Abstract
A key feature differentiating nonpathogenic SIV infection of sooty mangabeys (SMs) from pathogenic HIV/SIV infections is the rapid resolution of type I IFN (IFN-I) responses and IFN-stimulated gene expression during the acute-to-chronic phase transition and the establishment of an immune quiescent state that persists throughout the chronic infection. We hypothesized that low levels of IFN-I signaling may help to prevent chronic immune activation and disease progression in SIV-infected SMs. To assess the effects of IFN-I signaling in this setting, in the present study, we administered recombinant rhesus macaque IFNα2-IgFc (rmIFNα2) to 8 naturally SIV-infected SMs weekly for 16 weeks. Gene-expression profiling revealed a strong up-regulation of IFN-stimulated genes in the blood of treated animals, confirming the reagent's bioactivity. Interestingly, we observed an approximately 1-log decrease in viral load that persisted through day 35 of treatment. Flow cytometric analysis of lymphocytes in the blood, lymph nodes, and rectal biopsies did not reveal a significant decline of CD4+ T cells, a robust increase in lymphocyte activation, or change in the level of SIV-specific CD8+ T cells. The results of the present study indicate that administration of type I IFNs in SIV-infected SMs induces a significant anti-viral effect that is not associated with a detectable increase in chronic immune activation.
Introduction
In stark contrast to HIV infection in humans and experimental SIV infection of macaques, both of which lead to AIDS, SIV infection of natural host sooty mangabeys (SMs) is typically nonprogressive despite similarly high levels of virus replication.1,2 The reasons that SIV-infected SMs are resistant to AIDS remain incompletely understood, and it is hoped that their elucidation will help to define the mechanisms responsible for the development of AIDS in HIV-infected humans.3 Several studies have shown that 2 consistent features of naturally SIV-infected SMs are the absence of generalized immune activation during the chronic phase of the infection and a pattern of in vivo–infected cells that results in a preferential preservation of central memory CD4+ T cells from SIV infection.4,5
The low immune activation observed in chronically SIV-infected SMs represents a key phenotypic difference from pathogenic HIV/SIV infection of humans and macaques, in which chronic immune activation is a major marker and predictor of disease progression, both in the natural history and in the setting of antiretroviral treatment.6-11 In SIV-infected SMs, the low immune activation that is observed during the chronic phase of infection is established as the result of the relatively rapid resolution of a strong innate and adaptive immune response to the virus that occurs during the acute phase of infection and lasts approximately 4-6 weeks after the initial inoculation.4,12 Similar kinetics of the immune responses to SIV have been reported in another natural host, the African green monkey.13,14 The mechanisms by which SIV-infected SMs are able to tune down their immune activation remain unclear, but may involve specific virus properties, better ability to activate immune regulatory pathways, decreased sensing of viral antigens, preservation of mucosal immunity with consequent absence of microbial translocation, and differences in the pattern and anatomic location of infected cells.3
Regardless of the mechanisms involved, the low immune activation observed during the chronic phase of SIV infection in SMs is associated with the absence of up-regulation of type I IFN (IFN-I)–stimulated genes (ISGs), which is a consistent feature of the transcriptional profile of pathogenic HIV/SIV infections.4,13 At this time, however, it remains unknown whether and to what extent this lack of an IFN-I gene-expression signature in chronically SIV-infected natural hosts represents a cause or a consequence of the low immune activation. To address this issue, in the present study, we treated 8 naturally SIV-infected SMs for 16 weeks with a recombinant IFN-I agonist (a recombinant rhesus macaque [RM] IFNa2-Ig fusion protein, rmIFNα2) that induces a strong ISG up-regulation both in vitro and in vivo, thus demonstrating that SMs are not intrinsically resistant to IFN-I signaling. The main result of this treatment was a significant, approximately 1-log decline in viral load that persisted for approximately 6 weeks, coincident with the presence of a IFN-I transcriptional signature in the blood. No major immunologic effects were observed. rmIFNα2 treatment failed to induce a significant increase in immune activation and did not augment the level of SIV-specific CD8+ T cells. The results of the present study emphasize the important antiviral role of IFN-I and ISG up-regulation during SIV infection of SMs, and suggest that the mechanisms involved in the ability of these animals to maintain low immune activation are likely multifactorial and not entirely dependent on IFN-I and ISG expression.
Methods
Ethics statement
These studies were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, and were approved by the Emory University (AWA no. A3180-01) and University of Pennsylvania (AWA no. A3079-01) institutional animal care and use committees. All animals were anesthetized before the performance of any procedure, and proper steps were taken to ensure the welfare of and to minimize the suffering of all animals in these studies.
Animals and study design
Eight naturally (ie, not experimentally inoculated) SIV-infected SMs were selected for this study based on availability. Baseline viral quantification and immunophenotyping were performed 3 weeks before the beginning of treatment. On the first day of treatment and every week subsequently for a total of 16 weeks, each SM was given a subcutaneous dose of 500 000 units of recombinant RM IFN-α2-Ig fusion protein (median dose, 46 098 units/kg). Throughout the treatment period and afterward, PBMCs, lymph node (LN) biopsies, and rectal mucosa biopsies (RBs) were collected for virologic and immunologic analysis.
Recombinant RM IFN-α2-Ig fusion protein
The coding sequence of IFN-α was cloned and sequenced from several nonhuman primate species, including SMs and macaques, as described previously15 (http://pathology.emory.edu/Villinger/index.htm). The fusion of the IFNα to IgG was as described previously for PD-1–IgG and IL15RA-IgG2.16,17 Briefly, the Fc portion of a macaque IgG2 was mutated at 2 positions (L235A and P331S) to inactivate potential binding of the fusion protein to complement and to Fc receptors, respectively, to avoid the potential for complement and cell-mediated cytotoxicity of the cytokine targets. The mature IFN-α2 (165 aa) was amplified with primers IFNa12pMT (CC GGATCC TGT GAT CTA CCT CAA ACC) and IFNa13 Ig (GGT GG GCA CGT AGA TCT ACC TTC CTT ACT TCT TAA ACT TTC TTG C) and the IgG2 was amplified with primers PAmigg2b (GGTAGATCTACGTGCCCACCGTGCCCAGCTGAA) and IgG6ae (TATGACGTCGAATTCTCATTTACCCGGAGACACGGAGA). These 2 fragments were then concatamerized by another round of PCR using the primers IFNa12pMT and IgG6ae and the overlap created in the previous amplifications. The gene coding for this fusion construct was subcloned using BamHI and EcoRI into the pMT-BIP vector designed to produce soluble proteins from Schneider-2 (S-2) insect cells in spinner cultures (Invitrogen). All constructs were verified by sequencing. The protein released in the supernatant of theses culture at a concentration of 7-10 mg/L was then purified by passage over a protein G-Sepharose capture column and eluted with diluted acetic acid (pH 2.8). The approximately 50-kDa purified protein was then dialyzed extensively against PBS and tested for purity, presence of endotoxin, protein content, and biologic activity. The protein appeared > 90% pure and devoid of any significant endotoxin activity. Testing for rmIFNα2 bioactivity was performed using an EMCV neutralization bioassay on vero cells, as described previously.15
Detection of Abs against rmIFNα2 by Western blot
To verify that the SMs treated with rmIFNα2 did not generate an anti-cytokine–neutralizing response, we tested sequential plasma samples from all treated monkeys, including pretreatment to several weeks after the last administration, by Western blots against the protein. RmIFNα2 was separated through 12.5% SDS-PAGE gels transferred to a PVDF membrane. This membrane was then blocked with a 5% nonfat dry milk solution in PBS overnight before assembling into a Mini-PROTEAN II multiscreen apparatus (Bio-Rad). The plasma samples were tested at a 1:200 dilution. Bound Abs were then detected with a secondary goat anti–human kappa/alkaline phosphatase conjugate cross-reactive with monkey Ig and developed with a Bio-Rad substrate at room temperature.
Plasma RNA and cell-associated DNA viral quantification
Plasma viral quantification was performed as described previously.2 For the quantification of cell-associated viral DNA in CD4+ peripheral blood cells, frozen PBMCs were thawed in a 37°C water bath and immediately washed in DMEM supplemented with 10% FBS, l-glutamine, and penicillin/streptomycin. CD4+ peripheral blood cells were then separated by positive selection using CD4 microbeads for nonhuman primates (Miltenyi Biotec) on an LD column per the manufacturer's specifications. CD4+ peripheral blood cells were counted and resuspended in buffer RLT+ and extracted using the Blood DNA Mini Kit (QIAGEN). Quantitative real-time PCR was then performed on the extracted cell-associated DNA, as previously described.2 Viral genome copies were normalized by the number of cells.
Immunophenotyping by flow cytometry
Peripheral blood lymphocytes were separated by centrifugation in sodium citrate CPT tubes. LN and RB samples were processed as described previously using standardized procedures. Multicolor flow cytometric analysis was performed using predetermined optimal concentrations of the following fluorescently conjugated mAbs: anti–HLA-DR–PerCP-Cy5.5 (G46-6), anti–Ki67-FITC (B56), anti–CD69-APC–Cy7 (FN50), anti–CD25-APC-Cy7 (M-A251), anti–CD20-APC-H7 (L27), anti–CD3-Alexa Fluor 700 (SP34-2), anti–HLA-DR-APC (G46-6), anti–CCR5-APC (3A9), anti–CD14-PE-Cy7 (M5E2), anti–CD95-PE-Cy5 (DX2), and anti–CD62L-PE (SK11) from BD Biosciences; anti–CD28-ECD (CD28.2) and anti–CD16-PE (3G8) from Beckman Coulter; anti–CD8-PE–Texas Red (3B5) and the Aqua Blue Live/Dead Discriminator from Invitrogen; and anti–CD8-PerCP-Cy5.5 (RPA-T8), anti–CD28-PE–Cy7 (CD28.2), anti–CD4-Pacific Blue (OKT4) from eBiosciences. Flow cytometric acquisition and analysis of samples was performed on at least 100 000 events on an LSRII flow cytometer driven by the FACSDiva software package (BD Biosciences). Analysis of the acquired data were performed using FlowJo Version 7.6.5 software (TreeStar).
SIVmac239 gag and env peptide stimulations
Peptide stimulations were performed as described previously.18-20 The function of SIV-specific CD8+ T cells was assessed by flow cytometry after stimulation with peptide pools of 15-mers (overlapping by 11 amino acids) spanning the SIVmac239 Gag and Env proteins. Peptides were prepared from peptide stocks obtained from the National Institutes of Health AIDS Research and Reference Reagent Program, reconstituted in DMSO, and pooled. All peptides were used at a final concentration of 2 μg/mL. Purified PBMCs were thawed, resuspended, and stimulated as described previously. Anti-CD107a FITC (BD Biosciences) was added at the start of all stimulation periods, as described previously. The cocktail of Abs for surface staining included anti–CD8-APC–Cy7 (SK1), anti–CD3-Alexa Fluor 700 (SP34-2), and anti–CD95-PE–Cy5 (DX2) from BD Biosciences; anti–CD28-ECD (CD28.2) from Beckman Coulter; anti–CD4-Pacific Blue (OKT4) from eBiosciences; and Aqua Blue Live/Dead Discriminator from Invitrogen. The cocktail for intracellular staining included anti–CD107a-FITC (H4A3), anti–IL-2–APC (MQ1-17H12), anti–TNF-α–PE-Cy7 (Mab11), and anti–IFN-γ–PE (B27) from BD Biosciences.
RNA purification, array hybridization, and hemoglobin blocking
Methodology for purifying total RNA and microarray hybridization were described previously.4 Venous blood (2.5 mL) was collected into PAXgene blood RNA tubes (BD Biosciences) and stored at −80°C. Total RNA was purified with PAXgene Blood RNA kits (QIAGEN) according to manufacturer's protocol with on-column DNAse digestion. RNA quality was quantitated by Nanodrop analysis. Agilent Bioanalyzer capillary electrophoresis was used for quality assessment; all samples had an RIN > 8.0. RNA was hybridized to GeneChip Rhesus Macaque Genome Arrays (Affymetrix). A total of 0.5 μg of total RNA was amplified using the Affymetrix 3′ IVT Express Kit using techniques described previously,21 including the inclusion of a set of 5 peptide nucleic acid oligonucleotides (Bio-Synthesis) specific for regions of hemoglobin α and β mRNA into the reverse transcription cocktail, as described previously,4 to inhibit nonspecific binding.
Microarray data analysis
Background adjustment, normalization, and median polish summarization of .CEL files was performed using the robust multichip average algorithm.22 Robust multichip averaging was performed using Bioconductor and downstream analyses were performed using Partek Genomics Suite Version 6.4 software (Partek). One array with poor hybridization was excluded based on NUSE and RLE centroids outside of expected boundaries. To determine genes statistically changed after rmIFNα treatment, 2-way ANOVA (individual animal and time after treatment) was performed. Significantly expressed genes were defined by exhibiting a false discovery rate–corrected P < .00062. Differentially expressed genes with similar expression patterns were organized using agglomerative clustering with Pearson dissimilarity distance metric and average linkage.
Annotation of the Rhesus genome array with human gene symbol identifiers
Affymetrix annotation of the Rhesus GeneChip maps to the Rhesus genome and > 11 000 probe sets have provisional gene symbols in the National Center for Biotechnology Information Gene database (ie, “LOC” symbols). To increase the mapping of the array, probe sets lacking definitive annotation were cross-referenced to potential human orthologs identified by the online resources provided by the laboratory of Robert Norgren (Version 3, June 2010: http://www.unmc.edu/rhesusgenechip/) and the InParanoid ortholog database (release 7.0, June 2009: http://inparanoid.sbc.su.se)23 using probe set IDs and Ensembl Gene/Protein IDs, respectively.
GEO accession numbers
The microarray dataset was submitted to the Gene Expression Omnibus (GEO) online repository according to MIAME (Minimum Information About a Microarray Experiment) standards (GEO accession number GSE35460.)
Results
Treatment of chronically SIV-infected SMs with rmIFNα2 results in a strong increase in ISG expression in peripheral blood
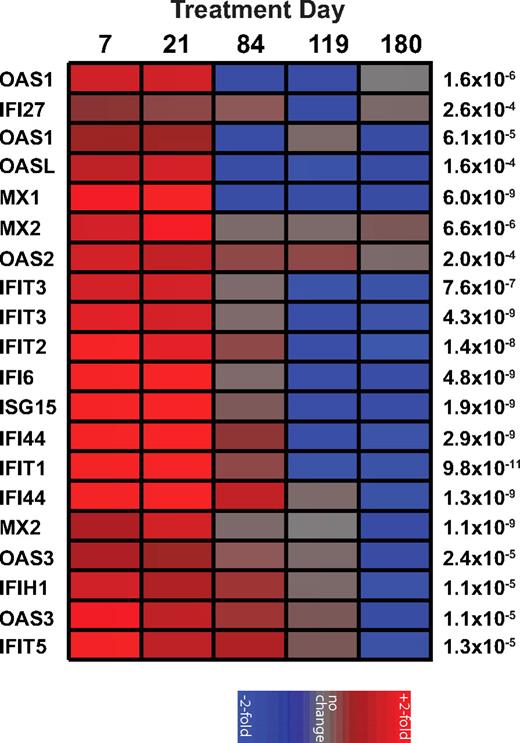
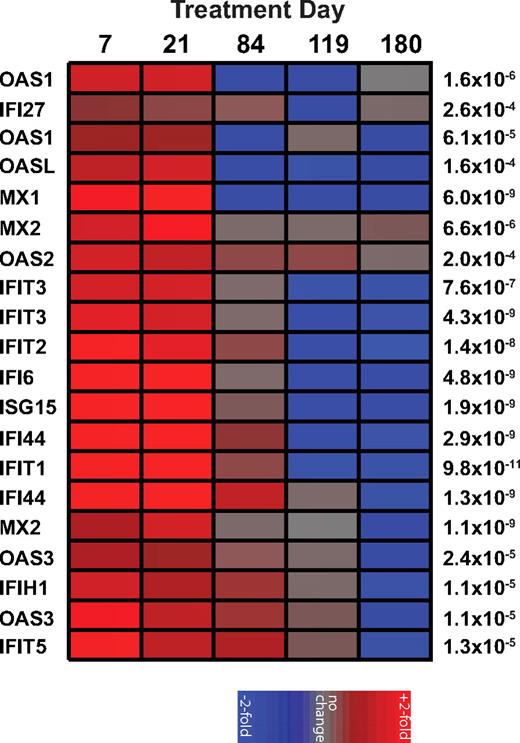
In contrast to pathogenic HIV and SIV infections of humans and macaques, chronic SIV infection of SMs is associated with low levels of immune activation and the absence of an IFN-I transcriptional signature.2,4 To test the hypothesis that low levels of IFN-I production and ISG expression contribute to the low levels of immune activation, 8 naturally SIV-infected SMs were treated with 500 000 units of rmIFNα2 subcutaneously once a week for 16 weeks. The fusion of the IFN-α2 to IgG was as described previously for PD-1-IgG and IL15RA-IgG216,17 with the Fc portion of a macaque IgG2 mutated at 2 positions (L235A and P331S) to inactivate binding to complement and to Fc receptors, respectively. Previous work from our laboratory showed that in vitro stimulation of SM PBMCs with rmIFNα2 resulted in a significant up-regulation of several ISGs as measured by real-time PCR.4 As expected based on these in vitro results, in vivo administration of rmIFNα2 induced a strong and specific up-regulation of numerous ISGs (eg, the antiviral genes OAS1, OAS2, and MX1) at days 7 and 21 of treatment relative to baseline (false discovery rate < 0.0006) as measured by gene array analysis using the Affymetrix RM gene chip that has been validated previously for use in SMs (Figure 1). The observed up-regulation of ISGs was transient, with expression levels returning to baseline by day 84 of treatment and at 2 posttreatment time points (days 118 and 180; Figure 1). Although the reasons that the rmIFNα2-induced ISG up-regulation lasted less than 12 weeks remain unclear, it is unlikely that Abs against the reagent were responsible for the decline in IFN-I sensitivity, because Western blots of serial serum samples (days −21, 7, 21, 42, 84, and 180) from the treated animals were inconclusive (data not shown). These observations indicate that rmIFNα2 was bioactive in vivo in SMs and capable of inducing significant ISG expression, demonstrating that chronically SIV-infected SMs are not intrinsically resistant to IFN-I stimulation.
Recombinant rmIFNα induces a transient increase of ISGs in vivo. A heat map of the fold change in gene expression relative to day −21 of treatment as measured from mRNA extracted from PBMCs of SIV-infected SMs (n = 8) treated longitudinally with recombinant rmIFNα2 and hybridized to Affymetrix Rhesus Arrays shows a significant up-regulation of ISGs during treatment. Genes with differential expression over time (P < .0006 by ANOVA) and documented function as ISGs are shown on the left; P values are shown on the right. Individual colored panels represent the average fold change. The color scale is indicated at bottom.
Recombinant rmIFNα induces a transient increase of ISGs in vivo. A heat map of the fold change in gene expression relative to day −21 of treatment as measured from mRNA extracted from PBMCs of SIV-infected SMs (n = 8) treated longitudinally with recombinant rmIFNα2 and hybridized to Affymetrix Rhesus Arrays shows a significant up-regulation of ISGs during treatment. Genes with differential expression over time (P < .0006 by ANOVA) and documented function as ISGs are shown on the left; P values are shown on the right. Individual colored panels represent the average fold change. The color scale is indicated at bottom.
Treatment of chronically SIV-infected SMs with rmIFNα2 results in an approximately 1-log decline in plasma viremia without changes in cell-associated viral load
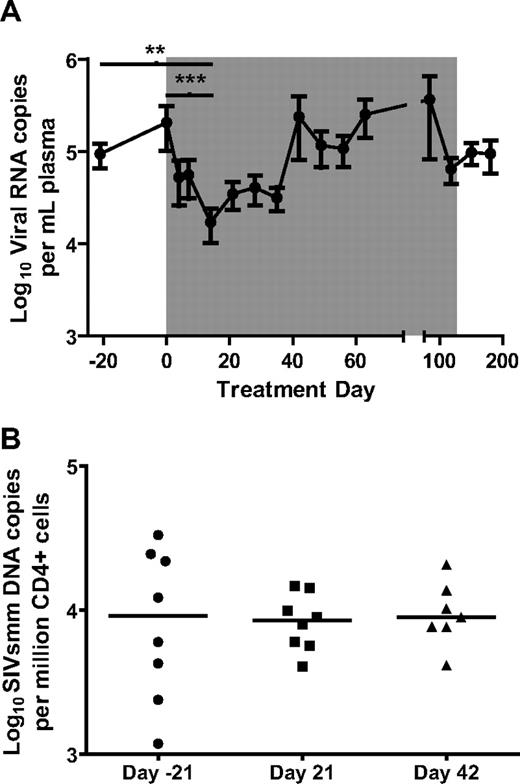
Whereas a series of recent studies have suggested that IFN-I is a potential cause for the aberrant chronic immune activation associated with pathogenic HIV/SIV infections,4,13,24,25 the canonical role of IFN-I is to induce a cellular antiviral state in response to viral infections by up-regulating the expression of several antiviral genes.26 In the context of HIV and SIV infection, IFN-I can suppress virus replication by up-regulating antiretroviral genes such as APOBECs, TRIMs, SAMHD1, and BST-2/tetherin.27-31 To determine the effect of rmIFNα2 on viral replication in the 8 treated SIV-infected SMs, viral RNA genome copy number was measured in plasma (Figure 2A) by real-time PCR. By day 14 of treatment, the mean viral load had declined significantly by approximately 1 log (Friedman test with Dunn multiple comparisons, P < .01), remained lower than baseline through day 35 and returned to pretreatment levels by day 42 of treatment (Figure 2A). Interestingly, the effect of rmIFNα2 on plasma viral load was consistent with the observation that the used reagent induced a significant up-regulation of ISGs, with elevated levels at day 7 and day 21 but not at day 84 of treatment compared with baseline (Figure 1). To better define the mechanism by which rmIFNα2 reduced plasma viral load, we also measured cell-associated SIV-DNA by real-time PCR in column-separated, CD4+ peripheral blood cells. In contrast to the significant decrease in plasma viral load observed at day 14 of treatment, the mean levels of cell-associated viral DNA did not change relative to pretreatment time points (Figure 2B). Overall, these data indicate that rmIFNα2 induced a significant but transient decline in virus replication in SIV-infected SMs.
Viral load decreased by approximately 1 log during the first 2 weeks of recombinant IFN-α treatment irrespective of the levels of infection of CD4+ cells. (A) Viral load remained low for 5 weeks, but returned to pretreatment levels by day 42 of treatment. The nadir of viral load at day 14 was significantly lower than day −21 and day 0 (Friedman test with Dunn multiple comparisons). **P < .01; ***P < .001. (B) CD4+ peripheral blood cells were sorted from PBMCs using CD4-Ig–labeled magnetic beads. Viral load was determined by real-time PCR.
Viral load decreased by approximately 1 log during the first 2 weeks of recombinant IFN-α treatment irrespective of the levels of infection of CD4+ cells. (A) Viral load remained low for 5 weeks, but returned to pretreatment levels by day 42 of treatment. The nadir of viral load at day 14 was significantly lower than day −21 and day 0 (Friedman test with Dunn multiple comparisons). **P < .01; ***P < .001. (B) CD4+ peripheral blood cells were sorted from PBMCs using CD4-Ig–labeled magnetic beads. Viral load was determined by real-time PCR.
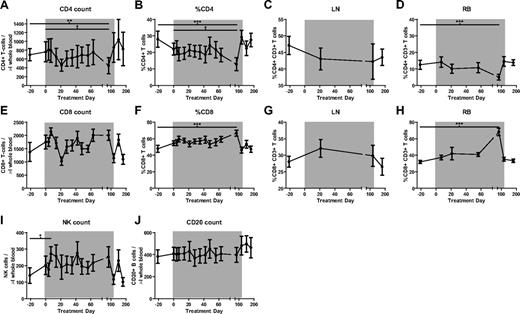
Administration of rmIFNα2 to chronically SIV-infected SMs does not result in CD4+ T-cell depletion
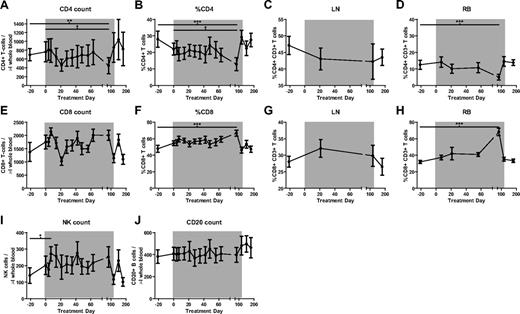
IFN-I treatment of HIV-HCV coinfected humans is used widely32,33 and has been found to be associated with a modest decrease in HIV viral load34 and a coincident development of mild lymphopenia.33,35-38 To determine the effects of IFN-I signaling augmentation as induced by rmIFNα2 on CD4+ T-cell homeostasis in chronically SIV-infected SMs, PBMCs and mononuclear cells derived from LNs and RBs were analyzed by multiparametric flow cytometry. This analysis revealed that rmIFNα2 did not induce any significant change in the absolute number or percentage of CD4+ T cells in any of the examined anatomic compartments (Figure 3A-D), aside from an isolated finding of lower CD4+ T-cell percentage and count at day 84 in peripheral blood and RBs (Friedman test with Dunn multiple comparisons, P < .05), which returned to baseline levels by the last time point during treatment. A similar analysis conducted on CD8+ T cells revealed no significant changes in their absolute numbers or percentage in peripheral blood and LNs relative to baseline, aside from the reciprocal changes expected in the percentage of CD8+ T cells in the peripheral blood and RB (Figure 3E-G). Finally, no changes were observed in the absolute numbers of circulating natural killer and B cells (Figure 3I-J), nor in the levels of plasmacytoid dendritic cells (data not shown). These immunophenotypic data indicate that treatment with rmIFNα2 was not associated with any major change in the levels of the main lymphoid subpopulations and, in particular, had no significant negative effect on CD4+ T-cell homeostasis.
The percentage of major lymphocyte populations does not change significantly during the course of recombinant IFN-α treatment of SIV-infected SMs but the numbers of CD8 T cells do decrease significantly between days 7 and 21 of treatment. Shown are counts and percentages of the major lymphocyte populations as determined by flow cytometry. (A) CD4+ T-cell counts. (B-D) % CD4+ T cells in blood (B), LNs (C), and RBs (D). (E) CD8+ T-cell counts. (F-I) %CD8+ T cells in blood (F), LNs (G), and RBs (H). (I) CD16+CD8+ natural killer (NK) cell counts. (J) CD20+ B-lymphocyte counts. Differences were determined using the Friedman test with Dunn multiple comparisons. *P < .05; **P < .01; ***P < .001.
The percentage of major lymphocyte populations does not change significantly during the course of recombinant IFN-α treatment of SIV-infected SMs but the numbers of CD8 T cells do decrease significantly between days 7 and 21 of treatment. Shown are counts and percentages of the major lymphocyte populations as determined by flow cytometry. (A) CD4+ T-cell counts. (B-D) % CD4+ T cells in blood (B), LNs (C), and RBs (D). (E) CD8+ T-cell counts. (F-I) %CD8+ T cells in blood (F), LNs (G), and RBs (H). (I) CD16+CD8+ natural killer (NK) cell counts. (J) CD20+ B-lymphocyte counts. Differences were determined using the Friedman test with Dunn multiple comparisons. *P < .05; **P < .01; ***P < .001.
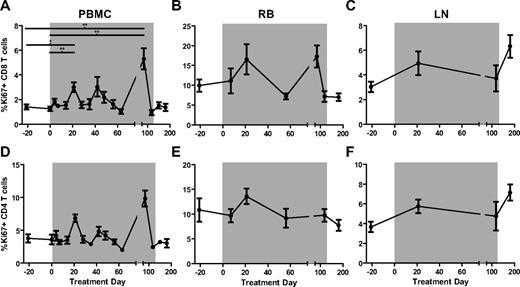
Treatment of chronically SIV-infected SMs with rmIFNα2 does not result in increased levels of lymphocyte proliferation and activation
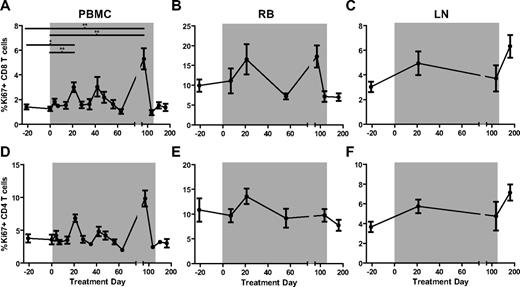
In chronically HIV-infected humans and SIV-infected RMs, high levels of immune activation are accompanied by strong ISG expression, whereas in chronically SIV-infected SMs, low levels of lymphocyte activation are associated with ISG expression similar to that observed in uninfected animals.4 To determine whether rmIFNα2 treatment and associated ISG up-regulation in chronically SIV-infected SMs results in increased levels of immune activation, we measured the percentages of CD8+ (Figure 4A-C) and CD4+ (Figure 4D-F) T cells expressing several markers of lymphocyte proliferation and activation longitudinally in the peripheral blood, RB, and LNs. We first examined the expression levels of the proliferation marker Ki67. This analysis revealed a significant but transient increase in the percentage of circulating CD8+ Ki67+ T cells at day 21 of treatment relative to baseline (Friedman test with Dunn multiple comparisons, P < .05), and no significant changes in the fraction of circulating CD4+Ki67+ T cells throughout treatment (Figure 4A,D). No significant changes in the fraction of CD8+Ki67+ or CD4+Ki67+ T cells were observed in LNs (Figure 4B,E) or RBs (Figure 4C,F). In addition, rmIFNα2 treatment was not associated with significant changes in the level of circulating or tissue-resident CD4+ or CD8+ T cells expressing the activation markers CD69, HLA-DR, and CD25 (data not shown). These data indicate that treatment with a bioactive IFN-I agonist did not induce a robust increase in the prevailing level of immune activation in chronically SIV-infected SMs.
T-cell activation measured by the proliferation antigen Ki67 that changed significantly relative to baseline (day −21 and day 0) only in CD8+ T cells sampled from peripheral blood. The levels of Ki67 on CD8+ (A-C) and CD4+ (D-F) T-cell subsets in PBMCs (A,D), RBs (B,E), and LNs (C,F) were determined by flow cytometry and analyzed using the Friedman test with Dunn multiple comparisons.
T-cell activation measured by the proliferation antigen Ki67 that changed significantly relative to baseline (day −21 and day 0) only in CD8+ T cells sampled from peripheral blood. The levels of Ki67 on CD8+ (A-C) and CD4+ (D-F) T-cell subsets in PBMCs (A,D), RBs (B,E), and LNs (C,F) were determined by flow cytometry and analyzed using the Friedman test with Dunn multiple comparisons.
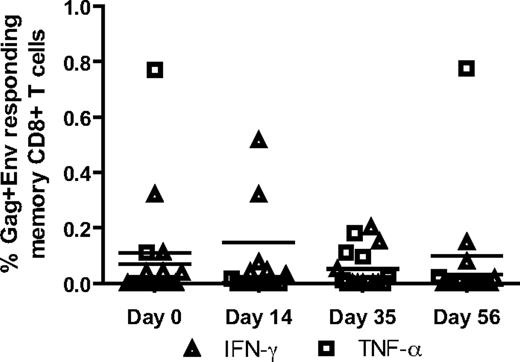
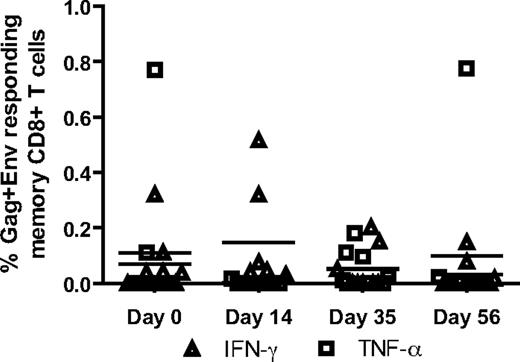
Treatment with rmIFNα2 is not associated with increased SIV-specific CD8+ T-cell responses
In several experimental systems, IFN-I provides an important third signal for the induction of robust antiviral CD8+ T-cell responses.39-41 Therefore, it is conceivable that in the present study the administration of rmIFNα2 may have resulted in an increased level of SIV-specific CD8+ T cells, which in turn could have contributed to the decrease in SIV viral load observed during treatment. To determine whether rmIFNα2 treatment resulted in an augmentation of SIV-specific CD8+ T-cell responses in our group of SIV-infected SMs, PBMCs collected at various time points before and after rmIFNα2 administration were stimulated in vitro with SIVmac239 gag and env peptide pools, and the levels of IFN-γ, TNF-α, IL-2, and CD107a were measured by intracellular staining and flow cytometric analysis. As shown in Figure 5, this analysis did not reveal any significant increase in the level of SIV-specific CD8+ T-cell responses in the tested animals, with only relatively minor fluctuations in the magnitude and functionality of the response observed between different time points. Therefore, these data do not support the hypothesis that the decline of viral load observed during rmIFNα2 treatment of SIV-infected SMs is mediated by the induction of stronger antiviral cellular immune responses.
Stimulated CD8 T cells did not produce more IFN-γ or TNF-α in response to stimulation with gag and env peptide pools during rmIFNα2 treatment. PBMCs were stimulated with gag and env peptides separately and analyzed by fluorescent Ab-mediated flow cytometry for CD8 T-cell IFN-γ and TNF-α production.
Stimulated CD8 T cells did not produce more IFN-γ or TNF-α in response to stimulation with gag and env peptide pools during rmIFNα2 treatment. PBMCs were stimulated with gag and env peptides separately and analyzed by fluorescent Ab-mediated flow cytometry for CD8 T-cell IFN-γ and TNF-α production.
Discussion
HIV and SIV infection of humans and RMs, respectively, results in a progressive immunodeficiency characterized by depletion of CD4+ T cells, destruction of lymphoid architecture, and chronic immune activation, all of which lead to an increased susceptibility to opportunistic infections. In striking contrast to the pathogenic infection of humans and macaques, SIV-infected SMs avoid AIDS despite high levels of virus replication.1,2 Whereas the mechanisms responsible for the lack of AIDS in these animals are incompletely understood, one recent study has shown that, in contrast to pathogenic SIVmac239 infection of RMs, SIV infection of SMs results in a rapid resolution of innate and adaptive antiviral immune responses coincident with the transition from the acute to the chronic phase of infection.4 This lower level of immune activation exhibited during the chronic phase of infection is associated with a dramatic down-modulation of ISGs, suggesting that resolution of the innate antiviral immune responses may be required to reach a state of nonpathogenicity in SIV-infected SMs.4 Consistent with this possibility is the growing body of experimental evidence suggesting that, despite the canonical antiviral mechanisms induced by IFN-I, long-term production of IFN-I and ISGs during a chronic viral infection can have detrimental effects on disease progression by fueling aberrant CD8+ T-cell responses,42,43 increasing apoptosis of uninfected CD4+ T cells44,45 and indirectly inducing higher viral loads.25 Whereas it is clear that IFN-I is an important contributor to the dynamics of virus-host interactions, additional studies are required to elucidate the direct and indirect influences of this pleiotropic immune effector molecule during pathogenic and nonpathogenic lentiviral infection and how the balance of these effects may be beneficial or detrimental to the host immune function.
In the present study, we administered rmIFNα2, a IFN-I agonist, to 8 chronically SIV-infected SMs in an effort to determine whether the addition of exogenous IFN-I could recapitulate some aspects of the pathogenic HIV and SIV infections of humans and macaques, such as induction of higher levels of immune activation, decline of CD4 T-cell count, and ultimately progression to AIDS. We did not use a group of control SIV-infected SMs treated with an “irrelevant” construct containing the IgG2 Fc portion coupled with an irrelevant protein. This decision was made because of the limited availability of SMs for this type of longitudinal in vivo study, which requires extensive tissue collections. Whereas this design is admittedly not ideal, it should be noted that the used fusion protein contains an IgG2-Fc that was mutated at 2 amino acid positions (L235A and P331S) to inactivate potential binding to complement and to Fc receptors, respectively.16,17 Therefore, the possibility that any biologic effect of rmIFNα2 was simply a consequence of an IgG2-Fc administration is extremely unlikely. Strikingly, we observed that 4 months of administration of IFN-I to chronically infected SMs did not result in any significant increase in immune activation nor in any signs of immune deficiency. Interestingly, IFN-I–treated SMs did exhibit an approximately 1-log decline in plasma viral load during the first 2 months of treatment, therefore confirming the well-known antiviral effect of these molecules. This virologic effect of IFN-I was transient, with levels of SIV viral load returning to baseline coincident with the loss of a IFN-I transcriptional signature in the blood. The observed suppression of virus load in SIV-infected SMs is similar in magnitude to that seen in HIV-hepatitis C virus (HCV)–coinfected patients who were treated for their HCV infection with pegylated-IFNα.34 The viral decline described in this study of HIV/HCV coinfection was more durable than what we observed in our cohort of IFN-I–treated, SIV-infected SMs.34 This discrepancy could be because of differences in the structures and bioavailabilities of rmIFNα2 and pegylated-IFNα or to the lack of chronic viral hepatitis in our SIV-infected SMs. Nevertheless, the predominant effect of IFN-I administration to SIV-infected SMs was to induce a heightened but transient antiviral state without resulting in chronic lymphocyte activation. The reason(s) that the effects of rmIFNα2 were transient and lost by day 84 of treatment in SIV-infected SMs may be complex, and include possible generation of anti-rmIFNα2 Abs and down-modulation of the expression of IFN-I receptors.
Whereas the results of the present study may downplay the pathogenic role of chronic IFN-I stimulation in immune activation and AIDS pathogenesis, it is important to note that 4 months of treatment (or, in fact, 2 months, if the rebound in viral load at day 42 is an indicator of the longevity of rmIFNα2 pharmacokinetics and bioavailability) may not have been sufficient to induce an immunopathologic state. However, although we acknowledge this limitation of the present study, we would have predicted that some effect on immune activation be observed within a few weeks of treatment if the used IFN-I agonist were to have a major impact on the phenotype of SIV infection in SMs. In particular, this effect would have been even more pronounced in the setting of a chronic viral infection in which low basal levels of IFN-I and ISG expression allow for a strong and immediate increase in ISG expression. One possible explanation for the lack of an activating effect of rmIFNα2 treatment is that infection of SMs with SIV triggers a species-specific gene-expression program that actively inhibits the activating effects of IFN-I stimulation. This phenomenon would be reminiscent of the rapid resolution of IFN-I and ISG responses observed during the transition from the acute to the chronic phase of SIV infection in SMs.4 An additional, nonmutually exclusive possibility is that the immunologic effect of “natural,” or virus-induced, IFN-I production is different from the effect of a rapid infusion of a single, massive dose of IFN-I. Indeed, on sensing the virus, plasmacytoid dendritic cells (pDCs), the primary IFN-I–producing cell during viral infections, traffic to LNs, where they secrete high levels IFN-I that appear to act mostly on cells located in close proximity.46,47 Conceivably, the dose (500 000 units) and/or route of administration (subcutaneous) of rmIFNα2 in our system, although similar to that used to treat HCV infection in humans, may not fully mimic the high levels of endogenous IFN-I observed in lymphoid tissues during pathogenic HIV and SIV. In the present study, no effect of rmIFNα2 on the levels of circulating pDCs was observed, and whether SIV pathogenesis is at least in part dependent on the presence of pDCs remains to be determined. Hopefully, the generation of reagents specific for this cell population may enable us to answer this important question.
The main goal of the present study was to provide insights on the mechanisms responsible for the striking differences in chronic immune activation observed between pathogenic and nonpathogenic primate lentivirus infections. In this regard, the presented data do not unequivocally support the hypothesis that lack of chronic IFN-I signaling is a key requirement to induce a state of nonpathogenicity in SIV-infected SMs. Instead, the observation that exogenous IFN-I treatment in SMs resulted in a significant, albeit transient, decline in SIV viral loads suggests that its antiviral effect is at least initially predominant among the many in vivo functions of this highly pleiotropic system of cytokines. In this context, the results of the present study are also consistent with a pathogenic model in which the chronic immune activation observed in HIV-infected humans or SIV-infected macaques is a complex and multifactorial phenomenon in which chronic IFN-I stimulation may simply be one of many players.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Stephanie Ehnert, Chris Souder, and all of the animal care and veterinary staff at the Yerkes National Primate Research Center; the Virology Core of the Emory Center for AIDS Research (P30-AI-50409); the University of Pennsylvania Center for AIDS Research; the University of Pennsylvania Flow Cytometry Core; the National Institutes of Health nonhuman primate reagent resource; Michaela Muller-Trutwin for helpful conversations; and Greg Tharp for bioinformatics assistance.
This work was supported by the National Institutes of Health (P01-AI076174 and R37-AI66998 to G.S.; P51-DRR00165 to the Yerkes National Primate Research Center; R24-016988 to F.V.; and T32-AI007632-10 to the University of Pennsylvania Department of Microbiology). The project was also funded by the National Center for Research Resources P51RR165 and is currently supported by the Office of Research Infrastructer Programs OD P510D11132.
National Institutes of Health
Authorship
Contribution: T.H.V. designed and performed the study, collected and analyzed the data, and wrote the manuscript; C.S., B.O.L., and R.O. collected and analyzed the data; K.A.R. and F.V. designed and produced the rmIFNα2 reagent and contributed to the study design; J.E. coordinated the veterinary needs for the animals in the study; S.E.B. collected and analyzed the microarray data; and G.S. designed and secured funding for the study and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Guido Silvestri, Division of Microbiology and Immunology, Yerkes National Primate Research Center, Department of Pathology & Laboratory Medicine, Emory University School of Medicine, 954 Gatewood Rd, Atlanta, GA 30329; e-mail: gsilves@emory.edu.