In line with the findings of Schoenwaelder and Jackson,1 we agree that the BH3 mimetics ABT-263 and ABT-737 inhibit normal platelet function. However, while Schoenwaelder et al attribute the inhibition of platelet activation on ABT-263 treatment to receptor ectodomain shedding,2 we believe that a modulation of cellular calcium flux contributes to the thrombocytopathy.3 Our hypothesis that ABT-263 may modify cellular calcium homeostasis was based on 2 observations. Firstly, exposure of platelets to ABT-263 resulted in an immediate increase in free cytosolic calcium and secondly, exposure of platelets to ABT-263 for 2 hours resulted in a depletion of intracellular calcium stores.3
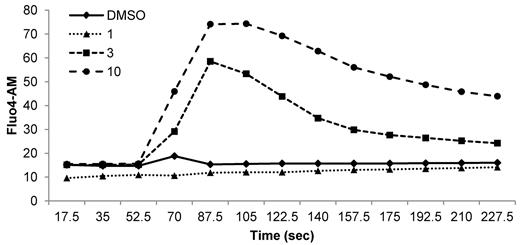
As stated in our previous publication,3 exposure to ABT-263 resulted in a concentration dependent increase in free cytosolic calcium. We are in agreement with the data presented by Schoenwaelder and Jackson, that ABT-263 (1μM) did not trigger a calcium response. However, in our hands concentrations of ABT-263 > 3μM induced an increase in intracellular free calcium (Figure 1), indicating that higher concentrations of ABT-263 are required to trigger the immediate calcium response. The precise reason that Schoenwaelder and Jackson did not observe an increase in cytosolic free calcium at higher concentrations of ABT-263 is unclear but may well relate to differences such as higher platelet density (3 × 108 cells/mL used by them compared with the lower cell density 5 × 107 cells/mL used in our study).3 In this regard we have previously reported that the efficacy of both ABT-263 (Navitoclax) and ABT-737 in inducing apoptosis of primary chronic lymphocytic leukemic (CLL) cells is markedly diminished both by increasing cell density as well as by protein binding.4
The increase in free cytosolic calcium is concentration dependent. Washed human platelets were stained with 1μM Fluo4/AM in HEPES-buffered saline for 30 minutes. Intracellular calcium levels were continuously monitored by flow cytometry. After establishing a baseline fluorescence signal for 1 minute, ABT-263 was added at 1, 3, or 10μM and the calcium response was monitored for an additional 3 minutes.
The increase in free cytosolic calcium is concentration dependent. Washed human platelets were stained with 1μM Fluo4/AM in HEPES-buffered saline for 30 minutes. Intracellular calcium levels were continuously monitored by flow cytometry. After establishing a baseline fluorescence signal for 1 minute, ABT-263 was added at 1, 3, or 10μM and the calcium response was monitored for an additional 3 minutes.
Schoenwaelder and Jackson assessed the calcium response after only 10 minutes of pretreatment with ABT-737. In our initial study,3 the depletion of cellular calcium stores was assessed only after exposure to ABT-263 or ABT-737 for 2 hours. This time point was selected because we did not consistently detect caspase activation earlier and wished to investigate the contribution of caspases to the depletion of intracellular calcium stores. We can only hypothesize that 10 minutes of treatment with ABT-737 is not sufficient to deplete the intracellular calcium stores, thus explaining the obvious difference in our results.
Taken together, our data consistently show that the BH3 mimetics, ABT-263 and ABT-737, can modulate the cellular calcium homeostasis in platelets. Further studies will be needed to address the functional consequences of this calcium response in vivo and its contribution to the thrombocypathy observed on ABT-263 administration.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gerald M. Cohen, MRC Toxicology Unit and Department of Biochemistry, Hodgkin Building, University of Leicester, P O Box 138, Lancaster Road Leicester, LE1 9HN, United Kingdom; e-mail: gmc2@le.ac.uk.