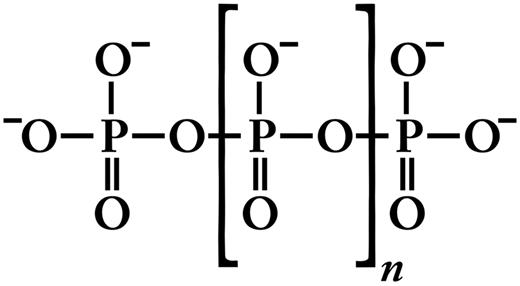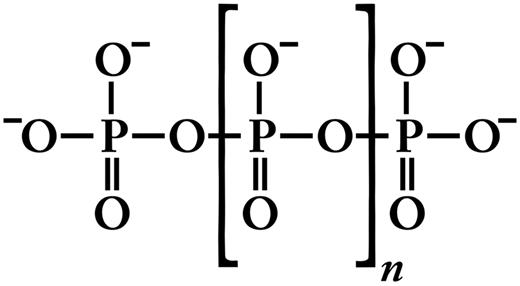Abstract
Inorganic polyphosphate is widespread in biology and exhibits striking prohemostatic, prothrombotic, and proinflammatory effects in vivo. Long-chain polyphosphate (of the size present in infectious microorganisms) is a potent, natural pathophysiologic activator of the contact pathway of blood clotting. Medium-chain polyphosphate (of the size secreted from activated human platelets) accelerates factor V activation, completely abrogates the anticoagulant function of tissue factor pathway inhibitor, enhances fibrin clot structure, and greatly accelerates factor XI activation by thrombin. Polyphosphate may have utility as a hemostatic agent, whereas antagonists of polyphosphate may function as novel antithrombotic/anti-inflammatory agents. The detailed molecular mechanisms by which polyphosphate modulates blood clotting reactions remain to be elucidated.
Introduction
Inorganic polyphosphate (polyP) is structurally very simple, consisting of linear polymers of orthophosphate linked by high-energy phosphoanhydride bonds (Figure 1). At physiologic pH, each internal phosphate unit of polyP carries a monovalent negative charge, making polyP an intensely anionic polymer. PolyP is ubiquitous in biology and can vary in polymer length from just a few phosphates to several thousand phosphate units long, depending on the organism and the tissue in which it is synthesized.1,2 PolyP is synthesized enzymatically from ATP; this reaction is fully reversible and may allow bacteria to synthesize ATP from stored polyP in times of starvation and environmental stress.3 PolyP can be degraded by endopolyphosphatases (which cleave within the polyP chain) and exopolyphosphatases (which sequentially remove terminal phosphates from polyP). Mammalian alkaline phosphatase is a potent exopolyphosphatase.4 PolyP has a half-life of approximately 1.5 to 2 hours in human blood or plasma, presumably because of exo- and/or endopolyphosphatase digestion.5,6
Structure of inorganic polyP. Inorganic polyphosphate (polyP) is a linear, highly anionic polymer of phosphates held together by high-energy phosphoanhydride bonds. Platelet polyP is approximately 60 to 100 phosphate units long,8,25,27 whereas microbial polyP can range up to thousands of phosphate units long.3,114
Structure of inorganic polyP. Inorganic polyphosphate (polyP) is a linear, highly anionic polymer of phosphates held together by high-energy phosphoanhydride bonds. Platelet polyP is approximately 60 to 100 phosphate units long,8,25,27 whereas microbial polyP can range up to thousands of phosphate units long.3,114
PolyP has been most extensively studied in prokaryotes and unicellular eukaryotes, but roles for polyP in mammalian systems are rapidly emerging. In mammalian cells, polyP has been identified in lysosomes,7 dense granules,8 mitochondria, and nuclei.9 PolyP associated with poly-3-hydroxybutyrate has been reported to be a component of the Ca2+-ATPase pump in human erythrocytes.10,11 PolyP has also been shown to induce proliferation and differentiation of mesenchymal stem cells via activation of the fibroblast growth factors.12 More recently, nucleoli of myeloma cells were shown to contain high levels of polyP compared with normal primary plasma cells.13 Other functions identified for polyP in mammalian cells include cell proliferation,14 angiogenesis,15 apoptosis,16 osteoblast function,17 bone mineralization,18,19 energy metabolism,20 and tumor metastasis.21,22
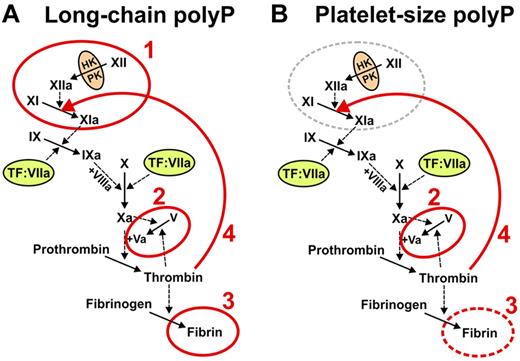
In this review, we explore the recently reported roles of polyP in blood clotting and inflammation. Studies from our laboratory and others have shown that polyP acts at the beginning, middle, and end of the blood clotting cascade (Figure 2), with prohemostatic, prothrombotic, and proinflammatory effects.5,23-30
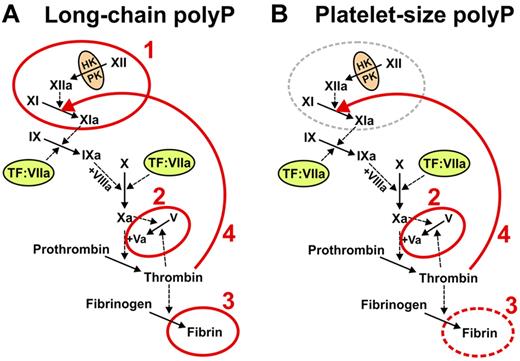
The roles of polyP in blood clotting vary depending on polymer length. (A) Microbial long-chain polyP (ranging from less than a hundred phosphates to several thousand phosphate units long) acts at 4 points in the clotting cascade, indicated in red: 1 initiates the contact pathway of blood clotting5,25,27 ; 2, accelerates factor V activation and abrogates TFPI function (the latter not shown explicitly)5,27 ; 3, enhances fibrin polymerization24,27,30 ; and 4, accelerates factor XI back-activation by thrombin.29 (B) Platelet-size polyP (∼ 60 to ∼ 100 phosphate units long) acts most potently at 3 points in the clotting cascade, indicated in red: 2 indicates abrogates TFPI function (and overlaps the minimal size necessary to accelerate factor V activation)5,27 ; 3, overlaps the minimal size necessary to enhance fibrin polymerization24,27,30 ; and 4, accelerates factor XI back-activation by thrombin.29 Reproduced from Choi et al with permission.29
The roles of polyP in blood clotting vary depending on polymer length. (A) Microbial long-chain polyP (ranging from less than a hundred phosphates to several thousand phosphate units long) acts at 4 points in the clotting cascade, indicated in red: 1 initiates the contact pathway of blood clotting5,25,27 ; 2, accelerates factor V activation and abrogates TFPI function (the latter not shown explicitly)5,27 ; 3, enhances fibrin polymerization24,27,30 ; and 4, accelerates factor XI back-activation by thrombin.29 (B) Platelet-size polyP (∼ 60 to ∼ 100 phosphate units long) acts most potently at 3 points in the clotting cascade, indicated in red: 2 indicates abrogates TFPI function (and overlaps the minimal size necessary to accelerate factor V activation)5,27 ; 3, overlaps the minimal size necessary to enhance fibrin polymerization24,27,30 ; and 4, accelerates factor XI back-activation by thrombin.29 Reproduced from Choi et al with permission.29
PolyP in microorganisms
Prokaryotic and eukaryotic microorganisms store high concentrations of polyP along with divalent metal ions, such as Ca2+, Mg2+, and Zn2+ in subcellular organelles termed acidocalcisomes (also known as volutin or metachromatic granules in some organisms).31 Microorganisms typically contain very long-chain polyP, ranging in length from hundreds to thousands of phosphate units,3 and furthermore, Neisseria species (including Neisseria meningitidis) express long-chain polyP on their capsule.32,33 In the bacteria in which it has been studied, polyP kinase 1 (PPK1), a membrane-associated enzyme, synthesizes polyP from ATP34 ; this reaction is fully reversible and may allow bacteria to resynthesize ATP from stored polyP in times of starvation.3 PolyP kinase is critical for cell viability in bacteria, as knocking out this enzyme results in defective motility and compromised survival during periods of environmental stress.35-39 PolyP activates the expression of Escherichia coli RpoS, the σ-factor responsible for activation of more than 50 genes required for survival during starvation and exposure to UV radiation, oxidative damage, and osmotic stress.40
Pseudomonas aeruginosa mutants lacking PPK1 are deficient in motility, quorum sensing, biofilm formation, and virulence; however, the mutants still possess as much as 20% of the wild-type levels of polyP, which points to an additional polyphosphate kinase, PPK2.41-43 An interesting feature of PPK2 is that it kinetically favors GTP synthesis from polyP over polyP synthesis in contrast to PPK1, which strongly favors synthesis of polyP from ATP.44,45 PPK2 may contribute to the GTP-dependent synthesis of alginate in bacterium, and its homologs have been identified in major bacterial pathogens.45 The importance of PPK1 and PPK2 in bacterial virulence and mucoid maintenance, respectively, suggests that the 2 polyP kinases may serve as possible targets for the discovery and design of novel antibiotics.
Dictyostelium discoideum, a heavily studied social slime mold, is one of a few eukaryotes known to possess a homolog of E coli PPK1.46 Similar to bacteria, D discoideum contains an additional PPK (termed DdPPK2), a tetramer that polymerizes into an actin-like filament concurrently with the synthesis of long-chain polyP.47,48 Interestingly, actin and polyP form a complex that masks the ends of the polyP chain, as the complex is susceptible to endopolyphosphatase, but not exopolyphosphatase.47 The vacuolar transporter chaperone (VTC) complex, a heteromeric membrane protein anchored in yeast vacuoles and acidocalcisomes of trypanosomatids, has been demonstrated to be essential for polyP synthesis.31 Ablation of VTC1 in Trypanosoma brucei has been shown to lead to reduced levels of polyP, osmotic sensitivity, and defects in cytokinesis.49 Furthermore, a recent report identified the VTC4 domain as the polyP-polymerizing component of the VTC complex.50
PolyP in platelets
In 1958, Hermansky and Pudlak described 2 albino patients who presented with increased bruising and prolonged bleeding.51 Subsequent studies demonstrated bleeding tendencies in patients with congenital deficiencies in platelet-dense granules (also called δ-granules).52,53 Ruiz et al noted that the dense granules of human platelets were strikingly similar in appearance to acidocalcisomes of unicellular organisms,8 as both are spherical, acidic,54 electron-dense,55 and contain divalent metal ions, including Ca2+, Mg2+, and Zn2+.56 Platelet-dense granules were known to contain inorganic phosphate and pyrophosphate,56 but whether they contained polyP was unknown. In 2004, Ruiz et al reported, for the first time, that platelet-dense granules have abundant polyP, at a concentration inside dense granules of approximately 130mM8 (expressed in terms of phosphate monomer, as polyP concentrations are typically reported in the literature). Consistent with this finding, Ruiz et al subsequently reported that patients diagnosed with platelet-dense granule defects (and experiencing bleeding symptoms) have platelet polyP levels approximately 10 times lower than normal.57 PolyP is efficiently secreted after platelet activation.8,25 Because human platelets contain 0.74 ± 0.08 μmol polyP/1011 platelets (expressed in terms of phosphate monomer),8 and given the normal range of platelets in human blood (1.5-4.5 × 1011 platelets/L), one can calculate that human blood will contain 1 to 3μM polyP after full platelet activation. In platelet-rich thrombi, local polyP concentrations are probably orders of magnitude higher than this. Unlike microbial polyP, platelet polyP is smaller and much less heterodisperse, having polymer lengths approximately 60 to 100 phosphate units long.8,25
Initiation of blood clotting via the contact pathway: a critical role for polyP
The classic blood clotting cascade can be triggered via either the tissue factor pathway or the contact pathway (Figure 2). Although the tissue factor pathway is essential for hemostasis,58 the contact pathway is not required because humans and animals lacking factor XII have no bleeding tendencies.59 As outlined in the next 2 paragraphs, however, the contact pathway plays other important roles and is triggered when factor XII, prekallikrein, and high molecular weight kininogen assemble on anionic polymers or surfaces. Factor XIIa then activates factor XI to XIa, which in turn activates factor IX, leading to propagation of the clotting cascade through the final common pathway.
Although the contact pathway is dispensable for hemostasis, it clearly participates in thrombosis. Clinical studies have associated elevated plasma factor XII with coronary heart disease, atherosclerosis,60 and recurrent coronary events after acute myocardial infarction.61 The Study of Myocardial Infarction-Leiden reported that men in the highest quintile for factor XI had an approximately 2-fold increased risk of myocardial infarction compared with the lowest quintile.62 Similarly, a Swiss case-control study showed a strong association between plasma kallikrein and factor XI activity with a history of myocardial infarction.63 Another recent study found a significant reduction in the incidence of ischemic stroke in patients with severe factor XI deficiency compared with the general Israeli population.64 Activation of the contact pathway of blood clotting thus has clinical implications in pathologic thrombus formation in humans.
Animal models also support a role for the contact pathway in thrombosis. Mice deficient in factor XII are protected against thrombus formation in a variety of models of arterial and venous thrombosis.65,66 Factor XII gene knockout in mice also results in defective immune responses to infection,67 suggesting that the contact pathway participates in host responses to pathogens. Consistent with this concept, several microbial activators of the contact pathway have been identified, including bacterial surface proteins,68,69 lipopolysaccharide,70 teichoic/lipoteichoic acid,70 and, as discussed here, long-chain polyP.5,27
The first reported role for polyP in blood clotting was a 2006 study from our laboratory that showed that polyP is strongly procoagulant, triggering clotting of plasma via the contact pathway as well as modulating downstream clotting reactions (see, in particular, step 1 in Figure 2A).5 For many years, the identity of the true (patho)physiologic activator(s) of the contact pathway has remained elusive, and indeed most published studies of this pathway have used artificial activators, such as glass, powdered clay (kaolin), diatomaceous earth, dextran sulfate, ellagic acid, or high concentrations of sulfatides. Most artificial contact activators are anionic surfaces or polymers. PolyP is also a highly anionic polymer, and we have shown that it binds tightly to the proteins responsible for initiating the contact pathway.5,27,71 Furthermore, activation of clotting via polyP has a bell-shaped concentration dependence, consistent with the idea that polyP serves as a template for assembling multiple contact factors.5,27
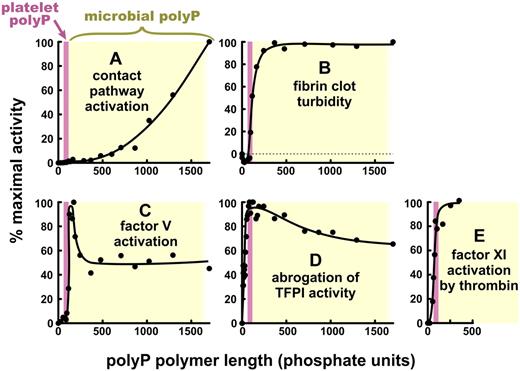
The ability of polyP to trigger the contact pathway exhibits a profound dependence on polymer length, with optimal specific activities requiring very long polyP polymers (Figure 3A).27 Consistent with this finding, polyP purified from Salmonella is extremely potent in triggering the contact pathway.27 We also found that, although platelet-derived polyP (and synthetic polyP of the same size, ie, 60-100 mers), could trigger clotting via the contact pathway,5,25 it is thousands of times less potent than very long-chain polyP (1000-2000 mers).27 These findings provide an explanation for reports, dating back to the 1960s, that activated human platelets express a weak but measurable ability to trigger the contact pathway in a factor XII-dependent manner (reviewed by Caen and Wu72 ). However, the low specific activity of platelet polyP toward the contact pathway is consistent with the idea that platelets are much more effective at accelerating clotting reactions than they are at initiating clotting (a concept elaborated in more detail in “PolyP accelerates thrombin generation” and “Platelet polyP promotes factors xl back-activated by thrombin”).
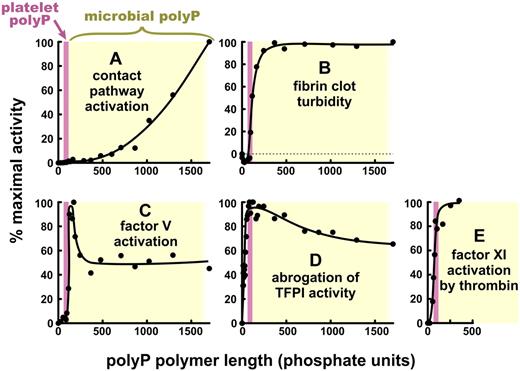
The various effects of PolyP on blood clotting are strikingly dependent on polymer length. Specific activities of polyP are plotted versus polyP polymer lengths, normalized in each case to 100% maximal activity. The data are replotted from Smith et al27 (but on linear axes) for: (A) triggering of plasma clotting via the contact pathway, (B) modulating fibrin clot turbidity, (C) accelerating factor V activation, and (D) abrogating TFPI anticoagulant function; and from Choi et al29 for (E) promoting factor XI back-activation by thrombin. (E) polyP longer than 350 mers was not tested. In each panel, the pink bar represents the approximate size of polyP secreted from activated human platelets (∼ 60 to ∼ 100 mers),8,25,27 and yellow rectangle, the approximate size of microbial polyphosphate (ranging from < 100 mers to > 1500 mers).27
The various effects of PolyP on blood clotting are strikingly dependent on polymer length. Specific activities of polyP are plotted versus polyP polymer lengths, normalized in each case to 100% maximal activity. The data are replotted from Smith et al27 (but on linear axes) for: (A) triggering of plasma clotting via the contact pathway, (B) modulating fibrin clot turbidity, (C) accelerating factor V activation, and (D) abrogating TFPI anticoagulant function; and from Choi et al29 for (E) promoting factor XI back-activation by thrombin. (E) polyP longer than 350 mers was not tested. In each panel, the pink bar represents the approximate size of polyP secreted from activated human platelets (∼ 60 to ∼ 100 mers),8,25,27 and yellow rectangle, the approximate size of microbial polyphosphate (ranging from < 100 mers to > 1500 mers).27
PolyP accelerates thrombin generation
As the essential cofactor for activation of prothrombin by factor Xa, factor Va occupies a central place in the clotting cascade (Figure 2). We found that polyP accelerates the proteolytic conversion of factor V to Va by both factor Xa and thrombin, resulting in an accelerated thrombin burst during plasma clotting reactions.5 This function of polyP requires much shorter polymers than those required for efficient triggering of the contact pathway. Indeed, we found that platelet-size polyP is within the range that will support this reaction, although optimal activity required polyP polymers slightly longer than those secreted by platelets (Figures 2B and 3C).27 Accelerating factor V activation has interesting consequences for blood clotting.5 When we added roughly platelet-sized polyP to plasma and triggered clotting with either tissue factor-liposomes or factor Xa plus phospholipids, the anticoagulant function of tissue factor pathway inhibitor (TFPI) was totally abrogated (Figure 3D).5,27 TFPI is a multifunctional serine protease inhibitor in plasma and platelets, and one of its most important targets is thought to be newly generated factor Xa.73 Mast and Broze showed that TFPI is a poor factor Xa inhibitor once factor Xa is bound to factor Va, especially in the presence of plasma concentrations of prothrombin.74 Consistent with this observation, we found that spiking plasma with factor Va also abrogates TFPI's anticoagulant activity.5 Interestingly, however, shorter polyP polymers are required to abrogate TFPI function than to enhance factor V activation (compare Figure 3C with Figure 3D), suggesting that polyP may be directly interacting with TFPI. We further found that platelet releasates strongly inhibit TFPI function and that almost all of this TFPI-abrogating activity is directly attributable to polyP.5,25 And finally, platelets from Hermansky-Pudlak syndrome patients exhibit reduced plasma clotting activity, which could be restored by adding polyP.25 Taken together, these studies show that polyP secreted by human platelets profoundly enhances blood clotting reactions.
PolyP enhances fibrin clot structure and stability
Thrombin converts fibrinogen into fibrin by limited proteolysis, and the resulting fibrin monomers spontaneously associate to form a 3-dimensional clot. When we mixed fibrinogen with polyP plus plasma concentrations of Ca2+, then added thrombin, the resulting fibrin clots were more turbid, had fibrils with higher mass/length ratios, were more resistant to elastic stretching, and were more resistant to fibrinolysis than were clots formed under identical conditions but without polyP (Figure 3B).24 After such clots were washed extensively with buffer, they exhibited strong, metachromatic staining with toluidine blue, characteristic for the presence of polyP.24 Thus, polyP appears to become incorporated into fibrin clots, although how polyP alters fibrin clot structure is not known. Heparin, another highly anionic linear polymer, also increases fibrin clot turbidity75 but unlike the case with polyP, clots formed in the presence of heparin have increased susceptibility to fibrinolysis.76,77 PolyP therefore alters fibrin clot structure in a manner different from that of heparin or other anionic polymers.78 PolyP of the size secreted by activated platelets is just within the range necessary to enhance fibrin clot structure (Figure 3B), although optimal fibrin enhancement requires longer polyP polymers.27
We also discovered that pyrophosphate abrogates the ability of polyP to enhance fibrin clot structure while having no discernible effect on fibrin clots formed in the absence of polyP.27 It has been known for many years that activated platelets secrete pyrophosphate in relatively large amounts (comparable with the amount of polyP that they secrete8 ); but to our knowledge, no convincing roles for platelet pyrophosphate have been proposed. Our studies suggest that pyrophosphate may be an overlooked regulator of fibrin clot structure.27
Mutch et al reported that polyP attenuates fibrinolysis by inhibiting the binding of fibrin to tissue-type plasminogen activator or plasminogen.30 PolyP also slows fibrinolysis in plasma clots in a manner that is dependent on the presence of thrombin-activatable fibrinolysis inhibitor, a carboxypeptidase that removes C-terminal lysine residues from fibrin.5 This latter function may be a consequence of the earlier thrombin burst when plasma is clotted in the presence of polyP.
Platelet polyP promotes factor XI back-activation by thrombin
In the classic “waterfall” model of the blood clotting cascade, factor XI is activated by factor XIIa (step 1 in Figure 2A). As noted in “Initiation of blood clotting via the contact pathway: a critical role for polyP,” severe factor XII deficiency does not result in a bleeding diathesis in humans or mice; but on the other hand, severe congenital deficiency in factor XI is associated with significant bleeding tendencies in humans, particularly after surgery or trauma and most particularly in tissues with a high fibrinolytic potential.79 Thus, in normal hemostasis, factor XI must be activated by a protease other than factor XIIa. In 1991, 2 groups reported that thrombin can feed back to activate factor XI, providing a potential solution to the riddle of how factor XI functions in hemostasis.80,81 This feedback loop could provide sustained thrombin generation and possibly also decreased fibrinolysis via thrombin-activatable fibrinolysis inhibitor activation. It was quickly noted, however, that the kinetics of factor XI activation by thrombin are extremely slow in the absence of nonphysiologic polyanions, such as dextran sulfate, heparin, or high levels of sulfatides.80-83 Given the high concentration of competing thrombin substrates in plasma, this led some to question the physiologic significance of factor XI activation by thrombin.84 We recently discovered that polyP potently accelerates factor XI activation by both thrombin (step 4 in Figure 2) and factor XIa (ie, factor XI autoactivation).29 PolyP of the size secreted by platelets strongly supports these reactions (Figure 3E). We also found that platelet releasates strongly promote factor XI activation by thrombin, and we showed that this activity is the result of polyP.29 PolyP binds to both thrombin and factor XI, and factor XI activation by thrombin exhibits a bell-shaped concentration dependence on polyP, consistent with a template-based mechanism.5,26,29,71 These studies therefore show that platelet polyP is a potent, natural cofactor for factor XI activation by thrombin, explaining how factor XI functions in normal hemostasis independent of factor XII.
PolyP drives inflammation and thrombosis
The contact pathway plays important roles in inflammation. Activation of the contact pathway results in proteolytic liberation of bradykinin (a potent vasoactive peptide) from high molecular weight kininogen. Furthermore, a body of work has shown that components of the contact pathway can be selectively activated on cell surfaces, with or without triggering of clotting via activation of factor XI by factor XIIa.85 Accordingly, the contact pathway is perhaps more accurately termed the kallikrein-kinin system, which is implicated in acute and chronic inflammation in a number of human diseases.86,87 The kallikrein-kinin system also represents a point of cross-talk between the clotting and complement systems. In addition to bradykinin generation, kallikrein has been shown to directly activate complement components C3 and C5,88,89 whereas factor XIIa also initiates the classic complement cascade.90 A tragic example of pathologic activation of the contact pathway in vivo occurred in 2008, when therapeutic heparin contaminated with oversulfated chondroitin sulfate entered the drug supply, resulting in 81 deaths.91 Follow-up studies confirmed that oversulfated chondroitin sulfate (a highly anionic glycosaminoglycan) triggers the contact system, resulting in bradykinin generation and activation of complement components C3 and C5 in a factor XII- and kallikrein-dependent manner.92
Our studies have shown that long-chain polyP is an extremely potent trigger of the contact pathway,5,25,27 raising the possibility that polyP could drive inflammatory reactions. A recent study from Müller et al investigated in vivo roles for polyP using mouse models of inflammation and thrombosis.25 PolyP administered intravenously at high doses to wild-type mice led to lethal pulmonary embolism, whereas factor XII-deficient mice survived, as did wild-type mice administered an inhibitor of factor XIIa (CSL829). In other experiments, platelets were activated in vivo in wild-type mice by intravenous injection of an agonist peptide that stimulates protease-activated receptors, resulting in death by pulmonary embolism.25 Factor XII-deficient mice were protected from this otherwise lethal challenge, as were wild-type mice given high doses of alkaline phosphatase intravenously before administration of agonist peptide (to digest polyP). These experiments demonstrate that polyP can be thrombogenic in vivo, in a factor XII-dependent manner.25
As with other activators of the contact system, polyP promotes proteolysis of high molecular weight kininogen with concomitant bradykinin release.25 In a mouse model of edema, mice were injected subcutaneously with polyP, which provoked localized capillary leak (quantified by extravasation of Evans blue dye). Mice lacking either factor XII or the bradykinin B2 receptor were protected from polyP-induced edema, as were wild-type mice that had been administered factor XIIa inhibitors. In another mouse model, intraperitoneal injection of E coli–derived polyP into wild-type mice led to a rapid drop in systemic arterial blood pressure and death of 90% of the mice in 15 minutes, whereas mice lacking either factor XII or bradykinin B2 receptors survived this challenge.25 These experiments show that polyP can be strongly proinflammatory in vivo, in a manner that depends on factor XII activation and bradykinin generation.
The role of polyP in inflammation independent of the contact pathway has also received increasing attention in recent years. Extracellular histones have been shown to exhibit potent proinflammatory and procoagulant activities.93 Semeraro et al have shown that polyP substantially augments the procoagulant activity of histones, resulting in enhanced platelet activation and thrombin generation independent of factor XII or tissue factor.28 The mechanism by which polyP enhances histones' procoagulant activity is not known. Another recent study from Bae et al reported that polyP exerts a proinflammatory effect via NF-κB activation.94 It is conceivable that polyP may also interact with pathogen-associated molecular pattern receptors on the cell surface or with chemokines to modulate inflammatory reactions. Future studies with pathogen-derived polyP are required to assess the multiple roles of polyP in host-pathogen responses.
Methods for preparing and analyzing polyP
An expanding body of research is investigating the roles of polyP in biologic systems, but difficulties facing researchers in this field include the relative scarcity of commercial sources of size-fractionated polyP, as well as a dearth of techniques for manipulating and detecting polyP at microgram and nanogram quantities (other than using radiolabeled polyP, which is not always practical). The late Dr Arthur Kornberg, in his last paper on polyP biology, stated “for more widespread research on polyP, improvements in assay methods are needed. Among the reasons for the neglect of polyP research has been the lack of sensitive and facile analytical methods to assess its concentration in biologic sources….”45 page 608 Recent studies from several groups, including our own, are starting to fill this gap.
PolyP is an industrial chemical with applications in water treatment, fertilizer production, food processing, and the production of flame retardants.95 It can be chemically synthesized in very large quantities (metric tons) simply by heating sodium orthophosphate to a few hundred degrees Celsius and then rapidly cooling.96 Commercial sources for heterodisperse polyP preparations of various sizes include Sigma-Aldrich and BK Giulini. A preparation of water-soluble, high molecular weight polyP can be isolated from very high molecular weight “insoluble” polyP by differential solubilization using LiCl solutions.96 Other size ranges of polyP can be prepared via partial alkaline or acidic hydrolysis of long-chain polyP, enzymatic degradation with endopolyphosphatases, or differential acetone precipitation under various salt conditions.96 Narrow size fractions of polyP are best prepared using preparative gel electrophoresis.27,97 Polymer lengths of short- to medium-chain polyP can be characterized using paper chromatography, ion-exchange chromatography (2-12 mers),98 gel-filtration, or 31P-NMR spectroscopy.8,25,27 Larger and more heterodisperse polymers are best sized using analytical gel electrophoresis.27,97 PolyP may be detected in polyacrylamide gels using either toluidine blue or 4,6-diamidino-2-phenylindole staining.96,99 PolyP can be quantified as monophosphate after complete hydrolysis by acid or by enzymatic digestion (with, for example, exopolyphosphatase from Saccharomyces cerevisiae or calf intestinal alkaline phosphatase).96,100 In solution and in cells and tissues, polyP can also be detected (and, to some extent, quantified) using the same dyes that are used to stain polyP in gels, including toluidine blue96,101,102 or 4,6-diamidino-2-phenylindole,100,103-105 which exhibit metachromatic staining of polyP. Low levels of polyP can be accurately quantified using enzymatic methods, although these approaches are laborious and some of them require using radioisotopes (32P) for high sensitivity.96,106
The ubiquitous nature of polyP throughout biology, together with its simple structure, make it unlikely that specific blocking antibodies could be raised against polyP. Alternative methods for targeting polyP are being developed, however. For example, we successfully used the isolated polyP-binding domain of E coli exopolyphosphatase (ie, lacking the phosphatase catalytic domain) to block the procoagulant function of polyP in platelet releasates,29 in a manner akin to using a blocking antibody. Alternatively, recombinant yeast exopolyphosphatase has been used to digest polyP.13 Alkaline phosphatase (a highly active exopolyphosphatase), which also enzymatically degrades polyP, has been successfully used to destroy polyP procoagulant activity in vitro.5,25 In vivo, treatment of polyP with alkaline phosphatase abrogated activation of the contact system and bradykinin generation, abolished procoagulant platelet activity, and blocked platelet-induced thrombosis in mice.25 A potential drawback to using a relative nonspecific enzyme, such as alkaline phosphatase, is that it can also enzymatically degrade other phosphate-containing compounds, such as ADP, an important platelet agonist. And finally, the isolated polyP binding domain of E coli exopolyphosphatase has been used successfully as a probe to localize polyP in yeast cells107 and in acidocalcisomes in eggs of sea urchins108 and insects.109
For a variety of experiments, it would be desirable to be able to covalently attach biotin, epitope tags, dyes, fluorophores, etc, to polyP, and also to covalently immobilize polyP onto solid supports, such as magnetic beads and multiwell plates. PolyP can be immobilized onto zirconia beads via Lewis acid/base interactions,110 a method we have used to quantify exosite II–mediated thrombin binding to immobilized polyP.26 Unfortunately, this coupling chemistry is not suitable for attaching any other sorts of probes to polyP. Recently, however, we identified reaction conditions under which the terminal phosphates of polyP can be made to enter into stable phosphoramidate linkages with almost any primary amine-containing compound, using the zero-length coupling reagent, 1-ethyl-3-[3-dimethylaminopropyl] carbodiimide.71 We have successfully used this coupling chemistry to covalently attach probes, such as biotin, fluorophores, and polyamines, to the terminal phosphates of polyP, and we have successfully coupled polyP to a variety of solid supports.71 This opens up a number of possibilities, including: identifying polyP-binding proteins in biologic samples, developing high-throughput screens for polyP inhibitors, using biotin- or fluorophore-tagged polyP to visualize polyP in vitro and in vivo (including flow cytometry), and developing therapeutics based on coupling polyP to solid supports (such as wound dressings) or to targeting molecules or nanoparticles. Interestingly, end-labeling polyP covalently with biotin or polyamines did not diminish its procoagulant activity but did protect it from exopolyphosphatase digestion.71
Conclusions and some future directions
The studies reviewed herein show that polyP secreted by activated human platelets accelerates factor V activation and abrogates the anticoagulant function of TFPI. Platelet polyP also potently supports factor XI activation by both thrombin and factor XIa (ie, factor XI autoactivation). These findings therefore have the potential to explain previously unexplained abilities of activated platelets to enhance blood clotting reactions.72 It is likely that polyP has other, as yet undiscovered, roles in blood clotting.
In a study not cited herein, we reported that polyP of approximately the size released by activated platelets can reverse the anticoagulant activity of a variety of anticoagulants, including unfractionated and low molecular weight heparins, as well as direct inhibitors of thrombin and factor Xa.23 PolyP can also shorten the clotting times of plasma from patients with hemophilia A or B, or patients taking vitamin K antagonists.23 It is thus tempting to speculate that polyP of the size secreted by human platelets (or suitable polyP derivatives) might be useful as injectable hemostatic agents. Long-chain polyP may have utility as a topical hemostatic agent, and indeed, adding polyP to chitosan-based wound dressings made them substantially more procoagulant.111
Although polyP and heparin are both linear, highly anionic polymers, they exhibit very different effects on blood clotting. Unlike heparin, polyP has no effect on antithrombin-mediated inhibition of thrombin or factor Xa.26 Short-chain polyP accelerates the autoactivation of factor seven-activating protease more potently than does unfractionated heparin; however, unlike heparin, polyP does not serve as a cofactor for factor seven-activating protease inhibition by antithrombin.112
Oscar Ratnoff, the late discoverer of factor XII, proposed the concept that blood coagulation, fibrinolysis, and inflammation are intimately related via surface contact, which he called “a seamless web of host defense reactions.”113 Together, the studies reviewed herein provide evidence that polyP is a key player in the web of host-pathogen interactions. Indeed, long-chain microbial polyP is a potent activator of the blood clotting system via the contact pathway and can trigger both thrombosis and inflammation (the latter via bradykinin generation and possibly complement activation). The clear contributions of polyP to thrombus formation and inflammation suggest that antagonizing polyP function in vivo may be an attractive approach for identifying novel antithrombotic/anti-inflammatory agents, perhaps with reduced bleeding side effects compared with conventional anticoagulant/antithrombotic drugs.
In conclusion, the detailed molecular mechanisms by which polyP acts as such a potent modulator of blood clotting and inflammation are still largely unknown, so this will be a fruitful and interesting topic for much future research.
Acknowledgments
This work was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health (grant R01 HL047014, J.H.M.; and grant F30 HL107089, S.H.C.).
National Institutes of Health
Authorship
Contribution: J.H.M., S.H.C., and S.A.S. performed literature reviews and designed and wrote the manuscript; and J.H.M. designed the original figures.
Conflict-of-interest disclosure: All authors are coinventors on patents and pending patent applications on medical uses of polyP.
Correspondence: James H. Morrissey, Biochemistry Department, University of Illinois, 417 Medical Sciences Bldg, MC-714, 506 S Mathews Ave, Urbana, IL 61801; e-mail: jhmorris@illinois.edu.