Abstract
Interactions between the multikinase inhibitor sorafenib and the BH3-mimetic obatoclax (GX15-070) were examined in human acute myeloid leukemia (AML) cells. Treatment with sorafenib/obatoclax induced pronounced apoptosis in and reduced the clonogenic growth of multiple AML lines and primary AML cells but not normal CD34+ cells. Sorafenib triggered rapid and pronounced Mcl-1 down-regulation accompanied by enhanced binding of Bim to Bcl-2 and Bcl-xL, effects that were abolished by obatoclax coadministration. Notably, shRNA knockdown of Bim, Bak, or Bax, but not Noxa, significantly attenuated obatoclax/sorafenib lethality, whereas ectopic expression of Mcl-1 exerted a protective effect. Furthermore, exposure of leukemia cells to sorafenib and obatoclax markedly induced autophagy, reflected by rapid and pronounced LC3 processing and LC3–green fluorescent protein (GFP) punctate formation. Multiple autophagy inhibitors or VPS34 knockdown, significantly potentiated sorafenib/obatoclax lethality, indicating a cytoprotective role for autophagy in this setting. Finally, studies in a xenograft mouse model revealed that combined sorafenib/obatoclax treatment markedly reduced tumor growth and significantly prolonged survival in association with Mcl-1 down-regulation and apoptosis induction, whereas agents administered individually had only modest effects. These findings suggest that combining sorafenib with agents that inhibit Mcl-1 and Bcl-2/Bcl-xL such as obatoclax may represent a novel and potentially effective strategy in AML.
Introduction
Members of the Bcl-2 family of apoptotic regulatory proteins are frequently dysregulated in diverse cancers, particularly hematologic malignancies such as acute myeloid leukemia (AML). Such aberrations include overexpression of antiapoptotic proteins such as Bcl-2, Bcl-xL, and Mcl-1, as well as decreases/loss of proapoptotic members such as Bim, Bax, natural born killer (Nbk)/Bcl-2–interacting killer (Bik).1-3 The ultimate consequences of these perturbations are defects in apoptosis that lead to enhanced cell survival as well as increased resistance to various chemotherapeutic drugs. To circumvent such problems, several strategies have been developed which directly target antiapoptotic Bcl-2 family members. Among these is obatoclax (GX15-070), a small molecule inhibitor that targets all prosurvival Bcl-2 members including Bcl-2, Bcl-xL, Bcl-W, as well as Mcl-1.4 Preclinical studies demonstrated that obatoclax exhibits potent antitumor activity in various cancer cell types including leukemia.5,6 It is currently undergoing phase 1 and 2 clinical evaluation.7,8 Obatoclax exerts its antitumor activity through multiple mechanisms. For example, it has been shown to trigger apoptosis by dissociating the proapoptotic protein Bak from both Mcl-14,6 and Bcl-xL9 in conjunction with release of Bim from Mcl-1 and Bcl-2.5,9 However, the ability of obatoclax to induce death in Bax/Bak-deficient cells5,10 prompted the search for additional mechanisms of lethality. In this context, obatoclax has been reported to induce autophagy- or necroptosis-dependent cell death.10,11 Finally, obatoclax may also inhibit cell growth by inducing cell-cycle arrest in S-G2 phase.5
Sorafenib was originally developed as a C-Raf and B-Raf inhibitor, but was subsequently shown to inhibit multiple other kinases, including FLT3, VEGFR-2, VEGFR-3, PDGFR-β, c-Kit, among others.12 It is currently approved for the treatment of refractory renal cell and hepatocellular carcinoma. When administered at standard doses (eg, 400 mg by mouth twice daily), steady-state levels in excess of 10μM have been reported.13 To date, interest in sorafenib in AML has focused on mutant FLT3 forms of the disease.14,15 However, several groups, including our own, have shown that pharmacologically achievable concentrations of sorafenib kill diverse malignant cell types, including wild-type FLT3 human leukemia cells, in association with down-regulation of Mcl-1 protein expression.16-21 In human leukemia cells, this stems from a translational inhibitory mechanism.16,22 In this setting, Mcl-1 down-regulation has been shown to play a significant functional role in sorafenib lethality.16,17,20
In addition to the well-established role of Mcl-1 in opposing sorafenib activity,16-21 recent evidence suggests that sorafenib lethality may also be attenuated by Bcl-2 and Bcl-xL,23,24 raising the possibility that an agent capable of inhibiting all 3 antiapoptotic proteins (ie, Mcl-1, Bcl-2, and Bcl-xL) might be particularly effective in potentiating sorafenib antileukemic activity. To test this hypothesis, we have examined antileukemic interactions between obatoclax and sorafenib in human leukemia cells, focusing on those with wild-type FLT3. Our results indicate that combined treatment with sorafenib and obatoclax exhibits potent antileukemic activity in vitro and in vivo, and suggest that this strategy warrants further investigation.
Methods
Cells
Human leukemia U937, HL-60, and MV4-11 cells were cultured as previously reported.25 U937 cells stably overexpressing wild-type Mcl-1 or Bim constructs were previously described.25 U937 cells stably expressing shRNA directed against Bax, Bak, or Noxa were generated as previously described.26,27 Knockdown of Bim was accomplished by transfecting U937 cells with 2 distinct microRNA-adapted shRNA constructs specifically designed against human Bim (shBim#1 and shBim#2; Open Biosystems). U937 cells transfected with shRNA constructs against green fluorescent protein (shGFP)26 were used as a control for various shRNA-expressing cells. To knockdown VPS34, lentiviral particles carrying a pKL01 shRNA construct (Open Biosystems) were generated using a Lenti-X HTX packaging system (Clontech) and transduced into U937 cells. Cells were selected in the presence of puromycin for 1 week and monitored for VPS34 expression level. U937 cells infected with lentiviruses carrying scrambled sequence constructs were used as negative controls.
Isolation of patient-derived leukemic blasts
Leukemic blasts were obtained from the BM of patients with AML, FAB subtype M2. These studies have been sanctioned by the Investigational Review Board of Virginia Commonwealth University/Medical College of Virginia, and all patients provided informed consent. In each case, the percentage of blasts in the peripheral blood was > 70%. BM was collected, and mononuclear cells isolated as previously described.16
FLT3 mutations analysis
FLT3 mutations analysis was performed on genomic DNA extracted from primary BM blasts isolated from patients with AML as previously described.25
Isolation of CD34+ cells
Normal BM CD34+ cells were obtained with informed consent from patients undergoing routine diagnostic procedures for nonmyeloid hematopoietic disorders. CD34+ cells were isolated from mononuclear cell preparations as previously described.28
In vivo studies
Animal studies were conducted under an approved protocol by the Virginia Commonwealth University Institutional Animal Care and Use Committee. Female athymic nude mice were purchased from Charles River Laboratories, and were inoculated subcutaneously with 2.5 × 106 parental or luciferase-expressing U937 cells. Mice were monitored for tumor growth visually or using the IVIS 200 imaging system (Xenogen Corporation). Once tumors became apparent, the mice were separated in 4 groups of 5 mice each and treated every 24 hours for 6 days a week with sorafenib (80 mg/kg) administered by gavage, obatoclax (3.5 mg/kg) by IM injection, or the combination of sorafenib and obatoclax. Control mice were treated with equal volumes of vehicle alone. Measurement of animal body weights were performed twice a week throughout the study as an indicator of toxicity. Tumor volumes were calculated using the formula (L × W2)/2, with L and W representing length and width, respectively, and when tumor size reached 2000 mm3, mice were euthanized.
Reagents
Sorafenib was provided by Bayer Pharmaceuticals and the National Cancer Institute (National Institutes of Health, Bethesda, MD). Obatoclax was provided by GeminX, Biotechnologies Inc, and the National Cancer Institute. HA14-1, gossypol, 3-methyladenine, chloroquine, and bafilomycin A1 were purchased from Sigma-Aldrich. All reagents were formulated as recommended by their suppliers.
Assessment of apoptosis
The extent of cell death was routinely assessed by 7-AAD staining assay as previously described.29 Parallel studies using annexin V/PI analysis yielded essentially equivalent results.
Autophagy
EGFP-LC3 fusion cDNA was cut from pEGFP-C2 vector, a gift from Dr T. Finkel (Addgene, Cambridge, MA)30 and subcloned into PLVX-puro lentiviral vector (Clontech). Lentiviral particles were generated as in “Cells” and used to infect U937 and MV4-11 cells. Cells expressing EGFP-LC3 were sorted by FACS before treatment. After exposure to the designated agents, cells were fixed with 4% paraformaldehyde, mounted onto slides in mounting medium containing DAPI (Southern Biotechnology Associates), and analyzed for autophagy using a Zeiss LSM 510 confocal microscope and confocal microscopy software (Zeiss LSM Data Server, Version 3.2.0.70, Carl Zeiss).
Fusion between autophagosomes and lysosomes was assessed by colocalization of LC3 (autophagosome marker) and LAMP1 (lysosome marker) using confocal microscopy. Briefly, cells expressing EGFP-LC3 were fixed with 4% paraformaldehyde and permeabilized with 0.25% Triton X-100. LAMP1 was immuno-detected with anti-LAMP1 Abs (H5G11; Santa Cruz Biotechnology) using Alexa Fluor 647–conjugated secondary Abs (Molecular Probes). LC3 was detected with GFP fluorescence amplified by Alexa Fluor 488–conjugated anti-GFP Abs (Molecular Probes).
Immunoprecipitation and immunoblotting
Cells were lysed in CHAPS buffer16 after which 500 μg of protein lysates were subjected to immunoprecipitation using designated Abs. Immunoblotting was performed using the immunoprecipitates or the whole-cell lysates as previously described in detail.29 Primary Abs were: polyclonal Bax, Bcl-2, and Mcl-1 (BD PharMingen), Noxa (Alexis), poly(ADP-ribose) polymerase (PARP; Biomol Research Laboratories), cleaved caspase-9, and cleaved caspase-3, ERK1/2, and ATG5 (Cell Signaling Technology), apoptosis-inducing factor, cytochrome c, polyclonal Bak, Bcl-xL, SQSTM1/p62, VPS34, and beclin-1, (Santa Cruz Biotechnology), LC-3 (Novagen), and Bim and α-tubulin (Calbiochem).
Bax and Bak conformational change
Cells were lysed in CHAPS buffer and protein lysates subjected to immunoprecipitation using anti-Bax 6A7 (Sigma-Aldrich) or anti-Bak Ab-1 Abs (Calbiochem) that recognize only conformationally changed Bax or Bak protein. Immunoprecipitates were then subjected to immunoblotting analysis using anti-Bax or anti-Bak polyclonal Abs.
Subcellular fractionation
Cytosolic and membrane fractions were separated as previously described.16
Statistical analysis
The significance of differences between experimental conditions was determined using the Student t test for unpaired observations. Synergistic interactions were evaluated using median dose effect analysis using the Calcusyn software program (Biosoft).31 Survival of mice after treatment was evaluated by Kaplan-Meier analysis.
Results
Combined exposure of human leukemia cells to sorafenib and obatoclax results in the pronounced induction of cell death in association with profound mitochondrial injury and caspase activation
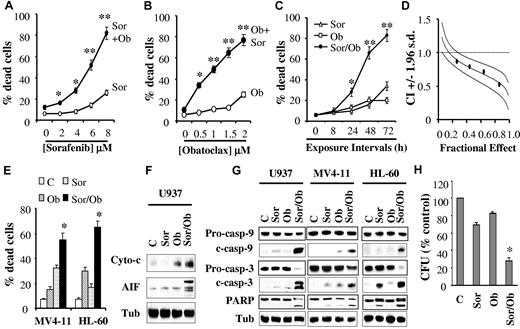
To examine interactions between sorafenib and obatoclax, U937 cells were exposed to various concentrations of these agents alone or in combination for 48 hours, after which the extent of cell death was monitored by the 7-AAD assay. While 1.5μM obatoclax was only minimally toxic by itself, it substantially increased cell death of pharmacologically achievable concentrations of sorafenib (eg, 4-8μM; Figure 1A). Analogously, the lethality of marginally toxic obatoclax concentrations (eg, 0.5-2μM) was significantly increased by coexposure to 7.5μM sorafenib (Figure 1B).
Combined treatment with sorafenib and obatoclax results in a marked induction of cell death in association with profound mitochondrial injury and caspase activation, and diminishes the colony-formation capacity of human leukemia cells. (A) U937 cells were exposed to the designated concentrations of sorafenib alone (○) or in combination with 1.5μM obatoclax (Ob, ●) for 48 hours after which the percentage of apoptotic cells was determined by the 7-AAD staining assay. *Significantly greater than sorafenib alone; P < .05; **P < .01. (B) U937 cells were exposed to the designated concentrations of obatoclax alone (○) or in combination with 7.5μM sorafenib (●), for 48 hours after which cell death was determined as in panel A. *Significantly greater than obatoclax alone; P < .02; **P < .01. (C) Cells were exposed to sorafenib (7.5μM) and obatoclax (1.5μM) alone or in combination for the indicated intervals, after which the percentage of dead cells was determined as above. *Significantly greater than either agent alone; P < .05; **P < .002. (D) Median dose effect analysis of cell death induction by sorafenib and obatoclax. U937 cells were exposed to varying concentrations of obatoclax and sorafenib at a fixed ratio (1:5), for 48 hours after which extent of cell death was monitored with the 7-AAD staining assay. Combination Index (CI) values were determined in relation to the fractional effect using the Calcusyn software program. CI values < 1.0 correspond to a synergistic interaction. (E) MV4-11 and HL-60 cells were exposed to sorafenib (75nM and 7.5μM, respectively) and obatoclax (0.5μM and 2μM, respectively), either individually or in combination for 48 hours, after which the percentage of dead cells was determined by the 7-AAD assay. *Significantly greater than values for either agent alone; P < .02. (F) U937 cells were exposed to sorafenib (7.5μM) and obatoclax (1.5μM) alone or in combination for 24 hours after which mitochondria-free cytosolic fractions were obtained and subjected to Western blot analysis to monitor the release of cytochrome c and AIF into the cytosol. For this and all subsequent Western blot analysis, blots were subsequently reprobed with antitubulin (Tub) Abs to document equivalent loading and transfer, and the blots shown are representative of at least 3 separate experiments. (G) U937, MV4-11 and HL-60 cells were exposed to sorafenib and obatoclax individually or in combination as in panels E and F for 48 hours after which protein lysates were prepared and subjected to Western blot analysis using the designated Abs. (H) U937 cells were plated in methylcellulose in the presence of sorafenib (7.5μM) and obatoclax (75nM) alone or in combination for 10 days after which CFUs were enumerated and expressed as a percentage of untreated cells. *Significantly less than values for either agent alone; P < .02. For panels A, B, C, E, and H, values represent the means ± SD for 3 separate experiments in which each sample was analyzed in triplicate.
Combined treatment with sorafenib and obatoclax results in a marked induction of cell death in association with profound mitochondrial injury and caspase activation, and diminishes the colony-formation capacity of human leukemia cells. (A) U937 cells were exposed to the designated concentrations of sorafenib alone (○) or in combination with 1.5μM obatoclax (Ob, ●) for 48 hours after which the percentage of apoptotic cells was determined by the 7-AAD staining assay. *Significantly greater than sorafenib alone; P < .05; **P < .01. (B) U937 cells were exposed to the designated concentrations of obatoclax alone (○) or in combination with 7.5μM sorafenib (●), for 48 hours after which cell death was determined as in panel A. *Significantly greater than obatoclax alone; P < .02; **P < .01. (C) Cells were exposed to sorafenib (7.5μM) and obatoclax (1.5μM) alone or in combination for the indicated intervals, after which the percentage of dead cells was determined as above. *Significantly greater than either agent alone; P < .05; **P < .002. (D) Median dose effect analysis of cell death induction by sorafenib and obatoclax. U937 cells were exposed to varying concentrations of obatoclax and sorafenib at a fixed ratio (1:5), for 48 hours after which extent of cell death was monitored with the 7-AAD staining assay. Combination Index (CI) values were determined in relation to the fractional effect using the Calcusyn software program. CI values < 1.0 correspond to a synergistic interaction. (E) MV4-11 and HL-60 cells were exposed to sorafenib (75nM and 7.5μM, respectively) and obatoclax (0.5μM and 2μM, respectively), either individually or in combination for 48 hours, after which the percentage of dead cells was determined by the 7-AAD assay. *Significantly greater than values for either agent alone; P < .02. (F) U937 cells were exposed to sorafenib (7.5μM) and obatoclax (1.5μM) alone or in combination for 24 hours after which mitochondria-free cytosolic fractions were obtained and subjected to Western blot analysis to monitor the release of cytochrome c and AIF into the cytosol. For this and all subsequent Western blot analysis, blots were subsequently reprobed with antitubulin (Tub) Abs to document equivalent loading and transfer, and the blots shown are representative of at least 3 separate experiments. (G) U937, MV4-11 and HL-60 cells were exposed to sorafenib and obatoclax individually or in combination as in panels E and F for 48 hours after which protein lysates were prepared and subjected to Western blot analysis using the designated Abs. (H) U937 cells were plated in methylcellulose in the presence of sorafenib (7.5μM) and obatoclax (75nM) alone or in combination for 10 days after which CFUs were enumerated and expressed as a percentage of untreated cells. *Significantly less than values for either agent alone; P < .02. For panels A, B, C, E, and H, values represent the means ± SD for 3 separate experiments in which each sample was analyzed in triplicate.
Time-course analysis of cells exposed simultaneously to 7.5μM sorafenib and 1.5μM obatoclax revealed ∼ 30% cell death at 24 hours, and more pronounced lethality after 48-72 hours (60%-80%, Figure 1C). Similar results were obtained with Wright-Giemsa staining or trypan blue assays (data not shown). Median dose effect analysis of cells exposed to sorafenib and obatoclax for 48 hours at a fixed ratio yielded combination index (CI) values considerably < 1.0, indicating a highly synergistic interaction (Figure 1D). Enhanced lethality after combined sorafenib/obatoclax exposure was also observed in other leukemia cell lines including the FLT3-ITD–dependent MV4-11, and promyelocytic leukemia HL-60 cells (Figure 1E). Notably, in MV4-11 cells, lower sorafenib concentrations were required (eg, 75nM), presumably because of inhibitory effects of sorafenib on FLT3.14,15 Combined exposure to sorafenib and obatoclax was associated with profound mitochondrial damage, reflected by the pronounced release of cytochrome c and AIF into the cytosol in U937 cells (Figure 1F), the marked cleavage/activation of caspases-3 and -9, and PARP in U937 as well as MV4-11 and HL-60 cells (Figure 1G), and the pronounced loss in mitochondrial membrane potential (Δψ, supplemental Figure 1A). In sharp contrast, individual exposure of cells to sorafenib or obatoclax had only minimal effects. Notably, pretreatment of cells with the pan-caspase inhibitor Q-VD-OPH sharply diminished sorafenib/obatoclax-mediated lethality (supplemental Figure 1B, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Combined treatment also markedly reduced cell growth and viability (supplemental Figure 1C). Finally, combined exposure to sorafenib and very low concentrations of obatoclax (75nM) resulted in a marked decline in U937 cell colony formation, whereas the agents administered alone exerted only modest effects (Figure 1H).
Parallel studies using the annexin V/PI assay revealed that coexposure of human leukemia cells to sorafenib and HA14-1, another BH3-mimetic Bcl-2 antagonist, for 24 hours also resulted in the marked induction of apoptosis (supplemental Figure 2A), in association with the striking release of cytochrome c and AIF into the cytosol (supplemental Figure 2B) and cleavage of caspase-3, caspase-9, and PARP (supplemental Figure 2C). In addition, median dose effect analysis performed in U937 cells revealed highly synergistic interactions between sorafenib and HA14-1 (supplemental Figure 2D) analogous to results obtained with sorafenib and obatoclax. Lastly, sorafenib also enhanced the lethality of the BH3-mimetic gossypol in U937 cells (supplemental Figure 2E).
Cotreatment with sorafenib and obatoclax increases lethality in primary AML blasts while largely sparing normal CD34+ cells
Parallel studies using the 7-AAD assay revealed that simultaneous administration of sorafenib and obatoclax resulted in a significant increase in cell death in primary leukemic blasts isolated from 4 patients with AML (FAB classification M2; 3 patients with wild-type FLT3 and 1 patient with a FLT3-ITD mutation; Figure 2A). Similar results were obtained when Wright-Giemsa–stained cells were evaluated (data not shown). Further analysis performed on blasts isolated from patient 1 showed enhanced cleavage of caspase-3 and PARP in cells cotreated with sorafenib and obatoclax compared with cells treated with the agents alone (Figure 2B). Interestingly, exposure to comparable or significantly higher concentrations of sorafenib and obatoclax alone or in combination exhibited minimal lethality toward normal CD34+ cells (Figure 2C). Furthermore, the clonogenic potential of primary AML specimens was markedly diminished by combined treatment with sorafenib and obatoclax (Figure 2D), whereas the clonogenicity of normal CD34+ cells was largely unaffected (Figure 2E).
Combined exposure to sorafenib and obatoclax results in enhanced lethality in primary AML cells. (A) Leukemic blasts were isolated from the BM of 4 patients with AML (FAB classification M2; AML#1, AML#2, and AML#4 with wild-type FLT3, and AML#3 with a FLT3-ITD mutation), exposed to sorafenib (7.5μM) and obatoclax (0.5μM) for 48 hours, after which the extent of cell death was assessed using the 7-AAD analysis. Results are presented as percentage of dead cells specific for each treatment using the formula ([treatment − control]/[100 − control]) × 100. Cell death for untreated control samples ranged from 10% to 25%. (B) Alternatively, protein lysates were prepared from AML patient #1 and subjected to Western blot analysis. Densitometric analysis of cleaved PARP and caspase-3 bands was performed using Adobe Photoshop. Values shown were normalized to ERK1/2 and represent relative changes compared with control. (C) Normal CD34+ cells were isolated as described in “Methods” from the BM of normal subjects (nonleukemic; N#1, N#2, and N#3) and exposed to increasing concentrations of sorafenib and obatoclax alone or in combination for 48 hours, after which the extent of cell death was determined using the 7-AAD analysis. (D) Two primary AML specimens were plated in methylcellulose in the presence of 5μM sorafenib and 75nM obatoclax alone or in combination for 14 days, after which CFUs were enumerated and expressed as a percentage relative to untreated cells. (E) Normal CD34+ cells from 2 subjects were plated in methylcellulose in the presence of increasing concentrations of sorafenib and obatoclax alone or in combination for 8 days, after which CFUs were enumerated and expressed as in panel D. For panels A, C, D, and E, data for each patient were obtained from a single experiment performed in triplicate; values represent the means ± SD.
Combined exposure to sorafenib and obatoclax results in enhanced lethality in primary AML cells. (A) Leukemic blasts were isolated from the BM of 4 patients with AML (FAB classification M2; AML#1, AML#2, and AML#4 with wild-type FLT3, and AML#3 with a FLT3-ITD mutation), exposed to sorafenib (7.5μM) and obatoclax (0.5μM) for 48 hours, after which the extent of cell death was assessed using the 7-AAD analysis. Results are presented as percentage of dead cells specific for each treatment using the formula ([treatment − control]/[100 − control]) × 100. Cell death for untreated control samples ranged from 10% to 25%. (B) Alternatively, protein lysates were prepared from AML patient #1 and subjected to Western blot analysis. Densitometric analysis of cleaved PARP and caspase-3 bands was performed using Adobe Photoshop. Values shown were normalized to ERK1/2 and represent relative changes compared with control. (C) Normal CD34+ cells were isolated as described in “Methods” from the BM of normal subjects (nonleukemic; N#1, N#2, and N#3) and exposed to increasing concentrations of sorafenib and obatoclax alone or in combination for 48 hours, after which the extent of cell death was determined using the 7-AAD analysis. (D) Two primary AML specimens were plated in methylcellulose in the presence of 5μM sorafenib and 75nM obatoclax alone or in combination for 14 days, after which CFUs were enumerated and expressed as a percentage relative to untreated cells. (E) Normal CD34+ cells from 2 subjects were plated in methylcellulose in the presence of increasing concentrations of sorafenib and obatoclax alone or in combination for 8 days, after which CFUs were enumerated and expressed as in panel D. For panels A, C, D, and E, data for each patient were obtained from a single experiment performed in triplicate; values represent the means ± SD.
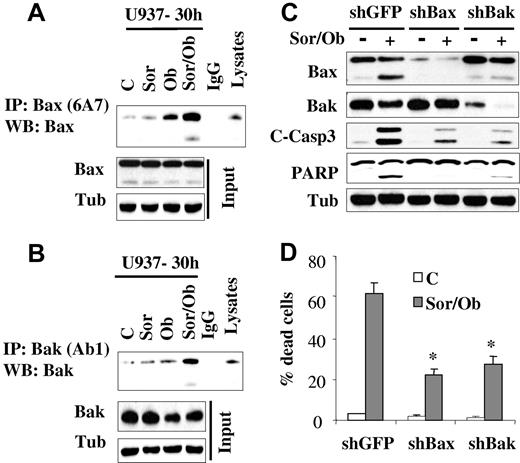
Sorafenib/obatoclax-mediated lethality involves Bax and Bak
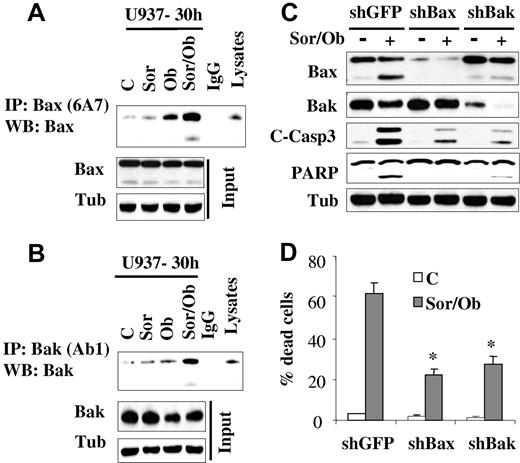
In view of the central roles that the multidomain proteins Bax and Bak play in apoptosis,32 activation of these proteins was examined. While treatment with sorafenib (7.5μM) or obatoclax (1.5μM) alone minimally induced Bak and Bax conformational change, effects of combined treatment were very pronounced (Figure 3A-B). In contrast, no major changes in Bax expression was observed in cells treated with obatoclax alone or in combination with sorafenib. On the other hand, a modest decrease in Bak protein level was observed after obatoclax treatment. To test the functional role of Bax and Bak in sorafenib/obatoclax lethality, U937 cells in which Bax or Bak were knocked down (Figure 3C) were used. Dose-response studies revealed that knockdown of Bax or Bak rendered cells more resistant to either sorafenib or obatoclax alone (supplemental Figure 3A-B). In addition, these cells displayed significant resistance to sorafenib/obatoclax-mediated caspase activation and cell death (Figure 3C-D). These findings indicate that Bax and Bak play significant functional roles in the antileukemic activity of the sorafenib/obatoclax regimen.
Exposure to sorafenib/obatoclax results in Bak and Bax conformational change while knockdown of these molecules markedly attenuates cell death. U937 cells were exposed to sorafenib (7.5μM) and obatoclax (1.5μM) alone or in combination for 30 hours after which cells were lysed in buffer containing 1% CHAPS; conformationaly changed Bax (A) and Bak (B) proteins were immunoprecipitated using anti-Bax 6A7 and anti-Bak Ab1 Abs, respectively, and subjected to Western blot analysis using polyclonal Bax or Bak Abs. Input lysates were also subjected to Western blot analysis to monitor Bax and Bak protein levels. (C) U937 cells stably transfected with shRNA against GFP (shGFP), Bax (shBax), or Bak (shBak) were treated with sorafenib (7.5μM) and obatoclax (1.5μM), for 48 hours after which protein lysates were prepared and subjected to Western blot analysis. Alternatively, the extent of cell death was determined using the 7-AAD staining assay (D). Values represent the means for 3 separate experiments ± SD * = significantly lower than values obtained for shGFP cells (P < .01).
Exposure to sorafenib/obatoclax results in Bak and Bax conformational change while knockdown of these molecules markedly attenuates cell death. U937 cells were exposed to sorafenib (7.5μM) and obatoclax (1.5μM) alone or in combination for 30 hours after which cells were lysed in buffer containing 1% CHAPS; conformationaly changed Bax (A) and Bak (B) proteins were immunoprecipitated using anti-Bax 6A7 and anti-Bak Ab1 Abs, respectively, and subjected to Western blot analysis using polyclonal Bax or Bak Abs. Input lysates were also subjected to Western blot analysis to monitor Bax and Bak protein levels. (C) U937 cells stably transfected with shRNA against GFP (shGFP), Bax (shBax), or Bak (shBak) were treated with sorafenib (7.5μM) and obatoclax (1.5μM), for 48 hours after which protein lysates were prepared and subjected to Western blot analysis. Alternatively, the extent of cell death was determined using the 7-AAD staining assay (D). Values represent the means for 3 separate experiments ± SD * = significantly lower than values obtained for shGFP cells (P < .01).
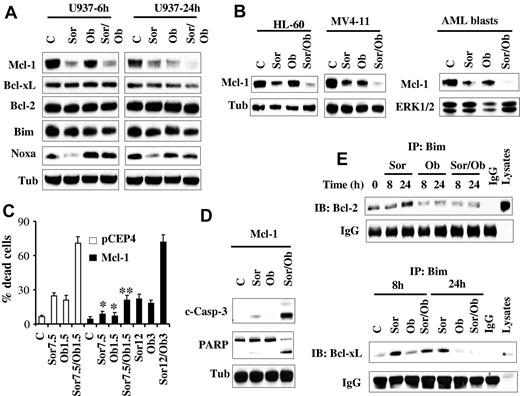
Mcl-1 down-regulation is required for sorafenib/obatoclax-mediated apoptosis
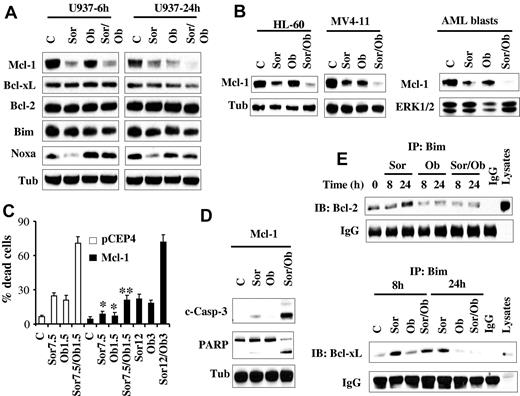
Previously, we and others reported that sorafenib diminishes Mcl-1 protein expression in human leukemia cells by inhibiting translation through a MEK1/2/ERK1/2 signaling-independent mechanism, and that this phenomenon plays a significant functional role in sorafenib lethality.16,17,20 Consequently, Mcl-1 expression and function was examined in relation to sorafenib and obatoclax responses. Consistent with our previous report,16 Western blot analysis revealed that sorafenib significantly reduced Mcl-1 protein levels. Interestingly, obatoclax induced similar effects, and combined treatment (24 hours) essentially abrogated Mcl-1 expression (Figure 4A). Comparable results were obtained in HL-60 and MV4-11 cells as well as primary AML blasts (Figure 4B). In contrast, no major changes were noted in the levels of other antiapoptotic family members, that is, Bcl-2 or Bcl-xL (Figure 4A). In addition, very modest down-regulation of Bim protein levels was observed after exposure to agents alone or in combination. However, in marked contrast, and consistent with a previous report,33 Noxa was markedly down-regulated by sorafenib (Figure 4A). However, this effect was largely prevented by cotreatment with obatoclax, which by itself modestly increased Noxa protein levels at 6 hours, but not at 24 hours.
Sorafenib/obatoclax-mediated lethality involves Mcl-1 down-regulation. (A) U937 cells were exposed to sorafenib (7.5μM) and obatoclax (1.5μM) alone or in combination for 6 or 24 hours after which protein lysates were prepared and subjected to Western blot analysis using the designated Abs. (B) HL-60, MV4-11 cells, and primary blasts were treated with sorafenib (7.5μM for HL-60 cells and primary blasts; 75nM for MV4-11cells) and obatoclax (2μM for HL-60, and 0.5μM for MV4-11 and primary blasts) for 28 hours after which cells were lysed and protein lysates were subjected to Western blot analysis. (C) U937 cells ectopically expressing Mcl-1 or their empty vector control cells (pCEP4) were treated with the designated concentrations of sorafenib and obatoclax alone or in combination for 48 hours after which the extent of cell death was determined using the 7-AAD staining assay. Values represent the means for 3 separate experiments ± SD. *Significantly less than values for pCEP4 control cells; P < .05. **P < .01. (D) Alternatively, cleavage of PARP and caspase-3 in U937/Mcl-1 cells exposed to 12μM sorafenib and 3μM obatoclax was monitored by Western blot analysis. (E) U937 cells were treated with sorafenib (7.5μM) and obatoclax (1.5μM) individually or together for 8 or 24 hours after which cells were lysed and subjected to immunoprecipitation using Bim Abs. The immunoprecipitates were separated by SDS-PAGE and immunoblotted with either Bcl-2 (top panel) or Bcl-xL (bottom panel) Abs.
Sorafenib/obatoclax-mediated lethality involves Mcl-1 down-regulation. (A) U937 cells were exposed to sorafenib (7.5μM) and obatoclax (1.5μM) alone or in combination for 6 or 24 hours after which protein lysates were prepared and subjected to Western blot analysis using the designated Abs. (B) HL-60, MV4-11 cells, and primary blasts were treated with sorafenib (7.5μM for HL-60 cells and primary blasts; 75nM for MV4-11cells) and obatoclax (2μM for HL-60, and 0.5μM for MV4-11 and primary blasts) for 28 hours after which cells were lysed and protein lysates were subjected to Western blot analysis. (C) U937 cells ectopically expressing Mcl-1 or their empty vector control cells (pCEP4) were treated with the designated concentrations of sorafenib and obatoclax alone or in combination for 48 hours after which the extent of cell death was determined using the 7-AAD staining assay. Values represent the means for 3 separate experiments ± SD. *Significantly less than values for pCEP4 control cells; P < .05. **P < .01. (D) Alternatively, cleavage of PARP and caspase-3 in U937/Mcl-1 cells exposed to 12μM sorafenib and 3μM obatoclax was monitored by Western blot analysis. (E) U937 cells were treated with sorafenib (7.5μM) and obatoclax (1.5μM) individually or together for 8 or 24 hours after which cells were lysed and subjected to immunoprecipitation using Bim Abs. The immunoprecipitates were separated by SDS-PAGE and immunoblotted with either Bcl-2 (top panel) or Bcl-xL (bottom panel) Abs.
To assess the functional significance of Mcl-1 down-regulation in sorafenib/obatoclax-induced lethality, U937 cells ectopically expressing Mcl-1 were used. As anticipated, these cells were significantly more resistant than control cells to sorafenib (supplemental Figure 3C). Interestingly, these cells were also significantly more resistant to obatoclax than control cells, although the degree of resistance was less than that observed in the case of sorafenib (supplemental Figure 3D). Notably, combined treatment with 7.5μM sorafenib and 1.5μM obatoclax, which resulted in pronounced cell death in empty-vector cells, only modestly increased cell death in Mcl-1–overexpressing cells (Figure 4C). However, Mcl-1–mediated resistance was fully reversed by increasing agent concentrations (ie, 12μM sorafenib and 3μM obatoclax; Figure 4C), accompanied by sharply increased caspase-3 cleavage/activation and PARP cleavage (Figure 4D). Together, these findings argue that Mcl-1 down-regulation in cells exposed to sorafenib and obatoclax plays a significant functional role in the lethality of this regimen.
Bim, but not Noxa, plays a critical functional role in antileukemic activity of sorafenib/obatoclax
Previously, we and others have reported that the BH3-only protein Bim (Bcl-2–interacting mediator of cell death) plays an important functional role in sorafenib-mediated apoptosis.28,34 Therefore, the hypothesis that sorafenib/obatoclax might enhance Bim activation by interfering with Bcl-2, Bcl-xL, and Mcl-1 interactions was examined. Interestingly, immunoprecipitation experiments revealed that sorafenib alone significantly increased Bim binding to Bcl-2 (Figure 4E top panel) and Bcl-xL (Figure 4E bottom panel), presumably because of release of Bim from Mcl-1 after down-regulation of the latter. Significantly, these effects were essentially abrogated by cotreatment with obatoclax. To test the functional role of Bim in sorafenib/obatoclax-mediated lethality, 2 shRNA constructs (shBim#1, shBim#2) designed against Bim were separately transfected into U937 cells, and stable clones exhibiting significant Bim knockdown with each construct were used (Figure 5A). Bim knockdown cells were significantly more resistant to sorafenib/obatoclax-mediated lethality than their control counterparts, reflected by diminished cleavage of caspase-3 and its downstream substrate PARP (Figure 5A), cytochrome c release into the cytosol (Figure 5B), and 7-AAD uptake (Figure 5C). Knockdown of Bim in MV4-11 cells also significantly diminished sorafenib/obatoclax lethality (supplemental Figure 4A-B). Furthermore, cells ectopically expressing Bim displayed significantly enhanced sensitivity to the sorafenib/obatoclax regimen compared with control cells transfected with a pcDNA3.1 construct as reflected by increased 7-AAD positivity (Figure 5D), and enhanced cleavage of caspase-3 and PARP (supplemental Figure 4C). Together, these findings suggest that Bim plays an important functional role in sorafenib/obatoclax-mediated lethality.
Knockdown of Bim, but not Noxa, significantly diminishes sorafenib/obatoclax-mediated cell death. (A) U937 cells were transfected with 2 shRNA constructs designed against Bim (shBim#1 and shBim#2), and one clone from each transfection was selected. These and shGFP control cells were exposed to sorafenib (7.5μM) and obatoclax (1.5μM) for 48 hours after which Western blot analysis was performed. (B) shBim#1 and shBim#2 cells were treated with sorafenib and obatoclax as in panel A for 6 hours after which the cytosolic fraction was isolated and subjected to Western blot analysis. (C) shBim#1, shBim#2, and shGFP cells were exposed to sorafenib and obatoclax as in panel A for 48 hours after which the extent of cell death was monitored by the 7-AAD staining assay. *Significantly less than values for shGFP control cells; P < .02. (D) U937 cells overexpressing wild-type Bim or their empty vector control (pcDNA3.1) were treated with sorafenib (7.5μM) and obatoclax (1.5μM) for 24 hours after which the percentage of dead cells was determined using the 7-AAD assay. *Significantly greater than values obtained for pcDNA3.1 cells (P < .05). (E) U937 cells in which Noxa was stably knocked down with shRNA and their control counterpart shGFP-transfected cells were exposed to sorafenib and obatoclax for 48 hours after which the extent of cell death was determined using the 7-AAD assay. *Not significantly different from values obtained for shGFP-transfected cells (P > .05). (F) Alternatively, cells were lysed and protein lysates subjected to Western blot analysis to monitor down-regulation of Noxa, and caspase-3 activation by Western blot analysis.
Knockdown of Bim, but not Noxa, significantly diminishes sorafenib/obatoclax-mediated cell death. (A) U937 cells were transfected with 2 shRNA constructs designed against Bim (shBim#1 and shBim#2), and one clone from each transfection was selected. These and shGFP control cells were exposed to sorafenib (7.5μM) and obatoclax (1.5μM) for 48 hours after which Western blot analysis was performed. (B) shBim#1 and shBim#2 cells were treated with sorafenib and obatoclax as in panel A for 6 hours after which the cytosolic fraction was isolated and subjected to Western blot analysis. (C) shBim#1, shBim#2, and shGFP cells were exposed to sorafenib and obatoclax as in panel A for 48 hours after which the extent of cell death was monitored by the 7-AAD staining assay. *Significantly less than values for shGFP control cells; P < .02. (D) U937 cells overexpressing wild-type Bim or their empty vector control (pcDNA3.1) were treated with sorafenib (7.5μM) and obatoclax (1.5μM) for 24 hours after which the percentage of dead cells was determined using the 7-AAD assay. *Significantly greater than values obtained for pcDNA3.1 cells (P < .05). (E) U937 cells in which Noxa was stably knocked down with shRNA and their control counterpart shGFP-transfected cells were exposed to sorafenib and obatoclax for 48 hours after which the extent of cell death was determined using the 7-AAD assay. *Not significantly different from values obtained for shGFP-transfected cells (P > .05). (F) Alternatively, cells were lysed and protein lysates subjected to Western blot analysis to monitor down-regulation of Noxa, and caspase-3 activation by Western blot analysis.
Finally, previous studies indicated that the proapoptotic protein Noxa may also contribute to cell death induced by treatment involving obatoclax35,36 or sorafenib33 in some settings. To determine whether Noxa played a functional role in sorafenib/obatoclax-mediated cell death, U937 cells in which Noxa protein was knocked down with shRNA were used. In contrast to results involving Bim, a 7-AAD staining assay revealed that U937 cells in which Noxa expression was markedly decreased (Figure 5F) remained fully sensitive to sorafenib/obatoclax lethality (Figure 5E). Consistent with this finding, no major changes were observed in caspase-3 or PARP cleavage (Figure 5F), arguing against the possibility that Noxa plays a major functional role in sorafenib/obatoclax-mediated apoptosis in human leukemia cells.
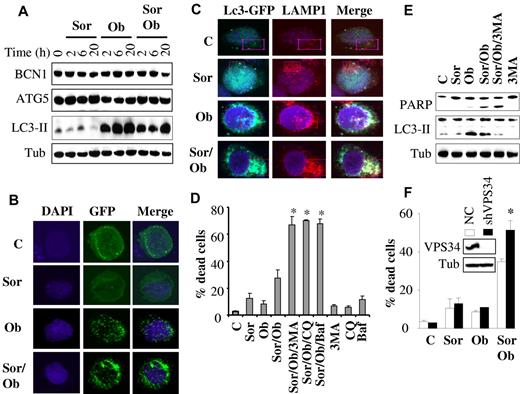
Autophagy plays a protective role against sorafenib/obatoclax lethality
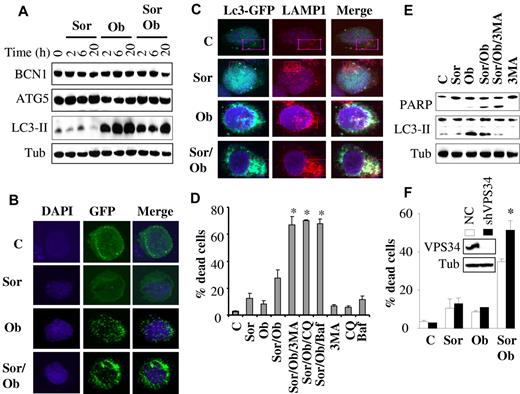
Because both sorafenib and obatoclax have been implicated in induction of autophagy in diverse systems,10,11 we examined whether this process might play a role in sorafenib/obatoclax antileukemic activity. As shown in Figure 6A, obatoclax elicited a rapid and robust induction of LC3 processing in U937 cells, which occurred as early as 2 hours after treatment and persisted over the ensuing 20 hours posttreatment. This effect was also observed with combined exposure to obatoclax and sorafenib, although it was slightly less pronounced. However, sorafenib alone had no discernible effect on LC3 processing. Similar results were obtained in HL-60 cells (supplemental Figure 5F). In contrast, no major changes were observed in the expression of beclin-1 (BCN1) or ATG5 protein levels with any treatments. Consistent with LC-3 processing, confocal microscopic analysis of U937 cells expressing an LC3-GFP fusion protein revealed a marked increase in autophagosomes, as indicated by enhanced LC3-GFP punctate formation/aggregation in cells exposed for 6 hours to obatoclax alone or in combination with sorafenib (but not to sorafenib alone; Figure 6B). Similar results were observed in MV4-11 cells (supplemental Figure 5A). Furthermore, immunofluorescence studies using LC3-EGFP (an autophagosome marker) and LAMP1 (a lysosome marker) revealed marker colocalization after treatment with obatoclax alone or in combination with sorafenib, indicating fusion between autophagosomes and lysosomes (Figure 6C, supplemental Figure 5B). Furthermore, p62/SQSTM1, a marker of autophagy flux, significantly decreased after treatment (24 hours) with obatoclax alone or obatoclax/sorafenib (supplemental Figure 5C).
Role of autophagy in sorafenib/obatoclax-mediated lethality. (A) U937 cells were exposed to sorafenib (7.5μM) and obatoclax (1.5μM) for the designated intervals after which protein lysates were prepared and subjected to Western blot analysis. (B) U937 cells were stably transfected with LC3-EGFP, and EGFP-positive cells were sorted by FACS and exposed to sorafenib and obatoclax for 6 hours. Cells were then fixed and subjected to confocal microscopy. (C) Representative images with confocal microscopy of colocalized LC3-GFP (green) and LAMP1 (red) in U937 cells after 6-hour treatment with sorafenib (7.5μM) and obatoclax (1.5μM). Enlarged images of outlined areas are shown in supplemental Figure 5B. (D) U937 cells were pretreated with 3-MA (2.5 mM), chloroquine (CQ; 40μM), or bafilomycin A (BAF; 75nM) for 30 minutes, and exposed to sorafenib (7.5μM) and obatoclax (1.5μM) alone or in combination for an additional 16 hours after which the extent of cell death was monitored by the 7-AAD assay. *Significantly greater than values for cells not exposed to 3MA, CQ, or BAF; P < .01. (E) Alternatively, protein lysates were prepared from the indicated samples and subjected to Western blot analysis. (F) U937 cells in which VPS34 was stably knocked down with lentivirus-mediated shRNA and their control scrambled sequence counterparts (NC) were exposed to sorafenib (7.5μM) ± obatoclax (1.5μM) for 24 hours, after which the extent of cell death was determined using the 7-AAD assay. *Significantly different from values obtained for control cells (P < .05).
Role of autophagy in sorafenib/obatoclax-mediated lethality. (A) U937 cells were exposed to sorafenib (7.5μM) and obatoclax (1.5μM) for the designated intervals after which protein lysates were prepared and subjected to Western blot analysis. (B) U937 cells were stably transfected with LC3-EGFP, and EGFP-positive cells were sorted by FACS and exposed to sorafenib and obatoclax for 6 hours. Cells were then fixed and subjected to confocal microscopy. (C) Representative images with confocal microscopy of colocalized LC3-GFP (green) and LAMP1 (red) in U937 cells after 6-hour treatment with sorafenib (7.5μM) and obatoclax (1.5μM). Enlarged images of outlined areas are shown in supplemental Figure 5B. (D) U937 cells were pretreated with 3-MA (2.5 mM), chloroquine (CQ; 40μM), or bafilomycin A (BAF; 75nM) for 30 minutes, and exposed to sorafenib (7.5μM) and obatoclax (1.5μM) alone or in combination for an additional 16 hours after which the extent of cell death was monitored by the 7-AAD assay. *Significantly greater than values for cells not exposed to 3MA, CQ, or BAF; P < .01. (E) Alternatively, protein lysates were prepared from the indicated samples and subjected to Western blot analysis. (F) U937 cells in which VPS34 was stably knocked down with lentivirus-mediated shRNA and their control scrambled sequence counterparts (NC) were exposed to sorafenib (7.5μM) ± obatoclax (1.5μM) for 24 hours, after which the extent of cell death was determined using the 7-AAD assay. *Significantly different from values obtained for control cells (P < .05).
Interestingly, interference with autophagy using a series of autophagy inhibitors (eg, 3MA, chloroquine, or bafilomycin A1) strikingly potentiated sorafenib/obatoclax lethality (Figure 6D). In contrast, these agents did not result in significant increases in the lethality of obatoclax or sorafenib alone (supplemental Figure 5D). Western blot analysis (Figure 6E) demonstrated diminished LC3 processing by sorafenib/obatoclax in the presence of 3MA, indicating inhibition of autophagy, accompanied by enhanced apoptosis, reflected by increased cleavage of PARP. Similar findings were observed in HL-60 cells (supplemental Figure 5E-F). Chloroquine, however, which inhibits late-stage autophagy by blocking lysosomal acidification, did not decrease LC3 processing (supplemental Figure 5G), as previously reported.37,38 In accord with these findings, knockdown of VPS34, an essential protein for autophagy,37 significantly enhanced obatoclax/sorafenib lethality (Figure 6F). Finally, consistent with a cytoprotective role for autophagy, Bim knockdown-mediated resistance to obatoclax/sorafenib lethality was associated with increased LC3 processing (supplemental Figure 6). Together, these findings argue that autophagy primarily plays a protective role against the lethality of this regimen.
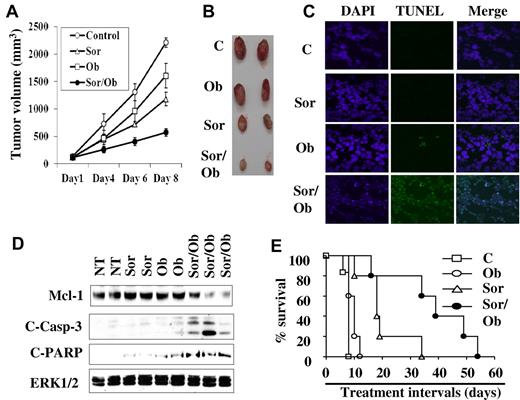
Combined treatment with sorafenib and obatoclax results in apoptosis induction, tumor growth reduction, and enhanced survival in an in vivo leukemia xenograft model
To determine whether cotreatment with sorafenib and obatoclax exhibits antileukemic activity in vivo, nude mouse xenografts inoculated with U937 cells were used. Notably, sorafenib (80 mg/kg) administered alone resulted in a clear inhibition of tumor growth (Figure 7A-B), and this effect was significantly enhanced by combined treatment with 3.5 mg/kg obatoclax, which by itself exhibited only modest effects (Figure 7A-B). Significantly, TUNEL assays and Western blot analysis performed on tumor tissue excised from animals treated with 2 doses of each agent alone or in combination over a 24-hour interval revealed that combined, but not individual, treatment induced apoptosis, reflected by a clear increase in TUNEL positivity (Figure 7C), and sharply increased caspase-3 processing and PARP cleavage in association with a marked decline in Mcl-1 levels (Figure 7D). After 8 days of treatment, Mcl-1 levels in tumors were significantly down-regulated by either agents alone or in combination (data not shown). These events correlated with significantly prolonged survival of tumor-bearing mice treated with sorafenib/obatoclax and, to a lesser extent, with sorafenib alone (Figure 7E). In contrast, obatoclax administered alone did not prolong survival. These findings were confirmed in studies of mice inoculated with luciferase-expressing U937 cells (supplemental Figure 7A). Finally, treatment with agents alone or in combination did not lead to major changes in mouse weights or in white or red blood cell counts (supplemental Figure 7B and C, respectively), nor were changes in behavior or hair loss observed. Together, these findings indicate that combined treatment with sorafenib and obatoclax significantly reduces tumor growth in association with Mcl-1 down-regulation, and prolongs survival in leukemia-bearing mice.
In vivo antileukemic activity of combined treatment with sorafenib and obatoclax. Nude mice were subcutaneously injected with U937 cells and subjected to treatment with sorafenib (80 mg/kg) and obatoclax (3.5 mg/kg) alone or together. Tumor volumes were measured at the indicated intervals (A), and pictures of 2 representative tumors for each group were obtained after 8 days of treatment (B). Xenograft-bearing mice were treated with sorafenib and/or obatoclax by IM administration twice over a 24-hour interval, after which tumors were excised, and either subjected to TUNEL analysis assays (C), or lysed, and subjected to Western blot analysis (D). (E) Kaplan-Meier survival plot for mice treated with sorafenib and obatoclax alone or in combination. The data shown are representative of 3 separate experiments each involving 5 mice/condition. The survival curves differed significantly between sorafenib/obatoclax and various other treatments (P = .001 to .04; log-rank test).
In vivo antileukemic activity of combined treatment with sorafenib and obatoclax. Nude mice were subcutaneously injected with U937 cells and subjected to treatment with sorafenib (80 mg/kg) and obatoclax (3.5 mg/kg) alone or together. Tumor volumes were measured at the indicated intervals (A), and pictures of 2 representative tumors for each group were obtained after 8 days of treatment (B). Xenograft-bearing mice were treated with sorafenib and/or obatoclax by IM administration twice over a 24-hour interval, after which tumors were excised, and either subjected to TUNEL analysis assays (C), or lysed, and subjected to Western blot analysis (D). (E) Kaplan-Meier survival plot for mice treated with sorafenib and obatoclax alone or in combination. The data shown are representative of 3 separate experiments each involving 5 mice/condition. The survival curves differed significantly between sorafenib/obatoclax and various other treatments (P = .001 to .04; log-rank test).
Discussion
Members of the Bcl-2 family of apoptotic regulatory proteins are frequently dysregulated in various transformed cells, including those of hematopoietic origin. In previous reports, we and others have demonstrated that the multikinase inhibitor sorafenib potently induces apoptosis in diverse cancer cell types, including leukemia, through a process involving Mcl-1 down-regulation.16,17 Interestingly, Mcl-1 down-regulation by sorafenib was found to be related to translation inhibition through a mechanism(s) independent of MEK1/2/ERK1/2 signaling.16,22 Recent evidence suggests that Bcl-2 and Bcl-xL may also confer resistance to sorafenib-mediated apoptosis in transformed cells23,39 raising the possibility that inhibition of the antiapoptotic proteins Bcl-2 and Bcl-xL may enhance sorafenib lethality. The present results indicate that combined sorafenib/obatoclax exposure triggers pronounced lethality in and markedly reduces the clonogenic survival of various primary AML blast specimens and cell lines, while exerting only modest effects on normal CD34+ cells. Importantly, in vivo studies demonstrate that combined sorafenib/obatoclax treatment markedly inhibits leukemia growth and significantly prolongs survival in association with diminished leukemia cell Mcl-1 down-regulation, and caspase activation, thus recapitulating effects occurring in vitro.
The observed Mcl-1 down-regulation by either sorafenib or obatoclax is in accord with previous results from our and other groups.16,17,35 While inhibition of MEK1/2/ERK1/2 has been shown to diminish Mcl-1 expression,40 sorafenib-mediated Mcl-1 down-regulation appears to proceed through a translational mechanism that does not depend on ERK1/2 inactivation.16,22,26 In contrast, relatively little is known about the mechanism(s) by which obatoclax down-regulates Mcl-1. Given evidence that obatoclax up-regulates Noxa expression in some cell types,35,36 it is possible that obatoclax may induce Noxa, which enhances Mcl-1 protein degradation.41 However, the observation that obatoclax diminished Mcl-1 levels in the absence of Noxa up-regulation (eg, in UPN-1 or Jeko mantle cell lymphoma lines)35 argues against a primary role for Noxa in this phenomenon, at least in some cell types. Regardless of the precise mechanism(s) by which Mcl-1 down-regulation occurred, the finding that ectopic Mcl-1 expression significantly attenuated sorafenib/obatoclax-induced lethality suggests that Mcl-1 down-regulation contributes functionally to cell death in this setting.
It is well established that Mcl-1 plays an important role in leukemia survival.42 However, it is unlikely that Mcl-1 down-regulation in isolation can trigger pronounced cell death.43 Instead, it is more likely that Mcl-1 down-regulation cooperates with other events (eg, MEK/ERK inactivation, down-regulation of cFLIPL, cIAP2, and survivin among others19,21,44 ) to lower the apoptotic threshold. In support of this notion, obatoclax, which inhibits Mcl-1 as well as Bcl-2/Bcl-xL,4 has been shown to induce apoptosis administered alone in human leukemia cells, but at considerably higher concentrations than those used here.5 Despite the observed cytoprotection conferred by ectopic expression of Mcl-1, it is noteworthy that increasing concentrations of sorafenib and obatoclax (∼ 2-fold) restored apoptosis to levels equivalent to those observed with lower drug concentrations in empty-vector control cells. Such findings raise the possibility that this strategy may be effective against some leukemia cells exhibiting increased Mcl-1 expression.
It is currently thought that Mcl-1 antiapoptotic actions primarily involve interactions with the proapoptotic proteins Bak and Bim.45 Of note, the BH3-only protein Bim binds with equivalent avidity to the antiapoptotic proteins Bcl-2, Bcl-xL, and Mcl-1.45,46 In this context, treatment with sorafenib increased binding of Bim to Bcl-2 and Bcl-xL, presumably a consequence of Mcl-1 down-regulation accompanied by release of Bim, and that this effect was essentially abrogated by obatoclax cotreatment. While obatoclax has been shown to interfere with Bim binding to Bcl-2,5,9 its capacity to inhibit the Bim-Bcl-xL association has not, to the best of our knowledge, previously been described. Together, these observations raise the possibility that combined treatment increases free Bim through 2 mechanisms: (1) reduction in Mcl-1 expression, thus freeing Bim; and (2) release of Bim from Bcl-2/Bcl-xL, to which it might otherwise bind after Mcl-1 down-regulation. The consequences of these events are activation of Bax/Bak and resulting induction of cell death. In addition to down-regulation of Mcl-1, obatoclax also has been shown to untether Bak from Mcl-1 and to promote Bak activation.4,6,9 Together, these findings support the notion that combined treatment leads to release of Bim from all 3 antiapoptotic proteins (ie, Bcl-2, Bcl-xL, and Mcl-1), leading to Bak and Bax activation and apoptosis. This interpretation is supported by the observations that knockdown of Bax, Bak, or particularly Bim with shRNA, or ectopic expression of Mcl-1, significantly diminished sorafenib/obatoclax-mediated lethality.
Recent studies have shown that Noxa plays an important role in cell death mediated by obatoclax when this agent is combined with bortezomib in mantle cell lymphoma cells35 or with tunicamycin in melanoma cells.36 In the latter, but not the former, report, modest up-regulation of Noxa was observed. In the present studies, while sorafenib sharply down-regulated Noxa protein levels as previously described,33 obatoclax, either alone or in combination with sorafenib, increased Noxa expression in accord with previous results.35,36 However, such increases were modest and transient, appearing at 6 hours but not at 24 hours. Significantly, knockdown of Noxa with shRNA failed to diminish obatoclax/sorafenib lethality, arguing that Noxa up-regulation does not play a critical functional role in this setting. This may be explained by evidence that the primary mechanism by which Noxa promotes apoptosis involves neutralization/down-regulation of Mcl-1,41,47 which under the present circumstances was largely eliminated through the actions of sorafenib.
Of note, obatoclax increased the activity of lower sorafenib concentrations to induce cell death in FLT3-mutated cells, which are known to be highly susceptible to the latter agent both in vitro and in vivo.14,15 In such cells, interactions between obatoclax and lower concentrations of sorafenib may involve mechanisms other than or in addition to translational inhibition of Mcl-1 (eg, potentiation of the lethal consequences of sorafenib-mediated FLT3-ITD inhibition by obatoclax). Separate studies designed to test this concept are currently in progress.
It is interesting that obatoclax administered either alone or in combination with sorafenib induced a marked increase in autophagic markers, including induction of LC3-II and formation of GFP-LC3 punctate/aggregates in human leukemia cells. Notably, LC3 processing was slightly reduced in cells exposed to combined treatment compared with obatoclax alone. However, the pronounced increase in LC3-GFP punctuate formation and fusion between autophagosomes and lysosomes observed with combined treatment was similar to that seen with obatoclax alone. Autophagy represents a versatile and dynamic cellular response to diverse noxious stimuli that in most cases protects cells, but can also, under some circumstances, contribute to their demise.48 It should be noted that increases in autophagic markers such as processed LC3 or the formation of LC3-GFP punctuate do not necessarily reflect increased autophagic flux, but can also represent inhibition of autophagosome maturation and lysosomal degradation.49 In this regard, LC3 processing can also be initiated by certain ill-defined autophagy-independent mechanisms.50 However, previous findings implicating obatoclax in the induction of autophagy,10 evidence of increased fusion between autophagosomes and lysosomes,11 and the observations that diverse autophagy inhibitors (eg, 3MA, chloroquine, bafilomycin A1) or knockdown of VPS3437 markedly potentiated sorafenib/obatoclax-mediated lethality, argue for a protective role for autophagy induction in the current setting. Finally, these findings raise the possibility that clinically relevant autophagy inhibitors (eg, chloroquine) may further enhance sorafenib/obatoclax antileukemic activity.
In addition to marked in vitro interactions, the sorafenib/obatoclax regimen elicited a pronounced reduction in tumor growth in a leukemia xenograft murine model accompanied by a significant increase in survival. Importantly, these events were also associated with Mcl-1 down-regulation and enhanced apoptosis in leukemia cells exposed to these agents in vivo. They also suggest that sufficiently high sorafenib and obatoclax concentrations can be achieved in vivo to recapitulate at least some of the actions observed in vitro eg Mcl-1 down-regulation. Interestingly, little toxicity was observed in animals with the doses and schedules used in these studies. Previous studies have described the development of neurotoxicity when obatoclax was administered intravenously to nude mice.6 We observed similar phenomena (data not shown), but this problem was largely resolved by using IM injections. Although the ability of this regimen to eradicate leukemia stem cells remains to be determined, the present in vivo findings, along with in vitro evidence that the sorafenib/obatoclax regimen is active against at least some primary AML cells and exhibits minimal toxicity toward normal CD34+ cells, suggest that this strategy warrants further consideration in AML. Accordingly, plans to evaluate the tolerability of this regimen through a phase 1 trial in patients with refractory AML, including patients with either FLT3-ITD–mutated or –wild-type disease, are currently under way.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by awards CA63753, CA93738, CA100866, Multiple Myeloma Research Foundation RC2CA148431 and P50CA130805 from the National Institutes of Health, award R6181-10 from the Leukemia & Lymphoma Society of America, Myeloma Specialized Program of Research Excellence (SPORE) award CA142509 and Lymphoma SPORE award CA130805, an award from The V Foundation and an award from Multiple Myeloma Research Foundation. Microscopy was performed at Virginia Commonwealth University Department of Neurology & Anatomy Microscopy Facility, supported, in part, with funding from NIH-NINDS center core grant 5P30NS047463.
National Institutes of Health
Authorship
Contribution: M.R. designed and performed the research, analyzed data, and wrote the manuscript; M.M.A. and E.A. performed the research; D.C.W. and A.F.-G. analyzed data; and S.G. designed research, identified patient samples, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Steven Grant, Division of Hematology/Oncology, Virginia Commonwealth University, MCV Station Box 230, Richmond, VA 23298; e-mail: stgrant@vcu.edu, or Dr Mohamed Rahmani, Massey Cancer Center, Virginia Commonwealth University, 401 College St, PO Box 980035, Richmond, VA 23298; e-mail: mrahmani@vcu.edu.

![Figure 2. Combined exposure to sorafenib and obatoclax results in enhanced lethality in primary AML cells. (A) Leukemic blasts were isolated from the BM of 4 patients with AML (FAB classification M2; AML#1, AML#2, and AML#4 with wild-type FLT3, and AML#3 with a FLT3-ITD mutation), exposed to sorafenib (7.5μM) and obatoclax (0.5μM) for 48 hours, after which the extent of cell death was assessed using the 7-AAD analysis. Results are presented as percentage of dead cells specific for each treatment using the formula ([treatment − control]/[100 − control]) × 100. Cell death for untreated control samples ranged from 10% to 25%. (B) Alternatively, protein lysates were prepared from AML patient #1 and subjected to Western blot analysis. Densitometric analysis of cleaved PARP and caspase-3 bands was performed using Adobe Photoshop. Values shown were normalized to ERK1/2 and represent relative changes compared with control. (C) Normal CD34+ cells were isolated as described in “Methods” from the BM of normal subjects (nonleukemic; N#1, N#2, and N#3) and exposed to increasing concentrations of sorafenib and obatoclax alone or in combination for 48 hours, after which the extent of cell death was determined using the 7-AAD analysis. (D) Two primary AML specimens were plated in methylcellulose in the presence of 5μM sorafenib and 75nM obatoclax alone or in combination for 14 days, after which CFUs were enumerated and expressed as a percentage relative to untreated cells. (E) Normal CD34+ cells from 2 subjects were plated in methylcellulose in the presence of increasing concentrations of sorafenib and obatoclax alone or in combination for 8 days, after which CFUs were enumerated and expressed as in panel D. For panels A, C, D, and E, data for each patient were obtained from a single experiment performed in triplicate; values represent the means ± SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/119/25/10.1182_blood-2011-09-378141/4/m_zh89991290590002.jpeg?Expires=1765055551&Signature=YUbJSFsU1FBQ9eD-aj9ov5VdocNG2qaDVOP4gK2WbgCJhD9PvNo0cM255VCbIzIvEL61CLv17a9MF1SJCTgAoAJFAkxIwlAwCDWhDlm2KAhfQoa9yZyqpJjlA4O6Y3f7yh3bnJiWFvHonkSa7u3eflS9DE3~VpsAm1aZk7vOp4v6~U3uL5bgK36J6F3zhnbuiXlFKEitzcRFIIR5ndtbd9T3L7FMD0tO4uv15n2XMG1L47DI3hA3ZLOUWQyOh9rYjlBHVit5I85cNL6-UZvn63gbcRy34qgCOheI6qsUz4B80dHSLoM-aNDNMqrc-Lqykk6n9q1GnpEkni3zXd3XlA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)




![Figure 2. Combined exposure to sorafenib and obatoclax results in enhanced lethality in primary AML cells. (A) Leukemic blasts were isolated from the BM of 4 patients with AML (FAB classification M2; AML#1, AML#2, and AML#4 with wild-type FLT3, and AML#3 with a FLT3-ITD mutation), exposed to sorafenib (7.5μM) and obatoclax (0.5μM) for 48 hours, after which the extent of cell death was assessed using the 7-AAD analysis. Results are presented as percentage of dead cells specific for each treatment using the formula ([treatment − control]/[100 − control]) × 100. Cell death for untreated control samples ranged from 10% to 25%. (B) Alternatively, protein lysates were prepared from AML patient #1 and subjected to Western blot analysis. Densitometric analysis of cleaved PARP and caspase-3 bands was performed using Adobe Photoshop. Values shown were normalized to ERK1/2 and represent relative changes compared with control. (C) Normal CD34+ cells were isolated as described in “Methods” from the BM of normal subjects (nonleukemic; N#1, N#2, and N#3) and exposed to increasing concentrations of sorafenib and obatoclax alone or in combination for 48 hours, after which the extent of cell death was determined using the 7-AAD analysis. (D) Two primary AML specimens were plated in methylcellulose in the presence of 5μM sorafenib and 75nM obatoclax alone or in combination for 14 days, after which CFUs were enumerated and expressed as a percentage relative to untreated cells. (E) Normal CD34+ cells from 2 subjects were plated in methylcellulose in the presence of increasing concentrations of sorafenib and obatoclax alone or in combination for 8 days, after which CFUs were enumerated and expressed as in panel D. For panels A, C, D, and E, data for each patient were obtained from a single experiment performed in triplicate; values represent the means ± SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/119/25/10.1182_blood-2011-09-378141/4/m_zh89991290590002.jpeg?Expires=1765182456&Signature=3GEZ~FR2UE74XkNiSZJ3dlYa7se7jRZT3c6D94YwfJWUlkWCtibdgMxnDx60o0WA9YUBKrwcToh1c5SiDcS1D29VyuEm8zUpIXXBSZKjqlYz9eroTRFcP4efUq8VBUIQatMHWcY1Ues7NSfHy~-vM-0DeZqVmlw8-dwuAocUUczn9qBpM3a9noa1HbxYeDtbsVBNI5P-5uu07-dsccyaFbxA9~PQShf-1k4VcxHP8oxCzK9cYBuvYrpD6AoBI5mOs4yppFMFDoS7S8e8dBm5QbGQdQnujyXe9XMKSBVyNIOYuwsug~OzUNuJu-88KcsRfdrh68YTJPY3bKBqcMhVKA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)