactivation of the FoxP3 gene in animal model systems leads to the development of fatal autoimmunity,19 and patients with the X-linked IPEX syndrome, which is caused by mutations in the FOXP3 gene, manifest severe autoimmunity with multiple symptoms.20,21 Deficiencies in CD4+ Treg function may also play a role in other autoimmune diseases such as APECED, which results from lack of a functional AIRE gene product, because mice deficient in the Aire protein also appear to have defects in CD4+ Treg function.22 However, the role of CD4+ Tregs in restraining immune responses directed against tumor antigens is unclear. Relatively high frequencies of infiltrating CD4+ Tregs have been observed in solid cancers such as metastatic melanoma and oral squamous cell carcinomas,23,24 and modestly increased frequencies of CD4+ Tregs have also been observed in the peripheral blood of cancer patients.23,25,26 The presence of relatively high levels of CD4+ Tregs infiltrating ovarian cancer specimens was associated with poor prognosis in these patients26,27 ; however, a similar study carried out in patients with colorectal carcinoma indicated that a high intratumoral density of CD4+FoxP3+ T cells was associated with enhanced survival.28
In the present study, the phenotypic and functional characteristics of CD4+ Tregs that reconstitute in melanoma patients receiving adoptive TIL transfer were examined. Analysis of samples from multiple clinical trials demonstrated that CD4+ Treg reconstitution was influenced by the number of administered IL-2 doses and the degree of patient conditioning and was negatively associated with patient response to therapy.
Methods
Patient samples
All clinical trials were approved by the National Cancer Institute institutional review board. PBMC samples were collected from patients with metastatic melanoma in 5 NMA clinical trials (Figure 1A). Blood samples were prepared over a Ficoll-Hypaque gradient and cryopreserved until analyzed. Samples obtained from between 85% and 95% of the patients enrolled on the NMA + 2-Gy TBI, NMA + 12-Gy TBI, CD8+ young TIL, and gp100(154) TCR trials were included in this analysis. Samples from only 56% of the patients in the NMA trial were analyzed because of the limited availability of samples from these patients. At the time of T-cell infusion, peripheral lymphocyte counts were close to zero as a result of the NMA conditioning regimen. The first dose of 720 000 IU/kg of IL-2 was administrated IV within 24 hours of treatment and was continued every 8 hours until tolerance was reached. A maximum of 15 doses were administered. Patient response was assessed using standard radiographic studies and physical examination at 4 weeks after cell transfer and at regular intervals thereafter.
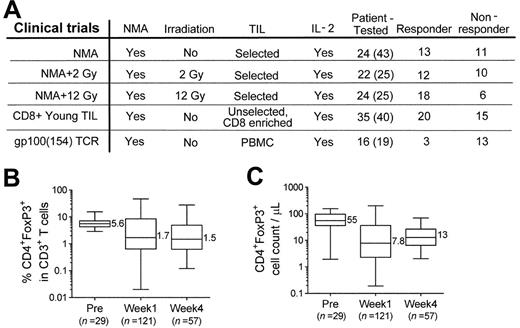
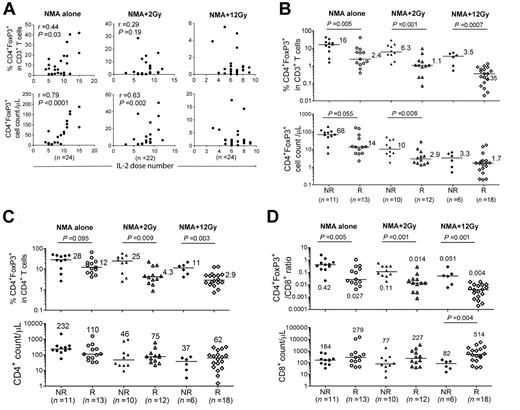
Widely varying frequencies of CD4+FoxP3+ T cells in patient PBMCs after adoptive immunotherapy. (A) Samples of patient PBMCs from 5 NMA clinical trials (detailed in “Methods”) were analyzed for the presence of CD4+FoxP3+ T cells. Numbers in parentheses indicate the total number of patients enrolled in these trials. (B) The percentages (% of CD3+ T cells) and (C) absolute cell count per microliter of CD4+FoxP3+ T cells in patient peripheral blood were analyzed before (PRE), approximately 1 week (week 1), and 4 weeks (week 4) after adoptive transfer. The maximum, 75th percentile, median, 25th percentile, and minimum values are shown. The number of CD4+FoxP3+ T cells represents the product of the absolute lymphocyte count, the fraction of the absolute lymphocyte count corresponding to CD3+ T cells, and the fraction of CD3+ T cells expressing FoxP3+ T cells.
Widely varying frequencies of CD4+FoxP3+ T cells in patient PBMCs after adoptive immunotherapy. (A) Samples of patient PBMCs from 5 NMA clinical trials (detailed in “Methods”) were analyzed for the presence of CD4+FoxP3+ T cells. Numbers in parentheses indicate the total number of patients enrolled in these trials. (B) The percentages (% of CD3+ T cells) and (C) absolute cell count per microliter of CD4+FoxP3+ T cells in patient peripheral blood were analyzed before (PRE), approximately 1 week (week 1), and 4 weeks (week 4) after adoptive transfer. The maximum, 75th percentile, median, 25th percentile, and minimum values are shown. The number of CD4+FoxP3+ T cells represents the product of the absolute lymphocyte count, the fraction of the absolute lymphocyte count corresponding to CD3+ T cells, and the fraction of CD3+ T cells expressing FoxP3+ T cells.
Experimental samples were obtained before therapy and 7-10 days after cell transfer (week 1), when absolute lymphocyte counts in the peripheral blood first exceeded 200 cells/μL and at least 2 days had elapsed between the final dose of IL-2 and sample collection, as well as approximately 1 month (week 4) after therapy. Peripheral blood samples from patients treated on 5 NMA protocols were analyzed for CD4+FoxP3+ T cells. Tumor-reactive TILs were administered to patients receiving NMA chemotherapy alone, NMA + 2-Gy TBI, or NMA + 12-Gy TBI.12 In the CD8+ young TIL trial, patients received minimally cultured autologous bulk TILs that were enriched for CD8+ T cells that had not been selected for tumor reactivity.6 In the gp100(154) trial, patients received autologous PBMCs engineered to express a TCR directed against the HLA-A2–restricted epitope corresponding to amino acids 154-162 of gp100.8 All patients received an NMA conditioning regimen consisting of treatment with cyclophosphamide (60 mg/kg) and fludarabine (25 mg/m2) as described previously.12 After cell transfer, up to 15 doses of IL-2 (720 000 IU/kg) were administered every 8 hours to tolerance. The average week-1 sampling time for each trial was 7.3 days for the CD8+ young TIL trial, 8.6 days for the NMA trial, 9.1 days for the NMA + 2-Gy trial, and 10.7 days for the NMA + 12-Gy trial. Pretreatment samples were collected 7 days before T-cell infusion. TILs were obtained from cryopreserved infusion samples used for the ACT treatment. All analyses of FoxP3 expression were carried out on freshly thawed samples of frozen cells. Patients in all trials were categorized into complete, partial, or nonresponders based on Response Evaluation Criteria in Solid Tumors (RECIST) guidelines, and complete and partial were combined as responders for statistical analysis. All patients enrolled had stage IV metastatic melanoma. Prior treatments and the duration of clinical responses observed in patients enrolled in these clinical trials have been described previously.29
Flow cytometric analysis
The following reagents were purchased from BD Biosciences and Pharmingen: mAbs to human CD3 (SK7), CD4 (SK3), CD25 (2A3), CD27 (M-T271), CD127 (hIL-7R-M21), CTLA-4 (BN13), Ki-67 (B56), CD45RO (UCHL-1), and IFN-γ (B27). mAbs to human FoxP3 (236A/E7) and mouse TCRβ (H57-597) were obtained from eBiosciences.
FoxP3 staining was performed using the FoxP3 staining buffer set (00-5523) and the intracellular staining protocol from eBiosciences. In brief, after surface staining for 20 minutes at 4°C with the Ab cocktails, cells were washed and incubated with freshly prepared fixation/permeabilization solution for 40 minutes at 4°C, washed twice with permeabilization buffer, and then stained with Ab to FoxP3 for 30 minutes at 4°C. Sample data were acquired by a FACSCalibur or FACSCanto cytometer (BD Biosciences) and analyzed using FlowJo Version 7.2.4 software.
Cell purification
For in vitro suppression assays, CD4+CD25+ and CD4+CD25− cells from patient PBMCs were separated by magnetic cell sorting using the IMag human CD4+ T-lymphocyte enrichment kit (BD Biosciences), followed by anti-CD25 magnetic particles. For DNA methylation assays, CD4+CD25 high, medium, and low fractions were sorted using the FACSAria cell sorter.
Intracellular cytokine induction
Details of the methods used for intracellular cytokine induction have been described previously.23 In brief, patient PBMCs were stimulated with or without phorbol 12-myristate 13-acetate (50 ng/mL) and ionomycin (1 μg/mL; P/I) in the presence of 1μM monensin (BD Biosciences) for 6 hours in medium supplemented with 1% (10 μg/mL) DNase (Genentech). Cells were then washed, incubated with Abs directed against cell-surface markers, fixed by Fix/Perm solution (eBiosciences), and incubated with anticytokine Abs.
In vitro suppression assays
Suppression assays were carried out using cells that were sorted using magnetic beads. For these assays, 2 × 104 CFSE-labeled CD4+CD25− effector cells were cultured with 2 × 104 (1:1) unlabeled CD4+CD25high cells, 60% of which expressed FoxP3+, and 2 × 105 irradiated feeder cells, and were stimulated with 500 ng/mL of plate-bound OKT-3 (anti-CD3) in 96-well round-bottom plate in IL-2–free medium (50% AIM-V/50% RPMI 1640 medium with 5% human serum; Invitrogen). Proliferation of CFSE-labeled T cells was assessed by flow cytometry after 96 hours of culture.
FOXP3 gene DNA methylation
Genomic DNA from FACS-sorted patient T-cell subsets was extracted with the DNeasy Blood & Tissue Kit (QIAGEN) and bisulfate converted by the EpiTect Bisulfite Kit (QIAGEN) according to the manufacturer's instruction. Converted DNA was then subjected to PCR with primers for amplification of specific targets in the first intron of the human FOXP3 gene. A 176-bp DNA fragment covering 11 CpG sites in this intron was amplified and sent for DNA pyrosequencing using designed primers for those sites. The sequence of the CpG region within intron 1 of human FOXP3 gene, with CpG dinucleotides that were shown previously to be unmethylated in CD4+ Tregs (shown in bold italics), is as follows: TCG(POS#1)GAACGAAACCTGTGGGGTGGGGTATCTGCCCTCTTCTCTTCCTCCGTGGTGTCGATGAAGCCCGGCGCATCCGGCCGCCATGACGTCAATGGCGGAAAAATCTGGGCAAGTCG(POS#11)GGG.
Statistical analysis
Statistical comparisons were performed with the nonparametric 2-tailed Mann-Whitney test and were generated using exact tests. The Holm method was used to adjust for multiple comparisons as appropriate. All reported P values are 2-tailed. We considered .05 > P > .01 as indicating a trend, whereas P < .01 was considered statistically significant. Nonparametric correlations were evaluated using the Spearman method and r and P values were reported. The following criteria were used for describing the strength of the association: strong association, r > 0.7; moderate association, 0.5 < r < 0.7; moderate to weak association, 0.3 < r < 0.5; and weak association, r < 0.3. Unbalanced 3-factor factorial repeated-measures ANOVA was performed on different variables, and time, treatment, and response were included in the initial model as the main effects (factors). Multivariable logistical regression analysis was performed to determine whether CD4+ Tregs and other parameters have the ability to predict clinical response. All statistical analyses were performed using SAS Version 9.2 and GraphPad Prism Version 5 software.
Results
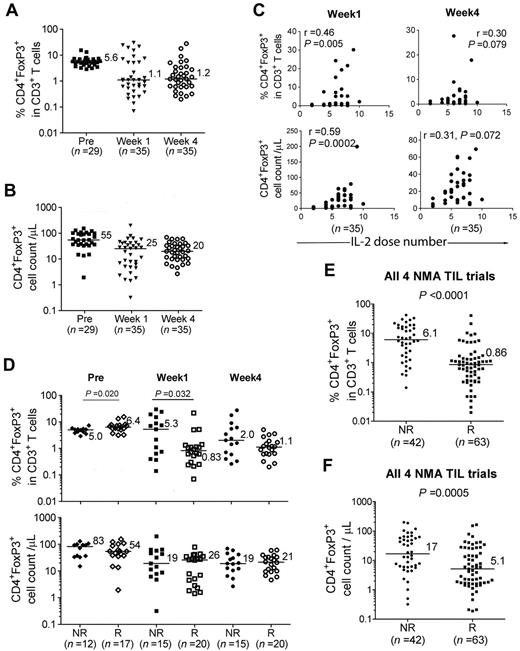
Widely varying frequencies of CD4+FoxP3+ T cells in patient PBMCs after adoptive immunotherapy
Peripheral blood samples from melanoma patients in multiple trials who had received adoptive cell transfer after NMA chemotherapy were studied (summarized in Figure 1A). Both the percentage (Figure 1B) and the absolute number (Figure 1C) of CD4+FoxP3+ T cells in patient PBMC samples obtained before treatment varied within a relatively narrow range and were similar to those reported in cancer patients in previous studies.17,23 There appeared to be a decline in the median percentage and number of peripheral CD4+FoxP3+ T cells in PBMCs analyzed between 7 and 10 days after treatment (week 1), as well as those obtained approximately 1 month after therapy (week 4), and a wider range in the values at these time points than those seen before therapy (Figure 1B-C).
Phenotype of CD4+FoxP3+ T cells in peripheral blood of ACT patients
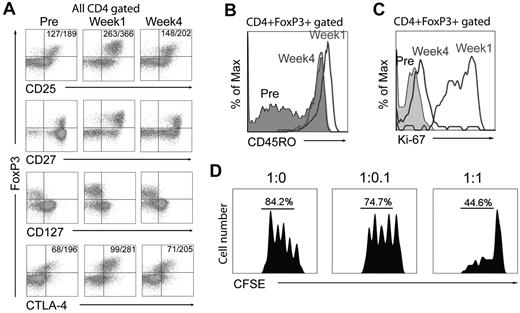
The CD4+FoxP3+ T cells present in week-1 samples expressed levels of FoxP3, CD25, and CTLA-4 that were high relative to those expressed in pretreatment samples, while retaining the low levels of the IL-7 receptor α chain (CD127) that were observed in pretreatment samples (Figure 2A). In contrast to pretreatment samples, expression of CD45RO was observed in more than 90% of the CD4+FoxP3+ T cells in week-1 and week-4 samples (Figure 2B and supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), indicating that the majority of CD4+ Tregs in posttreatment samples were effector Tregs.30 Furthermore, the majority of peripheral CD4+FoxP3+ T cells in week-1 samples expressed Ki-67 (Figure 2C and supplemental Figure 1), suggesting that these cells were in an active, proliferating state, whereas only a low percentage of cells in the pretreatment samples expressed Ki-67. The CD4+CD25high T cells present in a sample of peripheral blood 4 weeks after treatment suppressed the proliferation of autologous CD4+CD25− effector cells (Figure 2D), so it was not possible to carry out repeated assays because of sample limitations; nevertheless, these results provided direct evidence that the T cells present in the peripheral blood at this time represented regulatory T cells.
Phenotype of CD4+FoxP3+ T cells in the peripheral blood of ACT patients. (A) Cell-surface expression of CD25, CD27, and CD127, as well as intracellular CTLA-4 and FoxP3 expression, were evaluated on CD4-gated T cells from 1 of 29 patient PBMC samples analyzed before, 1 week, and 4 weeks after treatment. Numbers in the top left quadrants indicate the mean fluorescence intensity of CD25/FoxP3 or CTLA-4/FoxP3 expression. (B) Expression of CD45RO on gated CD4+FoxP3+ T cells from 1 of 8 representative patient PBMC samples obtained before, 1 week, and 4 weeks after treatment. (C) Expression of Ki-67 on gated CD4+FoxP3+ T cells from 1 of 29 representative patients before, 1 week, and 4 weeks after treatment. (D) CFSE dilution of labeled CD4+CD25− effector T cells stimulated with anti-CD3 Ab was assessed after a 96-hour coculture with CD4+CD25high T cells containing > 60% FoxP3+ T cells at varying effector T cell-to-Treg ratios. The percentages of T cells undergoing at least 1 division are shown, and data are representative of 2 experiments carried out with week-4 PBMC samples from 2 patients in the CD8+ young tumor-infiltrating lymphocyte (TIL) trial.
Phenotype of CD4+FoxP3+ T cells in the peripheral blood of ACT patients. (A) Cell-surface expression of CD25, CD27, and CD127, as well as intracellular CTLA-4 and FoxP3 expression, were evaluated on CD4-gated T cells from 1 of 29 patient PBMC samples analyzed before, 1 week, and 4 weeks after treatment. Numbers in the top left quadrants indicate the mean fluorescence intensity of CD25/FoxP3 or CTLA-4/FoxP3 expression. (B) Expression of CD45RO on gated CD4+FoxP3+ T cells from 1 of 8 representative patient PBMC samples obtained before, 1 week, and 4 weeks after treatment. (C) Expression of Ki-67 on gated CD4+FoxP3+ T cells from 1 of 29 representative patients before, 1 week, and 4 weeks after treatment. (D) CFSE dilution of labeled CD4+CD25− effector T cells stimulated with anti-CD3 Ab was assessed after a 96-hour coculture with CD4+CD25high T cells containing > 60% FoxP3+ T cells at varying effector T cell-to-Treg ratios. The percentages of T cells undergoing at least 1 division are shown, and data are representative of 2 experiments carried out with week-4 PBMC samples from 2 patients in the CD8+ young tumor-infiltrating lymphocyte (TIL) trial.
Characteristics of CD4+FoxP3+ T cells present in peripheral blood and in vitro–cultured T cells
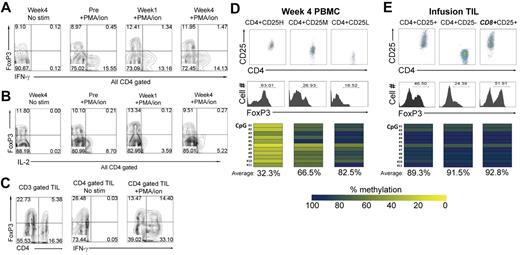
Previous studies have shown that peripheral CD4+ Tregs failed to produce inflammatory cytokines in response to polyclonal simulation with P/I.23 CD4+FoxP3+ T cells present in PBMCs obtained before and after adoptive transfer did not produce either IFN-γ (Figure 3A) or IL-2 (Figure 3B) upon P/I stimulation, suggesting that they were anergic. In patient PBMCs analyzed after transfer, expression of FoxP3 was only detected in CD4+ T cells, whereas both CD8+ and CD4+ T cells in infusion TILs expressed FoxP3 (Figure 3C). A significant portion of the CD4+FoxP3+ T cells, as well as the CD8+FoxP3+ T cells present in infusion TILs, produced IFN-γ in response to polyclonal stimulation (Figure 3C), as was observed previously in studies of activated human T cells.31,32 Additional studies were then carried out to determine the methylation status of CpGs within intron 1 of the FoxP3 gene, because the presence of undermethylated residues in this region represents one of the most reliable markers of stable CD4+ Tregs.17,18 The observation that 11 of the CpGs within intron 1 of the FOXP3 gene were undermethylated in DNA prepared from CD4+CD25hi T cells sorted from a week-4 PBMC sample (Figure 3D) further demonstrates that these cells represent CD4+ Tregs. In contrast, CpGs within the same region were highly methylated in CD4+CD25hi T cells isolated from in vitro–cultured infusion TILs (Figure 3E). These results indicate that CD4+FoxP3+ T cells present in the peripheral blood of patients receiving adoptive immunotherapy represent CD4+ Tregs, whereas CD4+FoxP3+ T cells within infused TILs may represent activated T cells that have transiently up-regulated FoxP3 expression.
Infused T cells lack characteristic features of CD4+ Tregs. (A-B) Intracellular cytokine expression was examined on CD4-gated PBMCs after 6 hours of stimulation with P/I. IFN-γ and IL-2 production were evaluated on pretreatment, week-1, and week-4 samples of PBMCs. Numbers indicate the percentage of cells in each quadrant. Representative data from 1 of 15 patient samples analyzed are shown. (C) Intracellular IFN-γ expression was examined after a 6-hour stimulation of infusion TILs with P/I. Representative data from 1 of 15 patient samples analyzed are shown. (D) DNA methylation assays of week-4 PBMC sample from 1 patient in the CD8+ young TIL trial. Cells were sorted into CD4+CD25H (high), CD4+CD25M (medium), and CD4+CD25L (low), and the frequency of FoxP3+ cells in each population is shown. The methylation status of 11 CpG dinucleotides within intron 1 of the FOXP3 gene shown previously to be undermethylated in CD4+ Tregs were analyzed. The percentages of the 11 CpGs that were methylated, evaluated as described in “Methods,” are shown in heat maps, and the average percentages of the 11 CpGs that were methylated re indicated below the heat maps. (E) DNA methylation analysis of a sample of infusion TILs. DNA isolated from CD4+CD25+, CD4+CD25−, and CD8+CD25+ T cells that were sorted using magnetic beads as described in “Methods” was subjected to methylation analysis.
Infused T cells lack characteristic features of CD4+ Tregs. (A-B) Intracellular cytokine expression was examined on CD4-gated PBMCs after 6 hours of stimulation with P/I. IFN-γ and IL-2 production were evaluated on pretreatment, week-1, and week-4 samples of PBMCs. Numbers indicate the percentage of cells in each quadrant. Representative data from 1 of 15 patient samples analyzed are shown. (C) Intracellular IFN-γ expression was examined after a 6-hour stimulation of infusion TILs with P/I. Representative data from 1 of 15 patient samples analyzed are shown. (D) DNA methylation assays of week-4 PBMC sample from 1 patient in the CD8+ young TIL trial. Cells were sorted into CD4+CD25H (high), CD4+CD25M (medium), and CD4+CD25L (low), and the frequency of FoxP3+ cells in each population is shown. The methylation status of 11 CpG dinucleotides within intron 1 of the FOXP3 gene shown previously to be undermethylated in CD4+ Tregs were analyzed. The percentages of the 11 CpGs that were methylated, evaluated as described in “Methods,” are shown in heat maps, and the average percentages of the 11 CpGs that were methylated re indicated below the heat maps. (E) DNA methylation analysis of a sample of infusion TILs. DNA isolated from CD4+CD25+, CD4+CD25−, and CD8+CD25+ T cells that were sorted using magnetic beads as described in “Methods” was subjected to methylation analysis.
Effects of TBI on CD4+ Treg reconstitution
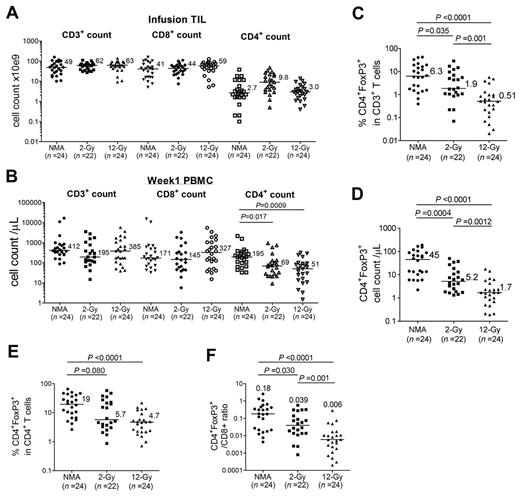
Reconstitution of CD4+ Tregs was examined in patients who received either NMA conditioning alone, NMA + 2-Gy TBI, or NMA + 12-Gy TBI before adoptive TIL transfer.12 The total number of infused CD3+ and CD8+ T cells were similar in the 3 protocols, but CD4+ cell counts were significantly higher in the NMA + 2-Gy trial compared with the NMA + 12-Gy trial (P = .004). CD8+ T cells represented more than 80% of the administered T cells in these trials (Figure 4A). One week after adoptive transfer, the absolute CD4+ T-cell counts in peripheral blood samples from the 2-Gy and 12-Gy protocols were significantly lower than those from the NMA-alone trial (Figure 4B), indicating that TBI may have resulted in depletion of endogenous CD4+ T cells. The ratio of CD4+ Tregs to CD3+ T cells and the absolute number of CD4+ Tregs in PBMC samples analyzed 1 week after transfer were significantly lower in the 2-Gy and 12-Gy trials than in NMA-alone trial, and these levels decreased with increasing radiation dose (Figure 4C-D). Furthermore, the ratios of peripheral CD4+ Tregs to total CD4+ and CD8+ T cells were significantly lower in the 12-Gy trials than in the NMA-alone trial 1 week after transfer (Figure 4E-F).
CD4+ Treg reconstitution in peripheral blood of NMA, NMA + 2-Gy TBI, and NMA + 12-Gy TBI TIL patients. (A) The total numbers of CD3+, CD8+, and CD4+ TILs that were infused into patients in the NMA-alone, NMA + 2-Gy TBI, and NMA + 12-Gy TBI TIL trials are shown. (B) The absolute CD3, CD8, and CD4 T-cell counts of week-1 PBMCs in the NMA alone, NMA + 2-Gy TBI, and NMA + 12-Gy TBI trials are indicated. Horizontal bars represent median values. (C-D) Percentages of Treg in CD3+ T cells and Treg absolute cell counts in the 3 NMA TIL trials were evaluated at week 1. (E) Percentages of CD4+ Treg as a percentage of total CD4+ T cells in the NMA-alone, NMA + 2-Gy TBI, and NMA + 12-Gy TBI TIL trials were compared at week 1. (F) The ratios of CD4+ Tregs to total CD8+ T cells in 3 NMA TIL trials were compared at week 1.
CD4+ Treg reconstitution in peripheral blood of NMA, NMA + 2-Gy TBI, and NMA + 12-Gy TBI TIL patients. (A) The total numbers of CD3+, CD8+, and CD4+ TILs that were infused into patients in the NMA-alone, NMA + 2-Gy TBI, and NMA + 12-Gy TBI TIL trials are shown. (B) The absolute CD3, CD8, and CD4 T-cell counts of week-1 PBMCs in the NMA alone, NMA + 2-Gy TBI, and NMA + 12-Gy TBI trials are indicated. Horizontal bars represent median values. (C-D) Percentages of Treg in CD3+ T cells and Treg absolute cell counts in the 3 NMA TIL trials were evaluated at week 1. (E) Percentages of CD4+ Treg as a percentage of total CD4+ T cells in the NMA-alone, NMA + 2-Gy TBI, and NMA + 12-Gy TBI TIL trials were compared at week 1. (F) The ratios of CD4+ Tregs to total CD8+ T cells in 3 NMA TIL trials were compared at week 1.
Association between number of administered IL-2 doses and CD4+ Treg reconstitution
High levels of CD4+FoxP3+ T cells have been observed previously in the peripheral blood of melanoma and renal cancer patients receiving high-dose IL-2 alone.17,33-35 Analysis of patients in the present trial who received NMA alone revealed that the number of IL-2 doses was strongly associated with the total number of CD4+ Tregs, but only moderately to weakly associated with the percentages of CD4+ Tregs in the peripheral blood at week 1 (Figure 5A first column). The number of IL-2 doses appeared to be weakly to moderately associated with peripheral CD4+ Treg reconstitution in the 2-Gy trial, but were not associated with CD4+ Treg reconstitution in the 12-Gy trial, perhaps as a consequence of the significant depletion of peripheral CD4+ Tregs observed in patients receiving TBI (Figure 5A).
Association between frequencies of CD4+ Tregs and patient response to therapy. (A) Nonparametric analysis of the relationship between doses of IL-2 administrated and percentages or absolute cell count of Tregs from week-1 PBMCs in 3 NMA trials. Spearman r and P values are shown. (B) The percentages of Tregs in CD3+ T cells and absolute Treg counts of week-1 samples from nonresponders and responders in 3 NMA TIL trials are shown. Horizontal bars represent the median values. (C) The percentages of CD4+ Tregs as a percentage of total CD4+ T cells in week-1 samples and absolute CD4+ T-cell counts in the peripheral blood of nonresponders (NR) and responders (R) in the 3 NMA TIL trials are shown. (D) Ratio of CD4+ Tregs to total CD8+ T cells in week-1 samples and absolute CD8+ T-cell counts in the peripheral blood of nonresponders and responders in the 3 NMA TIL trials are shown.
Association between frequencies of CD4+ Tregs and patient response to therapy. (A) Nonparametric analysis of the relationship between doses of IL-2 administrated and percentages or absolute cell count of Tregs from week-1 PBMCs in 3 NMA trials. Spearman r and P values are shown. (B) The percentages of Tregs in CD3+ T cells and absolute Treg counts of week-1 samples from nonresponders and responders in 3 NMA TIL trials are shown. Horizontal bars represent the median values. (C) The percentages of CD4+ Tregs as a percentage of total CD4+ T cells in week-1 samples and absolute CD4+ T-cell counts in the peripheral blood of nonresponders (NR) and responders (R) in the 3 NMA TIL trials are shown. (D) Ratio of CD4+ Tregs to total CD8+ T cells in week-1 samples and absolute CD8+ T-cell counts in the peripheral blood of nonresponders and responders in the 3 NMA TIL trials are shown.
Association between frequencies of CD4+ Tregs and patient response to therapy
One week after treatment, the ratios of peripheral CD4+ Tregs to total CD3+ T cells were significantly lower in responding patients than in nonresponders in the NMA-alone (P = .005), NMA + 2-Gy TBI (P = .001), and NMA + 12-Gy TBI (P = .0007) trials (Figure 5B). The absolute cell counts of CD4+ Tregs were significantly lower in responders than in nonresponders in the NMA + 2-Gy TBI trial and demonstrated a similar trend in the NMA-alone trial, but did not appear to differ in the 12-Gy trial, in which nearly all of the patients exhibited CD4+ Treg levels that were dramatically lower than those observed in the NMA-alone trial (Figure 5B). The ratios of peripheral CD4+ Tregs to both total CD4+ and CD8+ T cells at week 1 were also lower in responding patients than in nonresponders (Figure 5C-D). At the same time, the total numbers of CD4+ T cells present in the peripheral blood did not differ between responder and nonresponder groups in any of the trials, whereas the number of CD8+ T cells was significantly higher in responding patients in the 12-Gy TBI trial (Figure 5C-D).
CD4+ Treg reconstitution in CD8+ young TIL trial
Recent trials have employed the adoptive transfer of minimally cultured populations of autologous TILs that were not selected for their ability to respond to tumor, called “young” TILs,5 as well as young TILs that were enriched for CD8+ T cells.6 In the CD8+ young TIL trial, CD4+ T cells represented less than 1% of the administered T cells,6 and in this trial, the ratio of peripheral CD4+ Tregs to CD3+ T cells and the absolute number of CD4+ Tregs were lower 1 and 4 weeks after treatment than before therapy (Figure 6A-B). Given the substantial number of CD4+ Tregs observed in some patient PBMC samples (Figure 6A-B) and the relatively small number of CD4+ T cells transferred, it appears that most, if not all, of the CD4+ Tregs represented endogenous reconstituting cells. The number of doses of IL-2 administered to patients in the CD8 + young TIL trial was also moderately and positively associated with the percentage and the absolute number of CD4+ Tregs at week 1 (Figure 6C). In contrast, there was no correlation between the number of administered doses of IL-2 and absolute CD4+ or CD8+ T-cell counts at these times (data not shown), indicating that IL-2 administration may have promoted the growth of CD4+ Tregs selectively. The levels of CD4+ Tregs seen in the peripheral blood of nonresponders in the CD8+ young TIL protocol appeared to be higher than those observed in responders 1 week after treatment (P = .032, Figure 6D) after subtracting the pretreatment values, which were significantly different between the responders and nonresponders (P = .020).
Treg reconstitution in CD8+ young TIL trial. (A-B) Percentages and cell counts of Tregs in PBMCs from CD8+ young patients are shown. (C) Nonparametric correlation between doses of IL-2 administrated and percentages or absolute cell count of Treg from week-1 and week-4 PBMCs in CD8+ young TIL trial. Spearman r and P values are shown. (D) Percentages of Tregs in CD3+ T cells and Treg counts of nonresponders (NR) and responders (R) in the CD8+ young TIL trial are shown. (E-F) Nonparametric analysis was used to compare the percentages and absolute cell counts of CD4+ Tregs at week 1 in the peripheral blood of nonresponders and responders from the 4 NMA TIL trials. Horizontal bars represent the median values.
Treg reconstitution in CD8+ young TIL trial. (A-B) Percentages and cell counts of Tregs in PBMCs from CD8+ young patients are shown. (C) Nonparametric correlation between doses of IL-2 administrated and percentages or absolute cell count of Treg from week-1 and week-4 PBMCs in CD8+ young TIL trial. Spearman r and P values are shown. (D) Percentages of Tregs in CD3+ T cells and Treg counts of nonresponders (NR) and responders (R) in the CD8+ young TIL trial are shown. (E-F) Nonparametric analysis was used to compare the percentages and absolute cell counts of CD4+ Tregs at week 1 in the peripheral blood of nonresponders and responders from the 4 NMA TIL trials. Horizontal bars represent the median values.
Combined analysis of multiple trials indicates that peripheral CD4+ Treg levels are negatively associated with clinical responses
Combining the results obtained from analyzing the NMA, NMA + 2-Gy TBI, NMA + 12-Gy TBI, and CD8+ young TIL trials, the frequency and cell counts of CD4+ Tregs were significantly lower in peripheral blood samples obtained from responders than those obtained from nonresponders (P < .0001 and P = .0005, respectively; Figure 6E-F).
Multivariable logistical regression analysis was then performed for all 4 TIL trials, as shown in supplemental Table 1. The percentage of CD4+ Tregs in CD3+ T cells had a low R2 value (0.19), indicating that this represents a poorly fitting model and suggesting that the levels of CD4+ Tregs are not a significant predictor allowing separation of nonresponders from responders. The results of multivariable logistical regression analysis of treatment type, age, and IL-2 dose indicated that they also do not represent significant predictors of clinical outcome (supplemental Table 1). The odd ratios for IL-2 dose number and CD4+ Treg levels were 0.819 and 0.868, respectively, also indicating that they cannot be used as predictors of response to therapy.
A linear unbalanced 3-factor (time, treatment, and response) repeated-measures ANOVA was performed on log transformed data (supplemental Figures 2-5). The results of ANOVA analysis of CD4+FoxP3+ levels in CD3+ T cells showed that the counts were consistent with the nonparametric analyses shown in Figures 5 and 6, whereas total CD8 and CD4 cell counts were generally not significantly different between responders and nonresponders. ANOVA results of percentage of Tregs in CD3+ T cells (but not Treg) support the contention that nonresponders have higher mean levels than responders. In addition, the repeated-measures ANOVA occasionally revealed some higher-order interactions (which were masked by pooling over factors) that were deemed as trivial or minor.
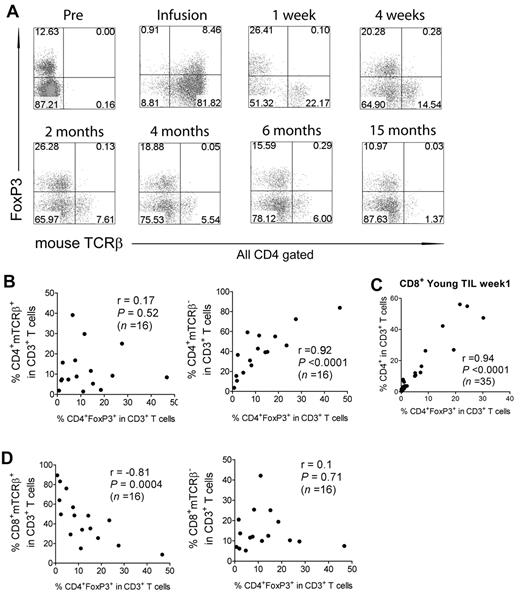
Evidence for lack of conversion of effector CD4+ T cells to CD4+FoxP3+ Tregs
The phenotype of adoptively transferred PBMCs that were engineered to express a mouse TCR directed against an epitope of the melanoma antigen gp1008 was examined. One week after transfer, persistent CD4+ T cells expressing the mouse TCRβ transgene lacked expression of FoxP3 (Figure 7A). Further analysis of persistent CD4+ T cells over a period of 15 months failed to provide evidence for conversion of the transferred effector T cells to CD4+ Tregs (Figure 7A). In addition, the frequency of CD4+ Tregs was strongly correlated with the frequency of CD4+ T cells that lacked expression of the murine TCRβ transgene (Figure 7B right panel), indicating that CD4+ Treg reconstitution is associated with the recovery of endogenous CD4+ T cells. A similar correlation was observed in samples from the CD8+ young TIL protocol (Figure 7C), in which the majority of CD4+ cells observed in the peripheral blood presumably represented endogenous reconstituting cells. Furthermore, there was a negative correlation between CD4+ Tregs and the percentage of persistent CD8+ T cells expressing the murine TCRβ transgene (Figure 7D). These results provide evidence that CD4+FoxP3+ Tregs present in the peripheral blood of adoptive cancer immunotherapy patients represent reconstituting endogenous T cells, and suggest that these cells may influence the response to therapy.
Evidence for lack of conversion of CD4+ T cells in TILs into CD4+ Tregs after adoptive transfer. (A) Cell-surface expression of the mouse TCRβ chain was analyzed on PMBCs from a representative patient (1 week to 15 months) from the gp100 TCR trial. Numbers indicate the percentages in each quadrant. The week-1 figure is a representative sample of 1 of 16 week-1 samples analyzed. Nonparametric correlation between the percentages of Tregs and persistent (mTCRβ+) or nonpersistent (mTCRβ−) CD4+ T cells from week-1 PBMCs in the gp100 trial (B), the percentages of Treg and CD4+ T cells from week-1 PBMCs in the CD8+ young TIL trial (C), and the percentages of Treg and persistent or nonpersistent CD8+ T cells from week-1 PBMCs in the gp100 TCR trial (D). Spearman r and P values are shown.
Evidence for lack of conversion of CD4+ T cells in TILs into CD4+ Tregs after adoptive transfer. (A) Cell-surface expression of the mouse TCRβ chain was analyzed on PMBCs from a representative patient (1 week to 15 months) from the gp100 TCR trial. Numbers indicate the percentages in each quadrant. The week-1 figure is a representative sample of 1 of 16 week-1 samples analyzed. Nonparametric correlation between the percentages of Tregs and persistent (mTCRβ+) or nonpersistent (mTCRβ−) CD4+ T cells from week-1 PBMCs in the gp100 trial (B), the percentages of Treg and CD4+ T cells from week-1 PBMCs in the CD8+ young TIL trial (C), and the percentages of Treg and persistent or nonpersistent CD8+ T cells from week-1 PBMCs in the gp100 TCR trial (D). Spearman r and P values are shown.
Discussion
The adoptive transfer of autologous TILs to patients who had received a prior NMA conditioning regimen can mediate tumor regression in 50%-70% of patients with metastatic melanoma.3 Adoptive T-cell immunotherapy allows for efficient in vitro activation of lymphocytes and enrichment of specific populations of cells such as CD8+ T cells,6 and facilitates the efficient modification of lymphocytes, as demonstrated in recent trials in which lymphocytes that were genetically engineered to express tumor-reactive TCRs were shown to mediate tumor regression7,8 Adoptive immunotherapy also provides an opportunity to modulate the effects of host conditioning before adoptive cell transfer. NMA chemotherapy and TBI before cell infusion provides a therapeutic benefit to patients receiving adoptive TIL transfer.12
In the present study, reconstitution CD4+FoxP3+ Tregs in cancer patients receiving adoptive immunotherapy following a lymphodepleting conditioning regimen were evaluated. Reconstituting CD4+FoxP3+ T cells expressed high levels of markers that have been found previously to be expressed on CD4+ Tregs and were anergic to polyclonal activation. The DNA that was isolated from CD4+CD25hi T cells obtained after adoptive transfer contained a region of unmethylated CpGs within intron 1 of the FOXP3 gene, a feature that has been associated with stable Tregs. FoxP3 expression was observed on the infused in vitro–cultured CD4+ TILs; however, several pieces of evidence suggest that the transferred cells do not contain significant numbers of Tregs and that they do not convert into Tregs after transfer. The FoxP3+ T cells present in the infusion samples were not anergic, and DNA prepared from these cells was fully methylated within the same region of the FOXP3 gene locus, which is consistent with previous results indicating that FoxP3 is transiently up-regulated on in vitro–activated T cells.23 Effector CD4+ T cells also did not convert into CD4+ Tregs after adoptive transfer, because the persistent TCR-transduced cells failed to express FoxP3. In addition, the reconstitution of CD4+ Tregs was associated with the recovery of endogenous effector CD4+ T cells, and CD4+ Treg reconstitution occurred at similar rates in patients receiving CD8+ young TILs and bulk young TILs (data not shown). Although all of the patients in NMA + 2-Gy TBI and NMA + 12-Gy TBI trials received CD34+ cells with adoptive cell transfer, 49 of 50 patients on these 2 protocols received an average of 1.0 × 106 CD3+ cells with their CD34+-selected cell infusion (range, 0.0-4.9 × 106 CD3+ cells). It also appears to be unlikely that the transferred HSCs would give rise to significant numbers of Tregs in 1-2 weeks. One patient, however, did receive unselected CD34+ cells derived from a BM harvest and received 1.1 × 109 CD3+ cells with the BM infusion, which could have contributed to the reconstituting pool of Tregs.
The levels of CD4+ Treg reconstitution were negatively associated with patient responses to adoptive therapy in 4 TIL clinical trials, indicating that CD4+ Tregs may play an inhibitory role in adoptive immunotherapy of human tumors. Although logistical regression analysis indicated that the levels of peripheral CD4+ Tregs did not have a high predictive value for clinical outcomes, ANOVA analysis confirmed that there was a significant association between CD4+ Treg levels and clinical responses. One potential explanation for these findings is that the levels of reconstituting CD4+ Tregs may influence either the persistence or the antitumor activity of adoptively transferred TILs. Alternatively, the levels of TIL persistence, which are affected by factors such as telomere lengths of the administered T cells,11 may influence the reconstitution of CD4+ Tregs by competing for homeostatic cytokines or other factors present in the lymphopenic environment present after NMA chemotherapy.
Our results indicated that reconstitution of CD4+FoxP3+ T cells was variable but depended at least in part on the number of doses of IL-2 that were administered to patients after adoptive transfer. The association between CD4+ Treg reconstitution and the number of administered IL-2 doses indicates that IL-2 may selectively promote the growth of CD4+ Tregs over effector T cells. These findings are consistent with a role for CD4+ Tregs, the numbers of which have been shown to increase in the peripheral blood of melanoma patients treated with IL-2 alone,33 in inhibiting antitumor immune responses, and suggest that the balance between effector T cells and CD4+ Tregs may have an impact on these antitumor treatments. In addition, high levels of differentiation and proliferation were observed in CD4+FoxP3+ and CD4+FoxP3− T cells, which might have resulted either from the IL-2 that was administered or from stimulation by homeostatic factors that are transiently elevated after NMA chemotherapy.12
In a recent study, patients experiencing an objective response received fewer IL-2 doses than nonresponders (P = .003) in the NMA-alone, NMA + 2-Gy TBI, and NMA + 12-Gy TBI trials combined, although the difference in dose numbers were small: responders received an average of 7.2 doses of IL-2, whereas nonresponders received an average of 8.8 doses.12 Only a subset of the patients in these trials were analyzed in the current study, leading to the lack of correlation observed in this study. Fewer IL-2 doses may lead to lower levels of Treg reconstitution after cell transfer, resulting in a better clinical outcome in some patients. However, an optimal dose of administered IL-2 was not observed, because a bell-shaped curve relating dose to clinical response was not observed (data not shown).
The addition of TBI to the NMA conditioning regimen resulted in a depletion of CD4+ Tregs 1 week after transfer. Further analysis revealed that the levels of CD4+ Tregs in week-4 samples from patients in the 12-Gy trial were similar to those observed at week 1 (data not shown), indicating that this may result in a relatively long-term loss of these cells. In addition, the intensity of TBI was associated with the degree of CD4+ Treg depletion. The treatment efficacy of ACT in a murine tumor model system was enhanced by increasing the intensity of TBI administered to mice,36 and the complete response rate of patients receiving 12-Gy TBI (40%) was higher than patients receiving 2-Gy TBI (20%) or NMA alone (12%),29 which may have been influenced in part by the nearly complete elimination of CD4+ Tregs seen in patients receiving 12-Gy TBI. However, the levels of Tregs did not appear to differ significantly between the complete responders and partial responders analyzed in the present study (data not shown), indicating that multiple factors are responsible for the complete responses observed in these patients.
The results of the present study provide some of the most direct evidence in humans for a role of CD4+ Tregs in blunting clinical cancer immunotherapy responses. Strategies for counteracting the potentially negative effects of CD4+ Tregs include TBI, drugs or Abs that selectively deplete CD4+ Tregs, and strategies that deplete all subpopulations of CD4+ T cells. The substitution of cytokines such as IL-15 or IL-7 for IL-2 may also help to minimize CD4+ Treg reconstitution and may lead to improved response to therapy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Arnold Mixon and Shawn Farid for assistance with cell sorting.
Authorship
Contribution: X.Y., M.A., S.A.R., and P.F.R. conceived the study, designed the experiments, performed the majority of the experiments, interpreted the data, and wrote the manuscript; Y.-C.L., F.L., and D.S.S. performed the experiments and contributed to data interpretation; D.J.L. and S.M.S. performed statistical analysis on all experiments; and M.E.D. prepared the clinical samples and performed the data analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Xin Yao or Paul F. Robbins, National Cancer Institute, National Institutes of Health, 10 Center Dr, CRC-10-3-5744, Bethesda, MD 20892; e-mail: xiny@mail.nih.gov or paulrobbins@mail.nih.gov.