Abstract
Constitutive activation of the kinases Akt or protein kinase C (PKC) in blood cancers promotes tumor-cell proliferation and survival and is associated with poor patient survival. The mammalian target of rapamycin (mTOR) complex 2 (mTORC2) regulates the stability of Akt and conventional PKC (cPKC; PKCα and PKCβ) proteins by phosphorylating the highly conserved turn motif of these proteins. In cells that lack mTORC2 function, the turn motif phosphorylation of Akt and cPKC is abolished and therefore Akt and cPKC protein stability is impaired. However, the chaperone protein HSP90 can stabilize Akt and cPKC, partially rescuing the expression of these proteins. In the present study, we investigated the antitumor effects of inhibiting mTORC2 plus HSP90 in mouse and human leukemia cell models and show that the HSP90 inhibitor 17-allylaminogeldanamycin (17-AAG) preferentially inhibits Akt and cPKC expression and promotes cell death in mTORC2 deficient pre-B leukemia cells. Furthermore, we show that 17-AAG selectively inhibits mTORC2 deficient leukemia cell growth in vivo. Finally, we show that the mTOR inhibitors rapamycin and pp242 work together with 17-AAG to inhibit leukemia cell growth to a greater extent than either drug alone. These studies provide a mechanistic and clinical rationale to combine mTOR inhibitors with chaperone protein inhibitors to treat human blood cancers.
Introduction
Constitutive activation of the AGC kinase protein kinase B/Akt is observed frequently in blood cancers and is associated with poor patient survival.1-3 Constitutive phosphorylation of Akt at residues S473 and T308, which are required for full Akt activation, is observed in most patients with acute myelogenous leukemia (70%-80%).1,2 Constitutive Akt activity promotes tumor-cell proliferation by phosphorylating and suppressing the cell-cycle inhibitor p27Kip1 and the F-box–containing transcription factor FoxO1.4-6 Akt activation also promotes tumor-cell survival by directly phosphorylating and inhibiting the proapoptotic protein BAD and by suppressing the degradation of the antiapoptotic protein Mcl-1 by inhibiting GSK3.3,7,8 Akt activity is regulated by mammalian target of rapamycin complex 2 (mTORC2), which phosphorylates Akt at S473.9-11 Pharmacologic or genetic inhibition of mTORC2 abolishes growth-factor–dependent Akt S473 phosphorylation and impairs Akt signaling.9-12
mTORC2 is composed of mTOR, Rictor, MAPK-associated protein 1 (Mapkap1/Sin1), mLST8, Protein observed with Rictor (Protor/PRR5,) and DEP-domain–containing mTOR-interacting protein (DEPTOR).13 mTORC2 function may be inhibited by rapamycin in cells that are chronically exposed to the drug; however, the precise mechanism through which this inhibition occurs is unclear.9,14 Genetic deletion of Rictor, Sin1, or mLST8 in mammalian cells results in the disruption of the mTORC2 complex and the loss of mTORC2 function.9,10,15 mTORC2 regulates the activity and stability of the AGC family kinases Akt and conventional protein kinase C (cPKC) in both a growth-factor–dependent and growth-factor–independent manner.9-11,15-17 Growth factor signals activate mTORC2, which in turn phosphorylates a conserved serine residue in the hydrophobic motif (HM, S473 in Akt1) of Akt and directs Akt kinase activity toward a selected subset of substrates such as FoxO1 and FoxO3a.9,13
The mTOR inhibitor rapamycin and the rapamycin-related rapalogs temsirolimus and everolimus suppress leukemic cell growth.18 However, these compounds generally do not promote tumor cell death due, at least in part, to the inability of these drugs to effectively block mTORC2 and Akt signaling.18 Small-molecule mTOR kinase inhibitors are capable of inhibiting the rapamycin-sensitive function of both mTOR and mTORC2 directly. Consequently, the mTOR kinase inhibitors impair Akt signaling by blocking mTORC2-dependent Akt S473 phosphorylation.12,19 mTOR kinase inhibitors exhibit an improved ability to suppress leukemic cell growth and to promote leukemic cell death in models of BCR-Abl+ B-cell acute lymphoblastic leukemia (B-ALL), T-ALL, and acute myelogenous leukemia.20-23 However, mTORC2 is not the sole kinase capable of phosphorylating Akt at the hydrophobic motif, and other kinases such as DNA-PK and TBK1 may function independently of mTOR to regulate Akt signaling.24,25 Therefore, the clinical utility of mTOR kinase inhibitors may be reduced against tumors that use alternative pathways to activate Akt signaling.
mTORC2 also regulates the stability of Akt and cPKC proteins in a growth-factor–independent manner.17 mTORC2 is required for the phosphorylation of the conserved turn motif (TM) of Akt (T450 in Akt1) and cPKC (T638 on PKCα and T641 in PKCβII).16,17 mTORC2 associates with actively translating ribosomes and phosphorylates the TM sites of nascent Akt and cPKC polypeptides during translation.26 TM site phosphorylation promotes the proper folding of newly synthesized Akt or cPKC polypeptides. In Sin1−/− or Rictor−/− cells, in which mTORC2 is disrupted, TM phosphorylation of Akt and cPKC is abolished, leading to reduced stability of Akt and cPKC.16,17 However, the stability of Akt proteins lacking TM phosphorylation is partially rescued through association with the chaperone protein HSP90.17 Inhibition of HSP90 in Sin1−/− mouse embryonic fibroblasts results in the rapid reduction of Akt protein expression.17
Because Akt is abnormally activated in many human cancers, in the present study, we explored the idea that inhibition of both mTORC2 and HSP90 in leukemia cells would synergistically decrease Akt expression and inhibit tumor cell proliferation and survival. We tested this novel therapeutic strategy by investigating the effect of 17-allylaminogeldanamycin (17-AAG) on Akt expression, cell proliferation, and survival in Sin1+/+ and Sin1−/− mouse v-Abl and p210 BCR-Abl–transformed pre-B leukemia cell lines. We show that 17-AAG inhibits Akt expression preferentially and promotes the cell death of Sin1−/−–deficient leukemia cells, but not wild-type leukemia cells. Furthermore, we demonstrate that 17-AAG specifically inhibits the in vivo tumor growth of Sin1−/−, but not Sin1+/+, p210 BCR-Abl–transformed leukemia cells in mice. Finally, we show that chronic rapamycin treatment inhibits mTORC2 and sensitizes wild-type mouse pre-B and human T-cell leukemia to 17-AAG–dependent inhibition of Akt expression and cell death. The results of the present study suggest that combining the chaperone protein inhibitor 17-AAG with mTOR inhibitors may be a promising anticancer strategy.
Methods
Mice
B6.SJL-Ptprca Pep3b/BoyJ mice were purchased from The Jackson Laboratory and used as recipients for the p210 BCR-Abl leukemia cell transfers. Mice receiving leukemia cells were irradiated with 300 cGy 24 hours before leukemia cell transfer, and 1 × 106 leukemia cells were injected via the tail vein. Mice were treated with vehicle only (sterile corn oil) or 17-AAG (80 mg/kg/d in sterile corn oil) delivered by 1 IP injection per day. All mice were housed in the animal facilities at Yale University and all animal procedures were approved by the Yale institutional animal care and use committee.
Abs
Anti–phospho-PKC α/βIIT638/641, anti–phospho-Akt T450, anti–phospho-Akt S473, anti–phospho-Akt T308, anti–pan-Akt, anti–phospho-S6 S235/236, and anti-S6 were purchased from Cell Signaling Technology. Anti–PKCβ2 and anti-ERK2 were from Santa Cruz Biotechnology. Anti-Sin1 Ab was described previously.9 Reagents used for flow cytometry were as follows: annexin V-PE (BD Pharmingen), propidium iodide (PI) and CD45.2-PE (eBiosciences), and NGFR-Alexa Fluor 647 (a kind gift of Warren Schlomchik, Yale University). All reagents were used at a 1:100 dilution of the stock from the company.
Inhibitors
17-AAG (10mM; LC Laboratories) stocks were prepared in DMSO and used at a final concentration of 5μM, which was used for the in vitro experiments. For in vivo experiments, 17-AAG was first prepared at a stock concentration of 150 mg/mL in DMSO, further diluted with corn oil to a final concentration of 8 mg/mL, and used at a dose of 80 mg/kg/d delivered by IP injection. Imatinib (10mM; LC Laboratories) stocks were prepared in sterile water and used at a final concentration of 10μM. Rapamycin (LC Laboratories) was prepared as a 10mM stock in ethanol and used at a final concentration of 100nM. Wortmannin and LY294002 (10mM; Sigma-Aldrich) stocks were prepared in DMSO and used at a final concentration of 20μM. pp24212 (20mM) stock was prepared in DMSO and used at a final concentration of 400nM.
Retroviral vectors and generation of virus stocks
Abelson murine leukemia virus supernatant was kindly provided by Dr Yuan Zhuang (Duke University, Durham, NC). The p210 BCR-Abl retroviral supernatant was prepared by transfecting 293T cells with the retroviral plasmid plus ecotropic packaging plasmids. All viral supernatants were sterile filtered and stored at 4°C.
Generation of transformed cell lines
Sin1+/+ and Sin1−/− pro-B cells were described previously.27 Wild-type or Sin1-knockout pro-B cells were infected with Abelson murine leukemia virus or p210 BCR Abl retrovirus supernatants in the presence of 4 μg/mL of polybrene and centrifuged for 90 minutes at 800g at 37°C with RPMI 1640 + 10% FBS culture medium supplemented with recombinant mouse IL-7 (PeproTech). Infected cells were cultured in RPMI 1640 medium supplemented with 10% FBS, 50μM β-mercaptoethanol, and antibiotics (without IL-7) at 37°C in a 5% CO2 incubator.
Cell lines and culture
Jurkat cells, Abelson murine leukemia virus (Ab-MuLV) cells, or p210 BCR-Abl–transformed mouse pre-B leukemia cells were maintained in RPMI 1640 medium with 10% FBS, 100 U/mL of penicillin, 100 μg/mL of streptomycin, 5 μg/mL of gentamicin, and 50μM β-mercaptoethanol at 37°C in a 5% CO2 incubator. Cells were monitored daily and fresh medium were exchanged when needed.
Analysis of cell death
Control or treated cells were incubated with propidium iodide (PI) and annexin V-PE in annexin V binding buffer (10mM HEPES, pH 7.3, 150mM NaCl, and 1.8mM CaCl2) at room temperature for 15 minutes, and then analyzed by flow cytometry.
Immunoblotting
Cells were washed with PBS and lysed in RIPA buffer containing 50mM Tris-HCl, pH 8.0, 150mM NaCl, 1% Triton X-100, 1% Na-deoxycholate, 0.1% SDS, 1mM EDTA, 1mM EGTA, 1mM PMSF, 10 μg/mL of aprotinin, 10 μg/mL of leupeptin, 25mM NaF, 1mM Na3VO4, 25mM β-glycerophosphate, and 2.5mM p-nitrophenyl phosphate. Total cell lysates were resolved on 8% SDS-PAGE gels and transferred to an Immobilon P membrane (Millipore). Membrane was blocked in 5% nonfat dry milk in TBS-T (0.1% Tween 20/TBS) and blotted with appropriate Abs, as per the manufacturer's instructions.
Flow cytometry
Single-cell suspensions were stained in cold FACS buffer (1× PBS, pH 7.4, with 2% FBS) with the appropriate fluorophore-conjugated Abs for 30 minutes on ice. All cells were washed and resuspended in FACS buffer for acquisition on an LSRII or FACSCalibur flow cytometer (BD Biosciences) using FACSDiva Version 6.0 or CellQuest Pro Version 6.0 software (BD Biosciences). Postacquisition analysis was performed with FlowJo Version 7.6.3 software (TreeStar).
In vivo transplantation experiments with mouse p210 BCR-ABL leukemia cells
BCR-Abl–transformed pre-B cells were harvested in culture and injected via tail vein into sublethally irradiated (300 cGy) syngeneic Peb3b (CD45.1+) mice. 17-AAG was administered by 1 IP injection immediately after cell transfer and was repeated daily for 5 days. Mice were killed at day 7 (mice were not treated on day 6) to harvest BM and spleens. Leukemic engraftment was determined by flow cytometry.
Statistical analysis
The Student t test was used to determine the statistical significance of the differences between groups of samples. P < .05 was considered statistically significant. The number of sample replicates and the number of experimental replicates are indicated in the figure legends.
Results
mTOR inhibitors show limited cytotoxicity against T-ALL and pre-B-ALL
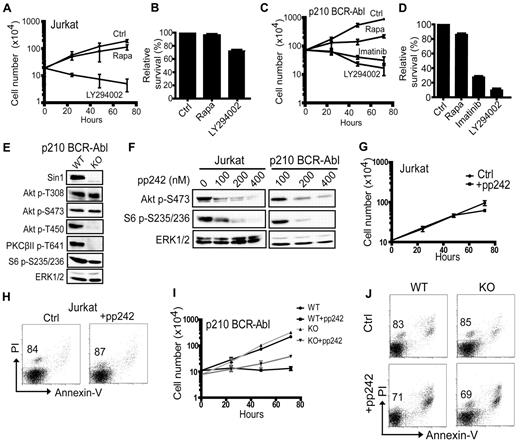
The mTOR signaling pathway is commonly activated in blood cancers to promote uncontrolled cellular growth and proliferation. Because mTOR is a master regulator of cell growth and metabolism, there is great interest in the clinical potential of mTOR inhibitors in the treatment of blood cancers.18,28-30 Rapamycin and the related rapalogs are approved for clinical use, and newly developed mTOR kinase inhibitors are currently in the early stages of clinical development. To determine the effect of mTOR inhibitors on leukemia-cell proliferation and survival, in the present study, we treated human Jurkat T-ALL cells with rapamycin or the dual PI3K/mTOR kinase inhibitor LY294002. We observed that rapamycin only slightly reduced Jurkat cell growth and had no effect on cell viability relative to mock-treated cells, whereas LY294002 dramatically reduced cell growth and promoted the death of Jurkat cells (Figure 1A-B). We also tested the effect of rapamycin and LY294002 on p210 BCR-Abl–transformed mouse pre-B-ALL cells. Similar to our observations in Jurkat cells, rapamycin reduced cell proliferation but had only a modest cytotoxic effect (Figure 1C-D). In contrast, LY294002 or the Abl kinase inhibitor imatinib were significantly more effective than rapamycin in inhibiting cell proliferation and promoting the death of p210 BCR-Abl leukemia cells (Figure 1C-D). These data show that rapamycin alone can suppress cell proliferation, but only induces minimal cytotoxicity in T-ALL and pre-B-ALL cells.
The role of mTOR in leukemia-cell proliferation and survival. (A) Jurkat cells were cultured with vehicle (Ctrl) only or with 100nM rapamycin (Rapa) or 25μM LY294002 as indicated. The total number of live cells at 0, 24, 48, and 72 hours was determined by a trypan blue exclusion cell-viability assay. Each data point shown is the average of triplicate samples from 1 of 3 independent experiments. (B) Jurkat cells from panel A were stained with PI and analyzed by flow cytometry at 24 hours. The number of viable cells in each drug-treated group relative to the untreated control (Ctrl) group (set as 100%) is shown. The data presented are the average of triplicate samples with SD and are representative of 3 independent experiments. (C) Wild-type p210 BCR-Abl–transformed mouse pre-B leukemia cells were cultured with vehicle (Ctrl) or with 100nM rapamycin (Rapa), 10μM imatinib, or 25μM LY294002 for the indicated times. The total number of live cells at 0, 24, 48, and 72 hours was determined as described in panel A. Each data point shown is the average of triplicate samples from 1 of 3 independent experiments. (D) Cells in panel C were stained with PI and analyzed by flow cytometry at 24 hours. The number of live cells in the drug-treated groups relative to the control (Ctrl) group (100%) is shown. The data shown are the average of duplicate samples and are representative of 3 independent experiments. (E) Total cellular proteins from Sin1+/+ (wild-type; WT) or Sin1−/− (knockout; KO) p210 BCR-Abl leukemia cells were analyzed by immunoblotting for the indicated proteins and phosphoproteins. (F) Jurkat or Sin1+/+ p210 BCR-Abl pre-B cells were cultured with the indicated doses of pp242 for 18 hours and then analyzed by immunoblotting for the indicated proteins. (G) Jurkat cells were cultured with (+pp242) or without (Ctrl) 400nM pp242 for the indicated periods of time and live cells were counted by a trypan blue exclusion assay. Fresh cell-culture medium with or without pp242 was added to the cells every 24 hours. The data shown are the average of triplicate samples from 1 of 2 independent experiments. (H) Cells from panel G were stained with PI and annexin V and analyzed by flow cytometry at the 24-hour time point to determine cell viability. The percentage of live cells (annexin V−PI−) is indicated. (I) Sin1 WT and KO p210 BCR-Abl cells were cultured with (+pp242) or without 400nM pp242 for the indicated periods of time and live cells were counted by a trypan blue exclusion cell-viability assay. Fresh cell-culture medium with or without pp242 was added to the cells every 24 hours. The data shown are the average of triplicate samples from 1 of 3 independent experiments. (J) Sin1 WT and KO cells from panel I were stained with PI and annexin V and analyzed by flow cytometry at the 24-hour time point to determine cell viability. The percentage of live cells (annexin V−PI−) is indicated.
The role of mTOR in leukemia-cell proliferation and survival. (A) Jurkat cells were cultured with vehicle (Ctrl) only or with 100nM rapamycin (Rapa) or 25μM LY294002 as indicated. The total number of live cells at 0, 24, 48, and 72 hours was determined by a trypan blue exclusion cell-viability assay. Each data point shown is the average of triplicate samples from 1 of 3 independent experiments. (B) Jurkat cells from panel A were stained with PI and analyzed by flow cytometry at 24 hours. The number of viable cells in each drug-treated group relative to the untreated control (Ctrl) group (set as 100%) is shown. The data presented are the average of triplicate samples with SD and are representative of 3 independent experiments. (C) Wild-type p210 BCR-Abl–transformed mouse pre-B leukemia cells were cultured with vehicle (Ctrl) or with 100nM rapamycin (Rapa), 10μM imatinib, or 25μM LY294002 for the indicated times. The total number of live cells at 0, 24, 48, and 72 hours was determined as described in panel A. Each data point shown is the average of triplicate samples from 1 of 3 independent experiments. (D) Cells in panel C were stained with PI and analyzed by flow cytometry at 24 hours. The number of live cells in the drug-treated groups relative to the control (Ctrl) group (100%) is shown. The data shown are the average of duplicate samples and are representative of 3 independent experiments. (E) Total cellular proteins from Sin1+/+ (wild-type; WT) or Sin1−/− (knockout; KO) p210 BCR-Abl leukemia cells were analyzed by immunoblotting for the indicated proteins and phosphoproteins. (F) Jurkat or Sin1+/+ p210 BCR-Abl pre-B cells were cultured with the indicated doses of pp242 for 18 hours and then analyzed by immunoblotting for the indicated proteins. (G) Jurkat cells were cultured with (+pp242) or without (Ctrl) 400nM pp242 for the indicated periods of time and live cells were counted by a trypan blue exclusion assay. Fresh cell-culture medium with or without pp242 was added to the cells every 24 hours. The data shown are the average of triplicate samples from 1 of 2 independent experiments. (H) Cells from panel G were stained with PI and annexin V and analyzed by flow cytometry at the 24-hour time point to determine cell viability. The percentage of live cells (annexin V−PI−) is indicated. (I) Sin1 WT and KO p210 BCR-Abl cells were cultured with (+pp242) or without 400nM pp242 for the indicated periods of time and live cells were counted by a trypan blue exclusion cell-viability assay. Fresh cell-culture medium with or without pp242 was added to the cells every 24 hours. The data shown are the average of triplicate samples from 1 of 3 independent experiments. (J) Sin1 WT and KO cells from panel I were stained with PI and annexin V and analyzed by flow cytometry at the 24-hour time point to determine cell viability. The percentage of live cells (annexin V−PI−) is indicated.
Rapamycin is an incomplete mTOR inhibitor that may not inhibit mTORC1 fully and may only inhibit mTORC2 in some cell types after chronic treatment.14 Therefore, we derived mTORC2-deficient, p210 BCR-Abl–transformed pre-B leukemia cell lines from a Sin1−/− mouse and analyzed the phosphorylation of Akt at the mTORC2 target site S473 in Sin1+/+ or Sin1−/− p210 BCR-Abl leukemia cells. We found that Sin1/mTORC2 deficiency did not abolish Akt S473 phosphorylation, suggesting that a kinase other than mTORC2 is responsible for phosphorylating the hydrophobic motif of Akt in p210 BCR-Abl pre-B leukemia cells (Figure 1E). However, we observed that mTORC2-dependent phosphorylation of the Akt and PKCβII TM (T450 and T641, respectively) was abolished in Sin1−/− p210 BCR-Abl pre-B leukemia cells (Figure 1E). To better understand the effect of complete mTOR inhibition on leukemia-cell proliferation and survival, we treated Jurkat and p210 BCR-Abl leukemia cells with the mTOR kinase inhibitor pp242. We found that a concentration of 400nM pp242 was sufficient to inhibit mTORC1-dependent S6 S235/236 phosphorylation in both Jurkat and wild-type p210 BCR-Abl leukemia cells (Figure 1F). This dose of pp242 inhibits Akt S473 phosphorylation in Jurkat cells, but only partially inhibits Akt S473 phosphorylation in p210 BCR-Abl leukemia cells (Figure 1F).
We also investigated whether mTOR inhibition with pp242 is sufficient to inhibit leukemia-cell proliferation and promote cell death. First, we cultured Jurkat cells in vitro with or without 400nM pp242 and counted live cells at 24, 48 and 72 hours. We observed that pp242 treatment did not inhibit Jurkat cell proliferation significantly compared with untreated cells (Figure 1G). Furthermore, pp242 did not reduce the viability of Jurkat cells significantly (Figure 1H). We also cultured Sin1+/+ and Sin1−/− p210 BCR-Abl pre-B leukemia cells with or without 400nM pp242 and counted live cells at 24, 48, and 72 hours. We observed that pp242 inhibited the proliferation of both Sin1+/+ and Sin1−/− p210 BCR-Abl pre-B cells significantly relative to untreated cells (Figure 1I). We also found that pp242 treatment resulted in a modest reduction in p210 BCR-Abl cell viability; however, the inner-cell viability between Sin1+/+ and Sin1−/− cells was similar (Figure 1J; 83% live Sin1+/+ untreated cells vs 71% Sin1+/+ pp242 treated and 85% live Sin1−/− untreated cells vs 69% Sin1−/− pp242 treated). These results suggest that mTORC2 is not required for p210 BCR-Abl leukemia cell growth and that inhibition of mTOR alone is insufficient to induce substantial cytotoxicity in pre-B-ALL and T-ALL compared with the dual PI3K/mTOR inhibitor LY294002 or the Abl kinase inhibitor imatinib. These data also suggest that leukemia cells may be able to survive despite the loss of mTOR activity.
17-AAG destabilizes Akt and preferentially induces cell death in Sin1−/− leukemia cells
Although Akt is critical for the survival of tumor cells, our data suggested that Akt HM and TM phosphorylation are not essential for leukemia cell survival. Rather, our results suggested that the Akt T-loop (T308) phosphorylation may be necessary and sufficient to promote tumor-cell survival. We have shown previously that Akt and PKCα/βII TM phosphorylation is abolished in Sin1−/− p210 BCR-Abl leukemia cells and Sin1−/− MEF cells (Figure 1E).17 Consistent with those results, we observed in the present study that Akt and PKCβII TM phosphorylation was absent in Sin1−/− Ab-MuLV–transformed pre-B-ALL cells (Figure 2A). In Sin1−/− MEF cells, the stability of Akt and PKCα/βII is partially maintained by HSP90, and inhibition of HSP90 with 17-AAG reduces Akt and PKC protein expression significantly.17 Therefore we investigated whether 17-AAG could inhibit Akt and PKCβII expression in Sin1−/− Ab-MuLV leukemia cells. Sin1+/− or Sin1−/− Ab-MuLV pre-B-ALL cells were treated with 17-AAG for 4 or 8 hours and total Akt or PKCβII protein levels were measured. We found that 17-AAG treatment reduced Akt and PKCβII expression in Sin1−/− Ab-MuLV pre-B-ALL cells to a greater extent than in Sin1+/− Ab-MuLV cells at the 4- and 8-hour time points (Figure 2B). We also observed that Akt and PKCβII expression was reduced Sin1−/− Ab-MuLV pre-B cells relative to Sin1+/− pre-B cells before the addition of 17-AAG (Figure 2B), which is consistent with our previous results showing that Akt and PKC protein stability is partially impaired in Sin1−/− MEFs.9
17-AAG destabilizes Akt and PKCβII and induces cell death of Sin1−/− Ab-MuLV pre-B leukemia cells preferentially. (A) Total cellular proteins from Sin1+/− (wild-type; WT) or Sin1−/− (knockout; KO) Ab-MuLV pre-B cells were assayed by immunoblotting for TM phosphorylation of Akt (p-T450) and PKCβII (p-T641). Sin1 expression is shown and ERK2 expression was used as a loading control. (B) Sin1 WT or KO Ab-MuLV pre-B cells were cultured in the presence or absence of 5μM 17-AAG for the indicated periods of time. Total Akt or PKCβII expression at each time point was measured by immunoblotting. ERK2 and β-actin expression served as loading controls. The Akt/actin or PKCβII/actin ratios were calculated by dividing the total pixel volume of Akt or PKCβII by the total pixel volume of β-actin. The results shown are representative of 2 independent experiments. (C) Sin1 WT or KO Ab-MuLV pre-B cells were cultured with or without 5μM 17-AAG for 24 hours. Cell viability was measured by flow cytometry with PI and annexin V staining. A representative FACS plot is shown on the left. The numbers in the plot show percentages of the gated populations in each quadrant. The data shown on the right graph are the average of triplicate samples from 1 of 3 independent experiments. (D) Sin1 WT or KO Ab-MuLV pre-B cells were cultured for 24 hours with or without 5μM 17-AAG and the relative change in viable cell number was determined. The data shown are the average of triplicate samples from 1 of 3 independent experiments. The P values shown were calculated by a 2-tailed t test.
17-AAG destabilizes Akt and PKCβII and induces cell death of Sin1−/− Ab-MuLV pre-B leukemia cells preferentially. (A) Total cellular proteins from Sin1+/− (wild-type; WT) or Sin1−/− (knockout; KO) Ab-MuLV pre-B cells were assayed by immunoblotting for TM phosphorylation of Akt (p-T450) and PKCβII (p-T641). Sin1 expression is shown and ERK2 expression was used as a loading control. (B) Sin1 WT or KO Ab-MuLV pre-B cells were cultured in the presence or absence of 5μM 17-AAG for the indicated periods of time. Total Akt or PKCβII expression at each time point was measured by immunoblotting. ERK2 and β-actin expression served as loading controls. The Akt/actin or PKCβII/actin ratios were calculated by dividing the total pixel volume of Akt or PKCβII by the total pixel volume of β-actin. The results shown are representative of 2 independent experiments. (C) Sin1 WT or KO Ab-MuLV pre-B cells were cultured with or without 5μM 17-AAG for 24 hours. Cell viability was measured by flow cytometry with PI and annexin V staining. A representative FACS plot is shown on the left. The numbers in the plot show percentages of the gated populations in each quadrant. The data shown on the right graph are the average of triplicate samples from 1 of 3 independent experiments. (D) Sin1 WT or KO Ab-MuLV pre-B cells were cultured for 24 hours with or without 5μM 17-AAG and the relative change in viable cell number was determined. The data shown are the average of triplicate samples from 1 of 3 independent experiments. The P values shown were calculated by a 2-tailed t test.
We also examined the effect of 17-AAG on Sin1+/− and Sin1−/− Ab-MuLV pre-B-ALL survival. Sin1+/− or Sin1−/− Ab-MuLV pre-B-ALL cells were cultured for 24 hours with 17-AAG or vehicle only and cell viability was assessed by flow cytometry using PI and annexin V staining. We observed that 17-AAG treatment decreased Sin1+/− cell viability by 10% (81% annexin V−PI− vehicle–treated cells vs 73% annexin V−PI− 17-AAG–treated cells) (Figure 2C). In contrast, 17-AAG treatment decreased Sin1−/− cell viability by 77% (64% annexin V−PI− vehicle–treated cells vs 15% annexin V−PI− 17-AAG–treated cells; Figure 2C). We also observed a reduced proportion of live, vehicle-treated, Sin1−/− Ab-MuLV pre-B cells compared with vehicle-treated Sin1+/− cells (81% live Sin1+/− cells vs 64% live Sin1−/− cells). However, 17-AAG treatment of Sin1−/− cells resulted in a significantly greater reduction in the total number of viable leukemia cells in culture compared with Sin1+/− cells (Figure 2D). These data show that loss of Sin1 and disruption of mTORC2-dependent Akt and PKCβII TM phosphorylation enhances the 17-AAG–mediated inhibition of Akt and PKCβII expression in Sin1−/− Ab-MuLV pre-B-ALL cells. Furthermore, disruption of Sin1/mTORC2 function sensitizes Ab-MuLV pre-B-ALL leukemia cells to 17-AAG–dependent cell death.
Restoration of mTORC2 function protects Sin1−/− pre-B-ALL cells from 17-AAG–mediated cell death.
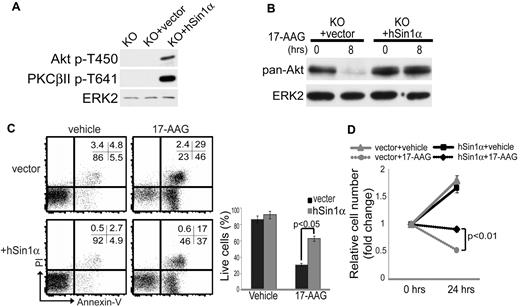
To confirm that the 17-AAG–dependent reduction in Akt expression observed in Sin1−/− Ab-MuLV pre-B-ALL cells was due to loss of Sin1/mTORC2 function, we reconstituted Sin1-deficient cells with human Sin1α (hSin1α). Retroviral reconstitution of hSin1α restored mTORC2-dependent TM phosphorylation of Akt and PKCβII in Ab-MuLV pre-B-ALL cells (Figure 3A) and prevented the 17-AAG–dependent decrease in Akt expression (Figure 3B). We next investigated whether hSin1α expression protects Sin1−/− Ab-MuLV pre-B-ALL cells from 17-AAG–dependent cell death. Sin1−/− Ab-MuLV pre-B-ALL cells were infected with an empty retrovirus or an hSin1α–expressing virus and then cultured for 24 hours with 17-AAG or vehicle only. Cell viability was assessed by PI/annexin V staining and flow cytometry. We observed that the reconstitution of Sin1−/− Ab-MuLV pre-B-ALL cells with hSin1α resulted in a 50% increase in the proportion of viable cells after 17-AAG treatment compared with the cells infected with a control retrovirus (23% annexin V−PI− viable control cells vs 46% annexin V−PI− viable hSin1α–expressing cells; Figure 3C). Furthermore, we observed that there were significantly more live hSin1α reconstituted cells in culture 24 hours after 17-AAG treatment compared with empty vector–infected Sin1−/− Ab-MuLV pre-B-ALL cells (Figure 3D). These results show that Sin1 protects pre-B leukemia cells from 17-AAG–mediated cell death and indicate that mTORC2 mediates this protection by phosphorylating the Akt and PKCβII TM site.
Rescue of mTORC2-dependent TM phosphorylation protects Sin1−/− Ab-MuLV pre-B cells from 17-AAG–mediated Akt degradation and cell death. (A) Total proteins from Sin1−/− (knockout; KO), empty vector (KO + vector), or human Sin1 (KO + hSin1α)–reconstituted Sin1−/− Ab-MuLV pre-B leukemia cells were assayed by immunoblotting for Akt and PKCβII TM phosphorylation as described in Figure 2A. ERK2 expression was used as a loading control. (B) Total cellular proteins were extracted from empty vector (+vector) or human Sin1 (+hSin1α)–reconstituted Sin1−/− Ab-MuLV–transformed leukemia cells that were treated with 5μM 17-AAG for 0 or 8 hours. Total Akt protein levels were then measured by immunoblotting, with ERK2 serving as a loading control. The data shown are representative of 2 independent experiments. (C) Empty vector (vector) or hSin1α–reconstituted Sin1−/− Ab-MuLV leukemia cells were treated with vehicle or 5μM 17-AAG for 24 hours. Cell viability was measured by PI and annexin V staining and flow cytometric analysis. A representative FACS plot is shown on the left. The numbers in the plot show the percentages of the gated populations in each quadrant. The graph on the right shows the relative proportion of live cells (PI−annexin V−) in each culture condition after 24 hours. The data shown are the average of triplicate samples with SD from 1 of 3 independent experiments. (D) The relative change in total cell number of vector or hSin1α–transduced Sin1−/− Ab-MuLV pre-B cells cultured with or without 5μM 17-AAG for the indicated amount of time is shown. The data are the average of triplicate samples with SD from 1 of 3 independent experiments. The P values shown were calculated by a 2-tailed t test.
Rescue of mTORC2-dependent TM phosphorylation protects Sin1−/− Ab-MuLV pre-B cells from 17-AAG–mediated Akt degradation and cell death. (A) Total proteins from Sin1−/− (knockout; KO), empty vector (KO + vector), or human Sin1 (KO + hSin1α)–reconstituted Sin1−/− Ab-MuLV pre-B leukemia cells were assayed by immunoblotting for Akt and PKCβII TM phosphorylation as described in Figure 2A. ERK2 expression was used as a loading control. (B) Total cellular proteins were extracted from empty vector (+vector) or human Sin1 (+hSin1α)–reconstituted Sin1−/− Ab-MuLV–transformed leukemia cells that were treated with 5μM 17-AAG for 0 or 8 hours. Total Akt protein levels were then measured by immunoblotting, with ERK2 serving as a loading control. The data shown are representative of 2 independent experiments. (C) Empty vector (vector) or hSin1α–reconstituted Sin1−/− Ab-MuLV leukemia cells were treated with vehicle or 5μM 17-AAG for 24 hours. Cell viability was measured by PI and annexin V staining and flow cytometric analysis. A representative FACS plot is shown on the left. The numbers in the plot show the percentages of the gated populations in each quadrant. The graph on the right shows the relative proportion of live cells (PI−annexin V−) in each culture condition after 24 hours. The data shown are the average of triplicate samples with SD from 1 of 3 independent experiments. (D) The relative change in total cell number of vector or hSin1α–transduced Sin1−/− Ab-MuLV pre-B cells cultured with or without 5μM 17-AAG for the indicated amount of time is shown. The data are the average of triplicate samples with SD from 1 of 3 independent experiments. The P values shown were calculated by a 2-tailed t test.
17-AAG preferentially inhibits the growth of Sin1−/− p210 BCR-Abl B-ALL cells in vivo
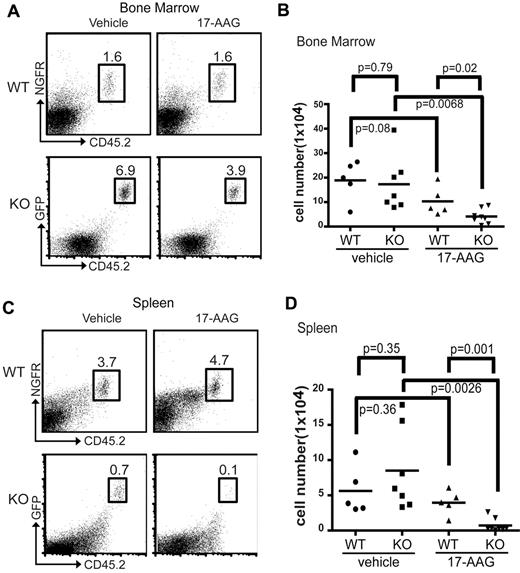
Our in vitro data strongly suggest that 17-AAG treatment will be highly effective at inhibiting the growth of tumors that lack mTORC2 function in vivo. To test this hypothesis, we transplanted 1 × 106Sin1+/+ or Sin1−/− p210 BCR-Abl–transformed mouse pre-B-ALL cells into wild-type CD45.1-congenic mice. Starting 24 hours after the leukemia cell transfer, the mice were treated with either vehicle or 17-AAG for 5 consecutive days. All of the mice were killed 1 day after the final drug treatment and analyzed for the presence of pre-B-ALL cells in the BM and spleen by flow cytometry. We observed that pre-B-ALL cells were present in the BM and spleen of all mice regardless of whether they received Sin1+/+ or Sin1−/− cells. However, we found that 17-AAG treatment resulted in a significant decrease in the proportion and total number of Sin1−/− pre-B-ALL cells in the BM, whereas 17-AAG treatment caused no significant decrease in the number of Sin1+/+ leukemia cells (Figure 4A-B). Furthermore, we observed that there was no difference in the number of Sin1+/+ and Sin1−/− pre-B-ALL cells obtained from the BM in the vehicle-treated cohort (Figure 4B). Similar to our observations in BM, we found that 17-AAG treatment resulted in a significant reduction in the proportion and total number of Sin1−/−, but not Sin1+/+, pre-B-ALL cells obtained from the spleen (Figure 4C-D) and, again, we observed no significant difference in the total number of Sin1−/− or Sin1+/+ pre-B-ALL cells obtained from the spleens of the vehicle-treated cohort (Figure 4D). These data demonstrate that inhibition of the chaperone pathway by 17-AAG works together with mTORC2 deficiency to suppress leukemia cell growth and survival in vivo.
17-AAG inhibits Sin1−/− leukemia growth preferentially in vivo. Sublethally irradiated wild-type CD45.1 congenic mice were transplanted with 1 × 106Sin1+/+ (CD45.2+NGFR+; wild-type; WT) or Sin1−/− (CD45.2+GFP+; knockout; KO) p210 BCR-Abl–transformed mouse leukemia cells by tail vein injection. The transplanted mice were treated with 17-AAG (80 mg/kg/d in corn oil) or vehicle (corn oil) daily for 5 consecutive days beginning 24 hours after tumor-cell transplantation. Mice were killed 24 hours after the last drug treatment and the percentage and total number of Sin1+/+ (wild-type; WT) or Sin1−/− (knockout; KO) pre-B leukemia cells in the BM (A-B) and spleen (C-D) were analyzed by flow cytometry. The total number of mice in each treatment group are as follows: WT vehicle-treated, n = 5; WT 17-AAG–treated, n = 5; KO vehicle-treated, n = 7; and KO 17-AAG–treated, n = 7. (A) Representative FACS plots showing the percentages of transplanted WT and KO leukemia cells (numbers in the boxes) in BM. (B) Summary of total number of transplanted leukemia cells in the whole BM extracted from one long leg bone (tibia) of WT or KO mice treated with vehicle or 17-AAG. Each symbol represents the cells from one tibia of the indicated recipient mice. (C) Representative FACS plots showing the percentages of transplanted WT and KO leukemia cells (numbers in the boxes) in the spleen. (D) Summary of total number of transplanted leukemia cells in the spleen of WT or KO mice treated with vehicle or 17-AAG. Each symbol represents the cells from the spleens of the indicated recipient mice.
17-AAG inhibits Sin1−/− leukemia growth preferentially in vivo. Sublethally irradiated wild-type CD45.1 congenic mice were transplanted with 1 × 106Sin1+/+ (CD45.2+NGFR+; wild-type; WT) or Sin1−/− (CD45.2+GFP+; knockout; KO) p210 BCR-Abl–transformed mouse leukemia cells by tail vein injection. The transplanted mice were treated with 17-AAG (80 mg/kg/d in corn oil) or vehicle (corn oil) daily for 5 consecutive days beginning 24 hours after tumor-cell transplantation. Mice were killed 24 hours after the last drug treatment and the percentage and total number of Sin1+/+ (wild-type; WT) or Sin1−/− (knockout; KO) pre-B leukemia cells in the BM (A-B) and spleen (C-D) were analyzed by flow cytometry. The total number of mice in each treatment group are as follows: WT vehicle-treated, n = 5; WT 17-AAG–treated, n = 5; KO vehicle-treated, n = 7; and KO 17-AAG–treated, n = 7. (A) Representative FACS plots showing the percentages of transplanted WT and KO leukemia cells (numbers in the boxes) in BM. (B) Summary of total number of transplanted leukemia cells in the whole BM extracted from one long leg bone (tibia) of WT or KO mice treated with vehicle or 17-AAG. Each symbol represents the cells from one tibia of the indicated recipient mice. (C) Representative FACS plots showing the percentages of transplanted WT and KO leukemia cells (numbers in the boxes) in the spleen. (D) Summary of total number of transplanted leukemia cells in the spleen of WT or KO mice treated with vehicle or 17-AAG. Each symbol represents the cells from the spleens of the indicated recipient mice.
Coadministration of mTOR inhibitors and 17-AAG promotes leukemia cell death
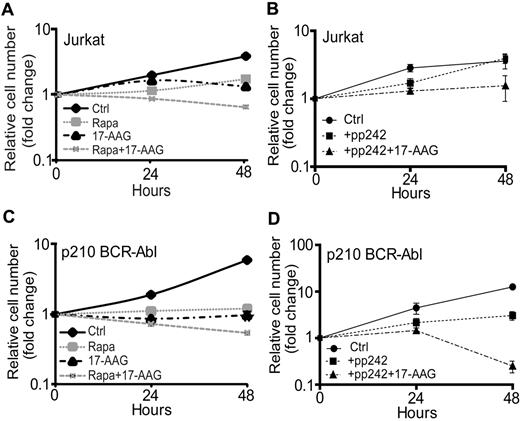
Our in vitro and in vivo data provide strong evidence that inhibition of both mTORC2 and HSP90 will produce a synergistic antitumor effect that is greater than the inhibition of the mTOR or chaperone pathway alone. Therefore, we chose to investigate whether 2 pharmacologic methods of mTORC2 inhibition, chronic exposure of cells to rapamycin17,27 or to pp242, could synergize with 17-AAG to suppress wild-type leukemia-cell proliferation in vitro. We first compared the growth rates of Jurkat cells treated with rapamycin alone, 17-AAG alone, or rapamycin plus 17-AAG with untreated cells. We observed that the combination of rapamycin plus 17-AAG inhibited Jurkat cell proliferation to a significantly greater extent than either drug alone (Figure 5A). We then treated Jurkat cells with the combination of pp242 plus 17-AAG, which also inhibited Jurkat cell proliferation; however, pp242 alone had little effect on cell proliferation (Figure 5B).
mTOR inhibitors synergize with 17-AAG to promote leukemia cell death. (A) Jurkat cells were treated with vehicle (Ctrl), 100nM rapamycin (Rapa), 5μM 17-AAG, or rapamycin (Rapa) plus 17-AAG for 0, 24, or 48 hours. The relative change in the number of viable cells at each time point was determined by a trypan blue exclusion assay. The total number of viable cells at 0 hours was set at 1. The data shown are the average of triplicate samples from 1 of 3 independent experiments. (B) Jurkat cells were treated with vehicle, 400nM pp242 (+pp242), or pp242 plus 5μM 17-AAG for 0, 24, or 48 hours. The relative change in the number of viable cells at each time point was determined by a trypan blue exclusion assay. The total number of viable cells at 0 hours was set at 1. The data shown are the average of duplicate samples from 1 of 2 independent experiments. (C) Wild-type p210 BCR-Abl mouse pre-B leukemia cells were treated with vehicle, 100nM rapamycin (Rapa), 5μM 17-AAG, or rapamycin (Rapa) plus 17-AAG for 0, 24, or 48 hours. The relative change in the number of viable cells at each time point was determined by a trypan blue exclusion assay. The total number of viable cells at 0 hours was set at 1. The data shown are the average of triplicate samples from 1 of 3 independent experiments. (D) Wild-type p210 BCR-Abl pre-B leukemia cells were treated with vehicle, 400nM pp242 (pp242), or pp242 plus 5μM 17-AAG for 0, 24, or 48 hours. The relative change in the number of viable cells at each time point was determined by a trypan blue exclusion assay. The total number of viable cells at 0 hours was set at 1. The data shown are the average of duplicate samples from 1 of 3 independent experiments.
mTOR inhibitors synergize with 17-AAG to promote leukemia cell death. (A) Jurkat cells were treated with vehicle (Ctrl), 100nM rapamycin (Rapa), 5μM 17-AAG, or rapamycin (Rapa) plus 17-AAG for 0, 24, or 48 hours. The relative change in the number of viable cells at each time point was determined by a trypan blue exclusion assay. The total number of viable cells at 0 hours was set at 1. The data shown are the average of triplicate samples from 1 of 3 independent experiments. (B) Jurkat cells were treated with vehicle, 400nM pp242 (+pp242), or pp242 plus 5μM 17-AAG for 0, 24, or 48 hours. The relative change in the number of viable cells at each time point was determined by a trypan blue exclusion assay. The total number of viable cells at 0 hours was set at 1. The data shown are the average of duplicate samples from 1 of 2 independent experiments. (C) Wild-type p210 BCR-Abl mouse pre-B leukemia cells were treated with vehicle, 100nM rapamycin (Rapa), 5μM 17-AAG, or rapamycin (Rapa) plus 17-AAG for 0, 24, or 48 hours. The relative change in the number of viable cells at each time point was determined by a trypan blue exclusion assay. The total number of viable cells at 0 hours was set at 1. The data shown are the average of triplicate samples from 1 of 3 independent experiments. (D) Wild-type p210 BCR-Abl pre-B leukemia cells were treated with vehicle, 400nM pp242 (pp242), or pp242 plus 5μM 17-AAG for 0, 24, or 48 hours. The relative change in the number of viable cells at each time point was determined by a trypan blue exclusion assay. The total number of viable cells at 0 hours was set at 1. The data shown are the average of duplicate samples from 1 of 3 independent experiments.
We also treated wild-type p210 BCR-Abl pre-B-ALL cells with rapamycin or 17-AAG alone or together. Although treatment with rapamycin or 17-AAG alone inhibited leukemia-cell proliferation significantly, cotreatment with rapamycin plus 17-AAG elicited an even greater suppression of leukemia cell growth than either drug alone (Figure 5C). Consistently, cotreatment of p210 BCR-Abl cells with pp242 plus 17-AAG also resulted in a significantly greater inhibition of leukemia cell growth compared with pp242 alone (Figure 5D). These data show that mTOR and chaperone inhibitors act in synergy to inhibit leukemia-cell proliferation and survival.
Discussion
Constitutively activated Akt signaling is found frequently in blood cancers, resulting in an increased resistance of tumor cells to cytotoxic chemotherapies. Therefore, the inhibition of Akt activity is a logical strategy to include in the treatment of these cancers. mTORC2 regulates the growth-factor–dependent activation of Akt by phosphorylating Akt HM at S473. However, we and others have shown that the inhibition of Akt HM phosphorylation does not fully inhibit Akt signaling.9,10,15,27 Furthermore, mTORC2 is not essential for the growth or proliferation of multiple cell types, including but not limited to embryonic fibroblasts, T cells, developing B cells, and v-Abl or p210 BCR-Abl oncogene-transformed pre-B leukemia cells.9,10,27,31,32 Furthermore, our analysis of Sin1−/− p210 BCR-Abl pre-B-ALL cells revealed that Akt S473 phosphorylation is not fully dependent on mTOR. This conclusion is supported by our observation the high dose of pp242 used herein failed to abolish Akt S473 phosphorylation completely in wild-type p210 BCR-Abl leukemia cells. Several recent studies have also shown that kinases in addition to mTORC2 may phosphorylate Akt in different type of cells.24,25 Therefore, the inhibition of mTOR alone may not be sufficient to induce the growth arrest and cell death of cancer cells.
mTORC2 also phosphorylates the TM of Akt and cPKC proteins.16,17 In the absence of mTORC2, Akt and cPKC TM phosphorylation is abolished and the stability of these proteins is reduced.16,17 HSP90 associates with Akt and cPKC proteins, which lack TM phosphorylation, and rescues the stability of the newly synthesized Akt and cPKC proteins.17 This is consistent with our observation that Akt and PKCβII expression is reduced by approximately 2-fold in Sin1−/− pre-B leukemia cells compared with Sin1+/+ leukemia cells (Figure 2B). In the present study, we have shown that HSP90 maintains Akt expression in Sin1−/− pre-B leukemia cells, but not in Sin1+/+ leukemia cells. We have also shown that treatment of Sin1−/− pre-B leukemia cells with the HSP90 inhibitor 17-AAG results in a dramatic reduction of Akt and PKCβII proteins. These data strongly support a model in which the sensitivity of Akt to 17-AAG is dependent on the loss of mTORC2-dependent Akt TM phosphorylation. Our present results also show that reexpression of Sin1 in Sin1−/− leukemia cells restores Akt TM phosphorylation and prevents the reduction of Akt expression on 17-AAG treatment in pre-B leukemia cells (Figure 3). Furthermore, chronic rapamycin treatment or pp242 inhibited mTORC2 in wild-type cells to sensitize p210-BCR-Abl or Jurkat leukemia cells to 17-AAG. These data demonstrate that the combined inhibition of mTORC2 and HSP90 destabilizes Akt and cPKC proteins and synergizes the ability of mTOR inhibitors and 17-AAG to elicit a more effective antileukemic effect.
Because Akt plays a critical role in regulating cell survival, we predicted that the 17-AAG–dependent reduction of Akt expression would promote the cell death of Sin1−/− pre-B leukemia cells preferentially over Sin1+/+ leukemia cells. Indeed, 17-AAG treatment induced substantially more cell death in Sin1−/− pre-B leukemia cells than wild-type pre-B leukemia cells. Furthermore, reexpression of human Sin1 in Sin1−/− pre-B leukemia cells led to increased resistance to 17-AAG–mediated cytotoxicity. These in vitro studies were supported by in vivo experiments in which we transplanted Sin1+/+ or Sin1−/− p210 BCR-Abl–transformed mouse leukemia cells into wild-type mice and treated the recipients with 17-AAG or vehicle for 5 days. Sin1 gene status does not alter tumor growth in vivo, becasuse we were able to recover equivalent numbers of Sin1+/+ and Sin1−/− leukemia cells from the BM and spleens of vehicle-treated mice (Figure 4). However, consistent with our in vitro studies, 17-AAG reduced Sin1−/− pre-B cell tumor burden significantly both in the BM and spleen, whereas the Sin1+/+ tumor cell numbers were not affected by 17-AAG. These data provide the first in vivo evidence that the inhibition of mTORC2 sensitizes leukemia cells to 17-AAG and strongly suggest that the dual inhibition of mTOR plus HSP90 may serve as an effective anticancer therapy.
Recently, De Raedt et al reported that the HSP90 inhibitor IPI-504 induced tumor regression when combined with rapamycin in a mouse model of Ras-driven lung cancer.33 The investigators presented evidence showing that IPI-504 induces reactive oxygen species in lung cancer tumor cells and that mTOR protects tumor cells from ROS by supporting tumor-cell production of the antioxidant glutathione. That study also showed that rapamycin/IPI-504 treatment promotes ER stress and mitochondrial damage, which ultimately results in tumor cell death. The mechanism of rapamycin-induced sensitivity to IPI-504 could be mediated through mTORC1, because knock-down of the essential mTORC1 component raptor mimicked the effect of rapamycin.33 However, the prolonged rapamycin treatment may have inhibited mTORC2 at later time points (> 1 day), which could also explain in part the synergistic antitumor effects of rapamycin and IPI-504 observed by De Raedt et al.33 It is unlikely that the enhanced cytotoxic effects of 17-AAG observed in Sin1−/− pre-B leukemia cells are due to impaired mTORC1 function, because Sin1 deficiency does not inhibit mTORC1-dependent phosphorylation of S6K and 4E-BP1 in pre-B leukemia cells27 (data not shown). Our data and those presented by De Raedt et al indicate that mTOR inhibitors could synergize with HSP90 inhibitors through 2 independent mechanisms, the first of which involves mTORC2-dependent regulation of Akt/cPKC protein stability and the second mTORC1-dependent regulation of the cellular antioxidant response.
Rapamycin and the rapalogs do not inhibit mTORC2 directly. However, accumulating evidence indicates that chronic rapamycin treatment may block mTORC2 complex assembly in many cell types.9,14,17,27,34 Therefore, it is reasonable to propose that HSP90 inhibitors such as 17-AAG will synergize with rapamycin to induce a cytotoxic response causing tumor regression or remission in human blood cancer patients. Substituting rapamycin with mTOR kinase inhibitors that block both mTORC1 and mTORC2 directly will likely increase the antitumor effectiveness of HSP90 inhibitors. Our present data also provide a rational for developing mTORC2-specific inhibitors that can be combined with 17-AAG to treat blood cancers. A therapeutic strategy that does not inhibit mTORC1 will prevent many of the immunosuppressive and metabolic side effects of rapamycin and preserve the mTORC1-mediated negative feedback regulation of PI3K. Based on the results of the present study, we predict that mTORC2 will be an important new target for the development of specific inhibitors that can be used in combination with chaperone inhibitors to treat a wide range of cancers.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Kevan M. Shokat (University of California, San Francisco) for generously providing the pp242; Warren Schlomchik (Yale University) for the generous gift of p210 BCR-Abl retrovirus and human NGFR–Alexa Fluor 647 Ab; and Dr Yuan Zhuang (Duke University) for generously providing the Abelson Murine leukemia virus supernatant.
This work was supported in part by the Department of Defense (grant PR093728 to B.S.). A.S.L. is the recipient of a Brown-Cox Fellowship from Yale University and is currently a Leukemia & Lymphoma Society fellow. F.Z. is partially supported by the Chinese Scholarship Council, Ministry of Education, China.
Authorship
Contribution: F.Z., A.S.L., and D.L. performed the experiments; F.Z., A.S.L., and B.S. analyzed the data, wrote the manuscript, and designed the experiments; and F.C. analyzed the data and assisted in manuscript preparation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Fangping Chen, Department of Hematology, Xiangya Hospital, Central South University, Changsha, Hunan 410078, PR China; e-mail: xychenfp@2118.cn; or Bing Su, Yale University School of Medicine, 10 Amistad St, Rm 401C, New Haven, CT 06520-8089; e-mail: bing.su@yale.edu.