In this issue of Blood, Elvington et al present a method to enhance complement activation during antibody therapy of cancer.1 Using a fusion protein of a complement receptor and an IgG Fc fragment, therapeutic outcome was improved in vivo.
The introduction of antibody therapy has successfully transformed the treatment of several malignancies. However, most patients are not completely cured and therefore novel strategies to improve the efficacy of antibodies are badly needed. The method presented by Elvington et al may help to advance antibody therapy through targeted amplification of complement activation on tumor cells.
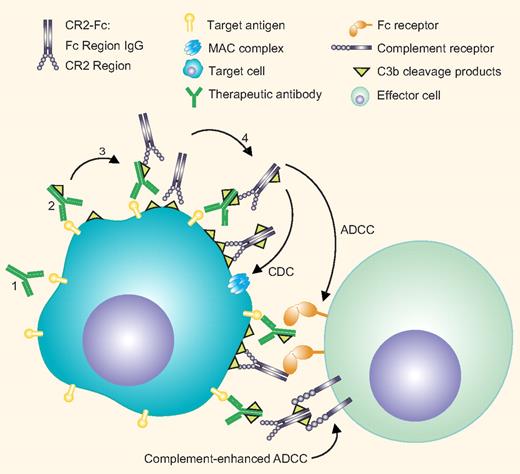
Activation of the complement system by antitumor antibodies can facilitate tumor cell destruction in several ways (see figure). Antibody-mediated complement activation results in the cleavage of C3, leading to the formation of the cleavage product C3b. Deposition of C3b on the tumor cells initiates a cascade that leads to complement-dependent lysis (CDC) via the formation of the membrane attack complex (MAC). C3b on the cell surface is rapidly degraded to inactive iC3b and more slowly to C3d and C3dg. C3 cleavage products are ligands to complement receptors (CRs), such as CR3, expressed by myeloid cells. The recognition of complement fragments by CRs on effector cells may promote direct complement-dependent cellular cytotoxicity (CDCC) or enhance Fc receptor-mediated antibody-dependent cellular cytotoxicity (ADCC).
Enhancement of complement-mediated antitumor antibody mechanisms by CR2-Fc. Antitumor antibodies bound to tumor antigens (1) activate the complement system leading to the deposition of activated C3 fragments. (2) These fragments may further activate the complement cascade eventually leading to tumor cell lysis via the formation of the membrane attack complex. Complement fragments can be recognized by complement receptors expressed on effector cells leading to direct cytotoxicity or enhanced FcR-dependent tumor killing. The CR2-Fc fusion protein targets deposited C3 fragments on the tumor cells, (3) activates complement, and generates its own ligand, thereby amplifying complement activation. (4) In addition, CR2-Fc binding increases the available Fc fragments leading to enhanced Fc receptor-mediated tumor killing. Professional illustration by Paulette Dennis.
Enhancement of complement-mediated antitumor antibody mechanisms by CR2-Fc. Antitumor antibodies bound to tumor antigens (1) activate the complement system leading to the deposition of activated C3 fragments. (2) These fragments may further activate the complement cascade eventually leading to tumor cell lysis via the formation of the membrane attack complex. Complement fragments can be recognized by complement receptors expressed on effector cells leading to direct cytotoxicity or enhanced FcR-dependent tumor killing. The CR2-Fc fusion protein targets deposited C3 fragments on the tumor cells, (3) activates complement, and generates its own ligand, thereby amplifying complement activation. (4) In addition, CR2-Fc binding increases the available Fc fragments leading to enhanced Fc receptor-mediated tumor killing. Professional illustration by Paulette Dennis.
Numerous in vitro and preclinical studies show that different antitumor antibodies can activate complement. It is, however, believed that complement-mediated effector functions are compromised in patients, mainly because tumor cells up-regulate complement regulatory proteins (CRPs) that control complement activation on normal tissues.
To improve the efficacy of therapeutic antibodies, a number of different strategies have been put forward to enhance complement-mediated tumor killing. These include selection of antibodies for potent CDC induction, engineering of existing antibodies, the use of a cocktail of antibodies recognizing multiple epitopes of onco-targets, and the use of soluble β-glycan to trigger CR3-dependent CDCC. To overcome tumor cell resistance from complement-mediated kill, down-regulation or blockage of CRPs or the use of bispecific antibodies to simultaneously engage CRPs and tumor antigen have been proposed (reviewed in Gelderman et al2 ).
Complement activation can be further enhanced by a secondary antibody directed against C3b and iC3b. An anti-iC3b antibody bound to deposited complement fragments induces the deposition of more complement, generating its own ligand and thereby further amplifying the complement cascade. This strategy has previously been shown to enhance CDC of lymphoma cell lines in vitro and to increase deposited complement when coadministered with rituximab in a cynomolgus monkey model.3,4
Imai et al have previously introduced CR2-Fc, a fusion protein between the extracellular domain of the complement receptor 2 (CR2) and an IgG Fc domain. In contrast to anti-iC3b antibodies CR2 also recognizes C3d and C3dg, the relatively long-lived complement degradation fragments. They showed that human CR2-Fc is able to target to C3 activation products, enhances CDC in vitro and is effective in a mouse xenograft model.5 The present study by Elvington et al provides data on therapeutic outcome with a murine CR2-Fc. Using 2 syngeneic mouse tumor models (metastatic lymphoma and melanoma), they demonstrate that coadministration of CR2-Fc with antitumor antibodies leads to better survival. The effect of either antibody or CR2-Fc required innate immune effector mechanisms and was dependent on the presence of macrophages.1
Interestingly, administration of CR2-Fc alone already prolonged the survival of the mice most likely due to the presence of natural antitumor IgM antibodies that efficiently activate complement. Antibody therapy of metastatic lymphoma by IgG anti-GD2 antibodies is primarily mediated by FcR.6 CR2-Fc binding also increases the number of available Fc fragments, and is shown to enhance ADCC in vitro5 and most likely also in vivo. CDC is involved in the action of CR2-Fc because no enhancement was seen in C6−/− mice that are unable to mediate CDC. However, this does not exclude the contribution of complement-enhanced ADCC to therapy. In the future this could be tested using CR3−/− mice. At present it is difficult to dissect the effector mechanisms recruited by the primary antibody and by CR2-Fc. This can be investigated by introducing mutations in the primary antibody and/or CR2-Fc that modify complement-, or FcR-binding. CR2-Fc deposition outside the tumor may trigger complement activation, which indirectly (eg, through cytokines) can lead to enhanced tumor killing by macrophages. This possibility should also be formally investigated in subsequent studies.
A possible caveat of this strategy is that CR2 ligands are constantly formed in the body in steady-state; therefore, CR2-Fc administration may potentially lead to excessive complement activation and inflammation. Human CR2-Fc was found to be accumulated in the kidneys, spleens, and livers of mice outside of the tumor,5 but preliminary results showed no toxicity.1 However, before CR2-Fc is taken into the clinical trials, thorough toxicologic investigations are required. Furthermore, the efficacy of CR2-Fc should be further tested in vivo using tumor cells engineered or selected to overexpress CRPs, mimicking the high-expression levels on complement-resistant human tumors.
This strategy is of clinical importance because CR2-Fc could potentially be used as adjuvant therapy to enhance efficacy and overcome tumor resistance in conjunction with any clinical antibody that is able to activate complement. Often complement activation is suboptimal due to low target expression or the presence of CRPs, in which case administration of CR2-Fc could tip the balance and facilitate complement activation. The requirement for both ADCC and complement-dependent mechanisms is more pronounced under suboptimal conditions, when the availability of the antibody is limited or the effector-to-target ratios are unfavorable.6,7 Certain antibodies may rely more on the help of complement than others for their action, which may underlie the discrepancy of clinical evidence regarding the role of complement during antibody therapy. This finding with CR2-Fc is a good example of how the combination of an antitumor and an immune-activating antibody can act synergistically.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■