Abstract
E-cadherin is best characterized as adherens junction protein, which through homotypic interactions contributes to the maintenance of the epithelial barrier function. In epithelial cells, the cytoplasmic tail of E-cadherin forms a dynamic complex with catenins and regulates several intracellular signal transduction pathways, including Wnt/β-catenin, PI3K/Akt, Rho GTPase, and NF-κB signaling. Recent progress uncovered a novel and critical role for this adhesion molecule in mononuclear phagocyte functions. E-cadherin regulates the maturation and migration of Langerhans cells, and its ligation prevents the induction of a tolerogenic state in bone marrow-derived dendritic cells (DCs). In this respect, the functionality of β-catenin could be instrumental in determining the balance between immunogenicity and tolerogenicity of DCs in vitro and in vivo. Fusion of alternatively activated macrophages and osteoclasts is also E-cadherin–dependent. In addition, the E-cadherin ligands CD103 and KLRG1 are expressed on DC-, T-, and NK-cell subsets and contribute to their interaction with E-cadherin–expressing DCs and macrophages. Here we discuss the regulation, function, and implications of E-cadherin expression in these central orchestrators of the immune system.
Introduction
Cadherins are transmembrane or membrane-associated glycoproteins that mediate Ca2+-dependent cell-cell adhesion and have mainly been described for their instrumental role during morphogenesis of a variety of organs.1 The cadherin superfamily composes classic type I cadherins, closely related type II cadherins, desmosomal cadherins, protocadherins, and several cadherin-related molecules. Cadherin-1 (encoded by Cdh1), also known as CD324, uvomorulin, or E-cadherin, in which the “E” stands for “epithelial,” is the founding member of the classic type I cadherin family, which also includes N-cadherin (neural, Cdh2), P-cadherin (placental, Cdh3), R-cadherin (retinal, Cdh4), and VE-cadherin (vascular endothelial, Cdh5). For several decades, E-cadherin has been known as a major constituent of adherens junctions (AJs), mediating strong homotypic adhesion between neighboring epithelial cells, thereby safeguarding epithelial barrier integrity.2 The lack of functional tight junction and desmosome formation in the absence of E-cadherin emphasizes its central role in the regulation of epithelial cell-cell contacts.3
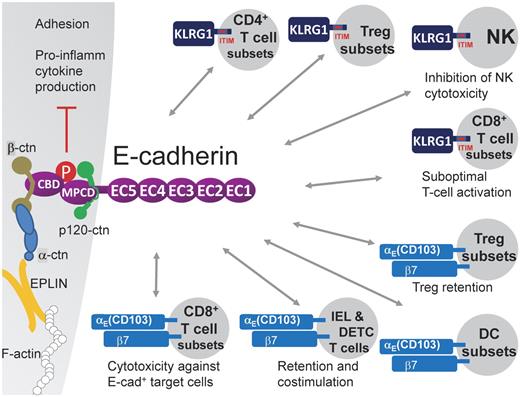
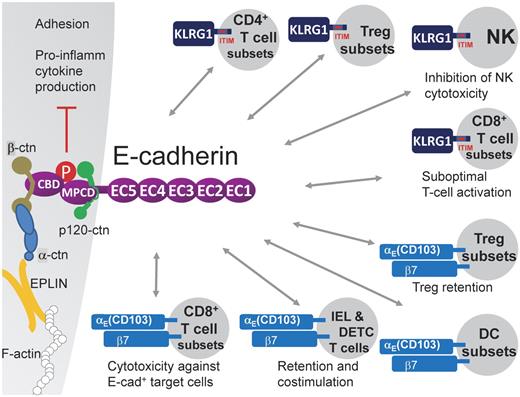
Cell-cell adhesion is mainly executed by the formation of E-cadherin trans-homodimers. Indeed, the E-cadherin molecule contains an ectodomain composed of 5 extracellular cadherin (EC1-5) repeats (550 aa in total), in which EC1 composes the HAV peptide sequence responsible for homophilic interactions, a transmembrane region, and a cytoplasmic tail (150 aa).2 The latter can be further subdivided into a β-catenin binding domain and a membrane proximal cytoplasmic/conserved domain, whose core sequence motif DEEGGGEED is important for p120-catenin binding, resulting in the stabilization of the E-cadherin/catenin complex at the cell surface.4 E-cadherin is transported from the Golgi complex to the cell surface as a pro-protein and associates already early during its biosynthesis with β-catenin, whereas p120-catenin and α-catenin (which binds to β-catenin) only associate after removal of the pro-domain, when the complex has reached the cell surface.2 It has long been assumed that the CCC complex, composing E-cadherin/β-catenin/α-catenin, links the actin cytoskeleton to the cell membrane, but this hypothesis was recently challenged. Indeed, α-catenin shuttles between an E-cadherin/β-catenin/p120-bound monomeric pool and an actin-bound dimeric pool but never binds β-catenin and F-actin at the same time.5 Instead, EPLIN was recently reported to be the missing link that connects the CCC complex to F-actin6 (Figure 1).
E-cadherin heterophilically interacts with KLRG1 and αE(CD103)β7. E-cadherin contains an ectodomain composed of 5 extracellular cadherin (EC1-5) repeats, a transmembrane region, and a cytoplasmic tail. The latter can be further subdivided into a β-catenin binding domain (CBD) and a membrane proximal cytoplasmic/conserved domain (MPCD) important for p120-catenin binding. EPLIN was reported to link the E-cadherin/β-catenin/α-catenin complex to F-actin.6 The E-cadherin ectodomain has been shown to bind to the inhibitory ITIM-containing receptor KLRG1, which is expressed on subsets of CD4+ T cells, including regulatory T-cell subsets, subsets of CD8+ T cells in distinct differentiation stadia, and mature NK cells. KLRG1 triggering results in reduced NK cytotoxicity and reduced CD8+ T-cell proliferation, suggesting that E-cadherin+ APCs might have the capacity to dampen lymphocyte functions. In addition, the KLRG1–E-cadherin interaction leads to E-cadherin phosphorylation and downstream signaling, resulting in reduced inflammatory cytokine production by the E-cadherin expressing cell. E-cadherin also binds to the αE(CD103)β7 integrin, possibly mediating adhesive interactions with αE(CD103)β7+ cells, such as IELs and DETCs, regulatory T-cell subsets, CD8+ T-cell subsets, and DC subsets. On T cells, this interaction was shown to mediate retention in tissues, costimulation, and enhanced cytotoxic activity (for CD8+ T cells).
E-cadherin heterophilically interacts with KLRG1 and αE(CD103)β7. E-cadherin contains an ectodomain composed of 5 extracellular cadherin (EC1-5) repeats, a transmembrane region, and a cytoplasmic tail. The latter can be further subdivided into a β-catenin binding domain (CBD) and a membrane proximal cytoplasmic/conserved domain (MPCD) important for p120-catenin binding. EPLIN was reported to link the E-cadherin/β-catenin/α-catenin complex to F-actin.6 The E-cadherin ectodomain has been shown to bind to the inhibitory ITIM-containing receptor KLRG1, which is expressed on subsets of CD4+ T cells, including regulatory T-cell subsets, subsets of CD8+ T cells in distinct differentiation stadia, and mature NK cells. KLRG1 triggering results in reduced NK cytotoxicity and reduced CD8+ T-cell proliferation, suggesting that E-cadherin+ APCs might have the capacity to dampen lymphocyte functions. In addition, the KLRG1–E-cadherin interaction leads to E-cadherin phosphorylation and downstream signaling, resulting in reduced inflammatory cytokine production by the E-cadherin expressing cell. E-cadherin also binds to the αE(CD103)β7 integrin, possibly mediating adhesive interactions with αE(CD103)β7+ cells, such as IELs and DETCs, regulatory T-cell subsets, CD8+ T-cell subsets, and DC subsets. On T cells, this interaction was shown to mediate retention in tissues, costimulation, and enhanced cytotoxic activity (for CD8+ T cells).
The maintenance of an epithelial barrier could be considered as a prime immunologic function of E-cadherin, compartmentalizing potentially harmful agents away from the underlying tissue. In addition, E-cadherin might actively regulate epithelial innate immune functions, such as the release of cytokines and chemokines, and modulate the transepithelial passage of innate and adaptive immune cells.7 However, in recent years, it became increasingly clear that E-cadherin and its associated catenins are also expressed in a variety of leukocytes, including conventional dendritic cells (DCs), Langerhans cells (LCs), and macrophages. These molecules gradually step into the spotlight as important markers and potential regulators of the immunogenic versus tolerogenic capacity of DCs, and of the pro- versus anti-inflammatory activation status of macrophages, 2 concepts that will be more amply discussed in this review. Of note, whereas extensive promoter studies have revealed regulatory sequences and transcription factors that activate or silence Cdh1 gene expression in epithelial cells, far less is known about Cdh1 gene regulation in nonepithelial cells, such as mononuclear phagocytes. An overview of available knowledge is given in Table 1 and will be elaborated on in later sections.
Here we first discuss the known adhesive and signaling functions of E-cadherin, which have mainly been studied in epithelial cells, and hint to a possible relevance of these data for the mononuclear phagocyte system. Subsequently, important concepts of DC and macrophage biology are highlighted and discussed in relation to E-cadherin function.
Functions of the E-cadherin/catenin complex: heterophilic adhesion with immune cells
E-cadherin binds KLRG1
Besides homophilic E-cadherin/E-cadherin interactions, E-cadherin can interact in a heterophilic manner with the inhibitory killer cell lectin-like receptor G1 (KLRG1)8 (Figure 1). KLRG1 recognizes the N-terminal homodimeric interface of E-cadherin EC1 and binds only the monomeric form of E-cadherin.9,10 KLRG1 is a type II transmembrane inhibitory receptor of the C-type lectin superfamily that contains an ITIM in its cytoplasmic domain. Phosphorylation of the ITIM recruits the SHIP-1 and SHP-2 phosphatases,11 thereby inhibiting NK cytotoxicity.8,12 Indeed, KLRG1 is predominantly expressed by NK cells with a mature phenotype13 and could be envisaged as a non–MHC-restricted NK receptor responsible for NK homeostasis in epithelia. Of note, in analogy with the frequent loss of MHC I molecules in cancer cells, development of human carcinomas is often associated with a loss or mutation of E-cadherin, rendering these cells susceptible to human NK-mediated lysis.14 In addition, KLRG1 is expressed by subsets of CD4+ and CD8+ T cells, including subtypes of CD4+ regulatory T cells, and is used as marker to distinguish short-lived effector CD8+ T cells from memory precursors15-17 (Figure 1). In highly differentiated CD8+ T cells, KLRG1 signaling results in defective Akt phosphorylation and leads to proliferative dysfunction, but only as long as KLRG1 and TCR/CD3 are coengaged in a spatially linked manner.18,19 However, high levels of soluble E-cadherin, produced on disruption of epithelial integrity, could also be sufficient to inhibit CD8+ T-cell function in a KLRG1-dependent manner.20 Together, these data hint to the possibility that antigen presentation by DCs or macrophages expressing high levels of E-cadherin results in suboptimal T-cell responses.
E-cadherin binds αE(CD103)β7 integrin
The integrin αE chain, also known as CD103, heterodimerizes with the integrin β7, thereby establishing an interaction partner for E-cadherin. Interestingly, the binding of the E-cadherin ectodomain to αE(CD103)β7 involves a loop at the tip of EC1 and in the EC5 domain, and hence interferes neither with homophilic adhesion nor with E-cadherin-KLRG1 interaction, suggesting that these various interactions could occur simultaneously.21 CD103 is abundantly expressed on intraepithelial αβ and γδ T cells (IEL and DETC, respectively) and mediates their adhesion to E-cadherin+ epithelial cells in vitro.22 The in vivo relevance of these findings is illustrated by CD103-deficient mice, which have reduced numbers of resident IELs and DETCs,23 probably because of a combination of mechanisms: (1) diminished retention in epithelia; (2) an altered motility24 ; and (3) a lack of αE(CD103)β7-mediated costimulation.25 Along the same line, retention of regulatory T cells in cutaneous wounds is mediated by CD103,26 and TGF-β-mediated expression of CD103 on CD8+ T cells promotes their cytotoxic activity against E-cadherin+ targets.27,28 Finally, CD103 expression distinguishes subpopulations of DCs that are found in lymphoid and various nonlymphoid organs and that display distinctive functions, such as cross-presentation of antigens in the draining lymph nodes (LNs), instruction of tissue-homing capacities on the activated T cells, and induction of regulatory T cells.29 Given these data, E-cadherin expression by mononuclear phagocytes would allow these cells to interact with a variety of CD103-expressing cell types, all of which play an important role in immune defense (Figure 1).
Functions of the E-cadherin/catenin complex: impact on intracellular signaling pathways
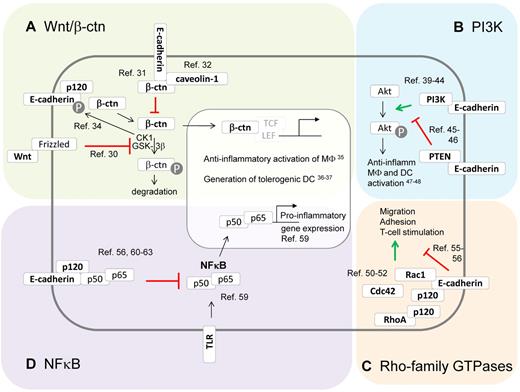
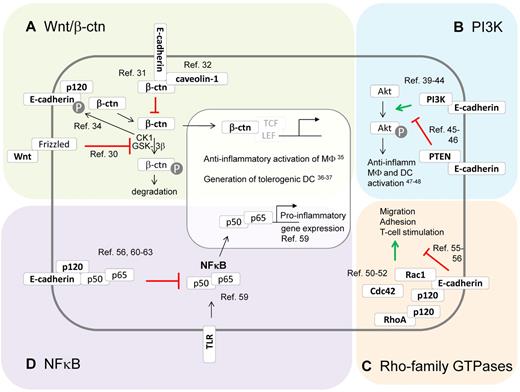
Besides their role in adhesion, E-cadherin and its associated catenins are known modulators of several signaling pathways and molecules in epithelial cells, including Wnt, phosphoinositide 3-kinase (PI3K), Rho-family GTPases, and NF-κB (Figure 2). Of importance, each of these pathways has been described to influence the DCs and macrophage phenotype as well, suggesting that expression of the E-cadherin/catenin complex in these cell types could actively modulate DC and macrophage behavior, as will be discussed later.
The E-cadherin/catenin complex and its impact on intracellular signaling pathways. Besides their function in adhesion, E-cadherin and its associated catenins are known modulators of several signaling pathways. (A) E-cadherin modulates canonical Wnt/β-catenin signaling. In the absence of Wnt, β-catenin gets phosphorylated by CKI and GSK-3β, which targets it for ubiquitination and proteasomal degradation.30 Once Wnt binds its receptor Frizzled, β-catenin phosphorylation is inhibited, leading to β-catenin nuclear translocation and activation of TCF/LEF-dependent gene expression. E-cadherin expression is able to inhibit β-catenin signaling via sequestering this molecule to the E-cadherin cytoplasmic tail31 or to caveolin-1.32 However, unbound E-cadherin might also stimulate Wnt/β-catenin signaling by assembling the Wnt signalosome, which phosphorylates E-cadherin and then releases β-catenin in the cytoplasm.34 Interestingly, β-catenin functioning has been shown to instruct anti-inflammatory macrophages35 and tolerogenic DCs.36,37 (B) E-cadherin modulates PI3K/Akt signaling. Phosphatidylinositol 3-kinases (PI3K) phosphorylate protein kinase B (Akt), thereby regulating various processes. Depending on the context, the E-cadherin/catenin complex might stimulate this cascade by recruiting and activating PI3K at the E-cadherin/catenin complex,39-44 or suppressing this cascade by recruiting PTEN to the E-cadherin/catenin complex.45,46 PI3K/Akt signaling typically instructs anti-inflammatory activation of macrophages and DCs.47,48 (C) The E-cadherin/catenin complex modulates Rho-family GTPases. The E-cadherin/catenin complex might bind and activate Rac-1, resulting in cytoskeletal reorganizations and cellular motility and adhesion. In addition, p120-catenin has been shown to modulate Rho GTPases, mainly via the inhibition of RhoA.50-52 Of note, inhibitory effects of E-cadherin on Rho GTPase activity and cell motility have been reported.55,56 Interestingly, macrophage and DC migration, adhesion, and T-cell stimulation are regulated by Rho-family GTPases, suggesting that E-cadherin expression might influence these phenomena. (D) The E-cadherin/catenin complex inhibits NF-κB. The transcription factor NF-κB is a master regulator of inflammatory gene regulation in macrophages and DCs.59 At least in epithelial cells, the E-cadherin/catenin complex is a potent repressor of NF-κB functions, by recruiting this transcription factor to the complex, possibly with p120-catenin as docking site. Knocking down E-cadherin and p120 catenin results in massive NF-κB activation and inflammation.56,60-63
The E-cadherin/catenin complex and its impact on intracellular signaling pathways. Besides their function in adhesion, E-cadherin and its associated catenins are known modulators of several signaling pathways. (A) E-cadherin modulates canonical Wnt/β-catenin signaling. In the absence of Wnt, β-catenin gets phosphorylated by CKI and GSK-3β, which targets it for ubiquitination and proteasomal degradation.30 Once Wnt binds its receptor Frizzled, β-catenin phosphorylation is inhibited, leading to β-catenin nuclear translocation and activation of TCF/LEF-dependent gene expression. E-cadherin expression is able to inhibit β-catenin signaling via sequestering this molecule to the E-cadherin cytoplasmic tail31 or to caveolin-1.32 However, unbound E-cadherin might also stimulate Wnt/β-catenin signaling by assembling the Wnt signalosome, which phosphorylates E-cadherin and then releases β-catenin in the cytoplasm.34 Interestingly, β-catenin functioning has been shown to instruct anti-inflammatory macrophages35 and tolerogenic DCs.36,37 (B) E-cadherin modulates PI3K/Akt signaling. Phosphatidylinositol 3-kinases (PI3K) phosphorylate protein kinase B (Akt), thereby regulating various processes. Depending on the context, the E-cadherin/catenin complex might stimulate this cascade by recruiting and activating PI3K at the E-cadherin/catenin complex,39-44 or suppressing this cascade by recruiting PTEN to the E-cadherin/catenin complex.45,46 PI3K/Akt signaling typically instructs anti-inflammatory activation of macrophages and DCs.47,48 (C) The E-cadherin/catenin complex modulates Rho-family GTPases. The E-cadherin/catenin complex might bind and activate Rac-1, resulting in cytoskeletal reorganizations and cellular motility and adhesion. In addition, p120-catenin has been shown to modulate Rho GTPases, mainly via the inhibition of RhoA.50-52 Of note, inhibitory effects of E-cadherin on Rho GTPase activity and cell motility have been reported.55,56 Interestingly, macrophage and DC migration, adhesion, and T-cell stimulation are regulated by Rho-family GTPases, suggesting that E-cadherin expression might influence these phenomena. (D) The E-cadherin/catenin complex inhibits NF-κB. The transcription factor NF-κB is a master regulator of inflammatory gene regulation in macrophages and DCs.59 At least in epithelial cells, the E-cadherin/catenin complex is a potent repressor of NF-κB functions, by recruiting this transcription factor to the complex, possibly with p120-catenin as docking site. Knocking down E-cadherin and p120 catenin results in massive NF-κB activation and inflammation.56,60-63
E-cadherin modulates canonical Wnt/β-catenin signaling
The canonical Wnt/β-catenin signaling pathway is best known for its role during developmental processes, but it can also regulate the function of various immune cells, including monocytes, macrophages, and DCs.30 In the absence of Wnt ligand binding to its receptor Frizzled and coreceptor LRP5 or LRP6, cytoplasmic β-catenin gets phosphorylated by the kinases CK1 and GSK-3β, targeting it for ubiquitination and subsequent proteasomal degradation. However, once Wnt signaling is initiated, β-catenin phosphorylation is inhibited, leading to the accumulation of free β-catenin and its translocation to the nucleus. Formation of the active β-catenin/TCF/LEF transcription factor complex finally results in the expression of Wnt target genes.30
It seems logical that E-cadherin expression can suppress Wnt/β-catenin signaling by sequestering β-catenin at sites of cell-cell contact. Evidence suggests that the mere presence of the E-cadherin cytoplasmic domain, rather than E-cadherin adhesive properties, is required to inhibit Wnt/β-catenin–dependent gene expression.31 Although direct binding of β-catenin to E-cadherin could be responsible for the sequestration effect, recent evidence suggests that E-cadherin also increases the interaction between caveolin-1 and β-catenin near the plasma membrane, resulting in reduced β-catenin/TCF/LEF-dependent transcription.32 Moreover, E-cadherin–based adhesion increases the turnover of cytoplasmic β-catenin by promoting the activity of a β-catenin phosphodestruction complex localized near AJs.33 However, outside AJs, unligated E-cadherin might actually be needed for initiating Wnt signaling, whereby the E-cadherin–p120-catenin complex controls the assembly of the Wnt signalosome.34 The activated CK1 kinase then phosphorylates E-cadherin, thereby weakening the E-cadherin–β-catenin association and turning the E-cadherin–bound β-catenin pool into a signaling β-catenin pool. The latter could be particularly relevant for E-cadherin–expressing macrophages and DCs. Indeed, Wnt/β-catenin signaling is generally considered to induce an anti-inflammatory phenotype in macrophages35 and a tolerogenic state in DCs,36,37 suggesting that unligated E-cadherin might contribute to these phenomena.
E-cadherin modulates PI3K/Akt signaling
Class IA PI3Ks are heterodimers consisting of a catalytic subunit (p110, existing in different isoforms) in complex with a regulatory subunit (p85, also existing in distinct isoforms), that generate phosphorylated phosphatidylinositol second messenger molecules (such as PtdIns(3,4,5)P3).38 As a consequence, PI3Ks are involved in a broad variety of cellular functions, including proliferation and differentiation, growth, survival, and motility, often linked to the PI3K-mediated activation of protein kinase B or Akt. Of note, the phosphatase PTEN catalyzes the reverse reaction and shuts down PI3K/Akt signaling.
Several lines of evidence indicate that the engagement of E-cadherin results in the activation of PI3K and Akt in keratinocytes, epithelial cells, and carcinoma cells.39-44 Although the exact molecular mechanisms involved are not entirely clear, current data suggest that nascent cell-cell contact formation via E-cadherin results in the activation of the c-Src kinase,39 leading to phosphorylation and subsequent recruitment of the PI3K p85 subunit to AJs.40-42 Next, PtdIns(3,4,5)P3 production in these nascent E-cadherin contacts triggers the recruitment of proteins containing pleckstrin homology domains, including Akt. Importantly, stimulation of PI3K/Akt is paralleled by a down-regulation of the growth-promoting MEK/ERK signaling, resulting in cell cycle arrest and differentiation.43,44 However, the link between E-cadherin expression and PI3K activity is not always unequivocal and might be context-dependent. Indeed, E-cadherin has been shown to up-regulate PTEN expression via β-catenin–mediated Egr1 regulation and mediates its recruitment to cell-cell junctions, leading to suppression of PI3K/Akt signaling and growth arrest in ovarian cancer cells and mammary epithelial cells.45,46
Importantly, the PI3K cascade is also a major player in determining the mononuclear phagocyte phenotype, whereby PI3K/Akt activity preferentially instructs anti-inflammatory macrophages47 and DCs48 (IL-10high/IL-12low), whereas PTEN activation exerts the opposite effect. Whether alterations in the levels of E-cadherin actually affect PI3K activity in DCs and macrophages and as such contributes to their anti-inflammatory polarization remains to be investigated.
E-cadherin modulates Rho-family GTPase activity
The mammalian Rho-family of GTPases is composed of 20 intracellular signaling molecules, the best documented of which are RhoA, Rac1, and Cdc42. These molecules switch between an active GTP-bound form and an inactive GDP-bound form and have mainly been studied as regulators of cytoskeletal dynamics involved in cellular motility, polarity, and vesicular trafficking, among others.49
Convincing evidence from multiple groups has demonstrated a recruitment and activation of Rac1 at sites of E-cadherin contact, but some controversy exists on the mechanism leading to Rac1 activation. Although Rac1 activation has been suggested to be a downstream event of c-Src and PI3K activity by some,50,51 other data suggest a PI3K-independent but rather p120-catenin–dependent mechanism.52 Irrespective of the mechanism, Rac1 appears to be instrumental in stabilizing AJs. p120-catenin itself displays intricate Rho GTPase-modifying properties. Free p120-catenin is able to inhibit RhoA, but this effect is lost on p120-catenin association with E-cadherin,53 possibly explaining the known RhoA activation by nascent E-cadherin cell-cell contacts. On the other hand, E-cadherin loss and release of p120-catenin activated a Rac1-MAPK signaling pathway promoting transformed cell growth.54 Of note, some studies show that E-cadherin negatively regulates cell proliferation and migration by inhibiting RhoA and Cdc42, again illustrating that E-cadherin outside-in signaling might be cell context-dependent.55,56 Interestingly, macrophage and DC migration/adhesion/T-cell stimulation are tightly regulated by Rho-family GTPases,57,58 but it remains elusive up to now whether E-cadherin is involved in this process.
E-cadherin inhibits NF-κB activity
In mammals, the NF-κB family is composed of 5 members, RelA (p65), RelB, c-Rel (Rel), NF-κB1 (p50 and its precursor p105), and NF-κB2 (p52 and its precursor p100), which can form homodimers and heterodimers. The canonical NF-κB activation pathway mainly applies to p65:p50 dimers, which are sequestered in a quiescent state in the cytoplasm by IκB family members under steady state. On stimulation by a broad range of inflammatory mediators, including cytokines and microbial or endogenous danger-associated molecules, p65:p50 is released after IκB phosphorylation by the IKK complex and subsequent degradation of this inhibitor. Finally, the heterodimer is translocated to the nucleus and activates the transcription of various target genes. Importantly, NF-κB regulates the phenotype of several cell types during inflammation, including epithelial cells and innate immune cells, such as macrophages and DCs, which has been shown instrumental to inflammation-associated carcinogenesis.59
Interestingly, the E-cadherin/catenin complex appears to possess the ability to down-modulate NF-κB activity. In distinct cellular systems, it was shown that a forced overexpression of E-cadherin reduces NF-κB activation, whereas loss of E-cadherin results in an increased activity of this transcription factor.56,60,61 Diversity might exist at the mechanistic level, with a possible requirement for p38 MAPK60 or RhoA-activated protein kinase D156 as intermediates between E-cadherin and NF-κB. In addition, NF-κB suppression might result from a physical association with the E-cadherin/catenin complex.61 In this respect, p120-catenin might serve as a docking site, considering the massive NF-κB activation, inflammation, and neoplasia formation in mice with a conditional p120-catenin deficiency in skin epidermis62 or squamous oral cavity/esophagus/forestomach.63
E-cadherin in conventional DCs and LCs
Immunogenicity/tolerogenity of conventional dendritic cell subsets
DCs play a central role in adaptive immunity. Conventional DCs are heterogeneous and compose cells that spend their whole life in secondary lymphoid tissues (LT-DCs), as well as DCs that reside first in the parenchyma of nonlymphoid tissues. Here they are known as interstitial DCs (Int-DCs), before migration to draining LNs, where they are called migratory DCs (Mig-DCs).64 In the mouse, conventional DCs have been categorized as CD8α+- and CD11b+-type DCs, a dichotomy that takes into account phenotypic, developmental, and functional attributes.65 Under steady-state (noninflammatory) conditions, DCs are described as being resting or “immature,” whereas in response to direct activation by protozoal, viral or bacterial stimuli, or by extrinsic inflammatory mediators, DCs undergo a terminal differentiation program (also called maturation) that renders them immunogenic.66 Such differentiation program encompasses the up-regulation of MHC class II and costimulatory molecules at the DC surface, the CCR7-dependent migration of Int-DCs to the T-cell zones of draining LNs, and the release of cytokines, altogether promoting the differentiation of naive antigen-specific T cells into effector cells.67,68 Importantly, under steady-state conditions, a fraction of Int-DCs undergoes a constitutive activation and migrates to the T-cell zones of draining LNs. However, those activated DCs are tolerogenic rather than immunogenic in that they generate induced regulatory T cells and lack the ability to drive the differentiation of naive self-reactive T cells into effectors.67
Immunogenicity/tolerogenicity of LCs
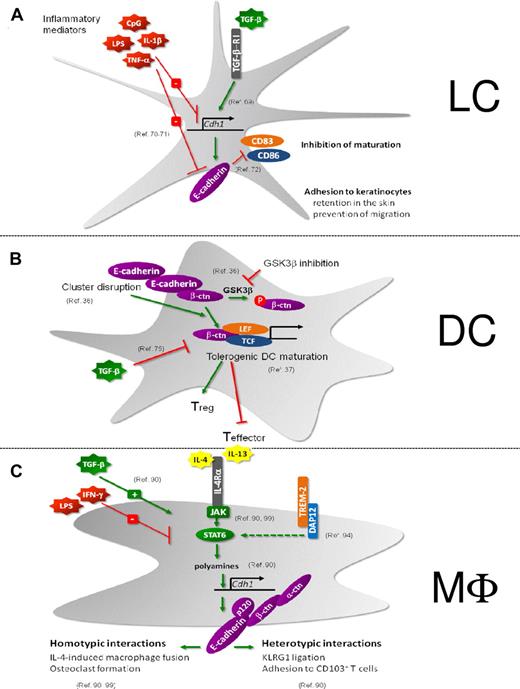
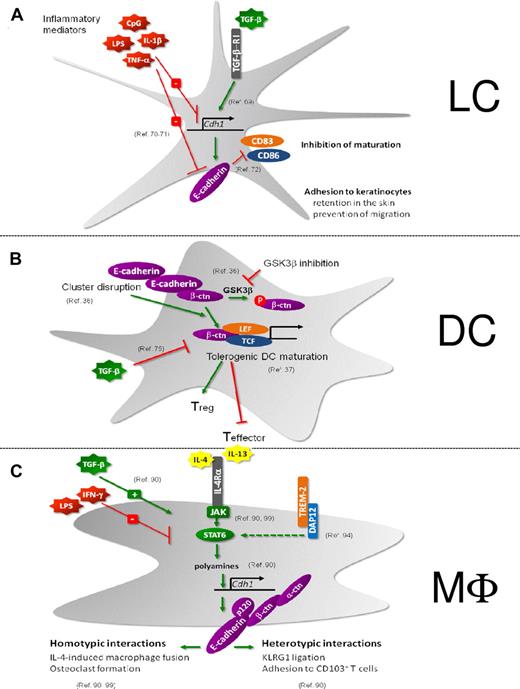
LCs constitute an additional type of DCs that reside in the epidermal layer of the skin where they tightly interact with the surrounding keratinocytes via homophilic interactions involving E-cadherin (Figure 3A). Maintenance of E-cadherin on the pool of immature LCs depends on TGF-β.69 On LC activation, E-cadherin expression is down-regulated and the E-cadherin–based LC-keratinocyte interactions are disrupted.70 As a result, LCs detach from keratinocytes and switch from a sessile to a mobile state, allowing them to migrate to draining LNs.71 E-cadherin is thus clearly involved in the physical retention of LCs in the skin. Moreover, LC-keratinocyte interactions may prevent LC maturation. For instance, using in vitro–generated immature LCs, it has been shown that ligation of E-cadherin inhibits their maturation and, conversely, that mechanical disruption of the clusters formed by cultured LCs up-regulated CD83 and CD86 expression and diminished CD1a surface expression.72 Therefore, E-cadherin interactions between immature LCs may specifically inhibit their maturation.
Regulation and function of E-cadherin. (A) LCs. (B) DCs. (C) Macrophages. (A) TGF-β induces E-cadherin in LCs, which is crucial for the maintenance of an immature LC pool in the epidermis.69 Retention in the skin is mediated by E-cadherin-dependent adhesion to keratinocytes. In addition, E-cadherin ligation on LCs prevents the maturation of these cells.72 Inflammatory stimuli decrease E-cadherin expression on LCs, resulting in their migration to the LNs and maturation.70,71 (B) On disruption of E-cadherin–mediated clusters or on GSK-3β inhibition, DCs undergo a partial maturation program toward tolerogenic DCs under the influence of β-catenin activity.36 This β-catenin–mediated induction of tolerogenic DC is inhibited by TGF-β.75 (C) E-cadherin is a selective marker for IL-4/IL-13–exposed macrophages and is STAT6/DAP12/polyamine-dependently induced.90,94,99 TGF-β synergizes with IL-4/IL-13 for maximal Cdh1 gene induction, whereas IFNγ and lipopolysaccharide inhibit E-cadherin expression.90 E-cadherin forms a functional complex with its catenins, allowing them to engage in homotypic interactions (IL-4–driven macrophage fusion and osteoclastogenesis)90,99 and heterotypic interactions (with CD103+ and KLRG1+ T cells).90
Regulation and function of E-cadherin. (A) LCs. (B) DCs. (C) Macrophages. (A) TGF-β induces E-cadherin in LCs, which is crucial for the maintenance of an immature LC pool in the epidermis.69 Retention in the skin is mediated by E-cadherin-dependent adhesion to keratinocytes. In addition, E-cadherin ligation on LCs prevents the maturation of these cells.72 Inflammatory stimuli decrease E-cadherin expression on LCs, resulting in their migration to the LNs and maturation.70,71 (B) On disruption of E-cadherin–mediated clusters or on GSK-3β inhibition, DCs undergo a partial maturation program toward tolerogenic DCs under the influence of β-catenin activity.36 This β-catenin–mediated induction of tolerogenic DC is inhibited by TGF-β.75 (C) E-cadherin is a selective marker for IL-4/IL-13–exposed macrophages and is STAT6/DAP12/polyamine-dependently induced.90,94,99 TGF-β synergizes with IL-4/IL-13 for maximal Cdh1 gene induction, whereas IFNγ and lipopolysaccharide inhibit E-cadherin expression.90 E-cadherin forms a functional complex with its catenins, allowing them to engage in homotypic interactions (IL-4–driven macrophage fusion and osteoclastogenesis)90,99 and heterotypic interactions (with CD103+ and KLRG1+ T cells).90
Although LCs have long been regarded as capable of taking up the pathogens and allergens that penetrate the epidermis and of conveying them in an immunogenic form to the draining LNs, several recent studies tend to suggest that LCs are also endowed with suppressive functions intended to dampen the immunogenic responses that are elicited by other DCs.73 Rare LCs manifest a motile behavior in the steady-state epidermis and probably contribute to the continuous replenishment of the Mig-LCs that are present in steady-state cutaneous LNs. Such continuous LC migration was observed in gnotobiotic mice and when DCs were incapable of responding to TLR signals.74 The fact that such migration occurs in the absence of intended inflammation supports a role for Mig-LCs in the maintenance of tolerance to peripheral self-proteins.
Regulation of E-cadherin/β-catenin function influences the DC immunogenic/tolerogenic state
Until recently, the sterile triggers that exist under steady-state conditions and result in the continuous activation of Int-DCs and epidermal LCs has remained an enigma. In the case of LCs, such triggers might correspond to endogenous inflammatory compounds that are released at low levels by stressed or malfunctioning keratinocytes in the steady state. Using cultures of immature BM-derived DCs (BMDCs), Jiang et al showed that BMDCs express E-cadherin and that mechanical disruption of E-cadherin–mediated DC-DC interactions induced BMDC activation as documented by the up-regulation of costimulatory molecules and of the CCR7 chemokine receptor, the down-regulation of macropinocytosis, and the redistribution of MHC II molecules from lysosomes to the cell surface36 (Figure 3B). In contrast to BMDCs activated via TLR engagement, BMDCs activated by disruption of E-cadherin–mediated intercellular contacts produced no inflammatory cytokines. After adoptive transfer, they expanded antigen-specific CD4+ T cells to the same extent as BMDCs activated via TLR engagement but failed to elicit IFN-γ–producing effector T cells. Instead, responding T cells developed into IL-10 producers with characteristics of regulatory T cells. Disruption of E-cadherin–mediated BMDC interactions led to the accumulation of β-catenin and to the activation of the β-catenin–dependent transcriptional activator TCF. Along the same line, pharmacologic inhibition of GSK3β resulted in an increase in the levels of cytosolic β-catenin and in BMDC maturation. Conversely, BMDCs depleted of β-catenin responded normally to lipopolysaccharide stimulation but failed to respond to mechanical or integrin-mediated stimulation. β-catenin is thus a necessary component of the signaling pathway induced by mechanical signals, and such pathway can be blocked by TGF-β.75 Based on these observations, Jiang et al suggested that the disruption of E-cadherin–mediated DC interactions constitutes one of the sterile triggers capable of generating tolerogenic DCs under steady-state conditions.36 In that model, immunogenic TLR-mediated signals prevail over the E-cadherin–β-catenin–mediated tolerogenic signals. Whether the model proposed by Jiang et al applies to in vivo conditions remains, however, to be documented by determining whether DC-specific deletion of β-catenin prevents the constitutive in vivo activation of Int-DCs.
Consistent with the view that β-catenin signaling mediates the induction of tolerogenic DCs (Figure 3B), Manicassamy et al showed that DC-specific deletion of β-catenin in mice led to a reduction in the numbers of regulatory T cells and in enhanced frequencies of Th1 and Th17 cells in the intestine but not in the spleen.37 Indeed, in contrast to splenic DCs, the Wnt signaling pathway is constitutively active in intestinal DCs and macrophages found in the large and small intestine. Intestinal DCs deficient in β-catenin displayed reduced levels of anti-inflammatory cytokines, and mice with a DC-specific deletion of β-catenin were more susceptible to dextran sulfate sodium-induced colitis. However, because the promoter used to drive the deletion of β-catenin was also active in macrophages, it is thus possible that macrophages also contribute to maintain tolerance in the intestine in a β-catenin–dependent manner.76 Regardless of that limitation, whether the suppressive role of β-catenin signaling depends on the coexpression of E-cadherin remains to be documented.
E-cadherin as marker for colitogenic DCs
In vivo, LCs have been thought to be the only conventional DCs to express high levels of E-cadherin.77 Interestingly, LCs also express tight junction proteins, such as claudin-1, enabling these cells to penetrate their dentrites through epithelial tight junctions and catch Ag without disturbing tight junction integrity.78 In addition in the intestine and the lung mucosa, DC subtypes (CD103+Langerin+ DCs for lung mucosa) were reported to express tight junction proteins contributing to the sampling of micro-organisms at the luminal side of the epithelium.79,80 It thus seems likely that these DCs also express E-cadherin, although this has not been formally tested in vivo. Minor and poorly characterized populations of E-cadherin–positive DCs have been observed under steady-state conditions in secondary lymphoid tissues.81 A comprehensive survey of E-cadherin expression among the CD8α+- and CD11b+-type DCs found in lymphoid and nonlymphoid tissues remains, however, to be done (overview of current knowledge in Table 2).
T cell–mediated intestinal inflammation is characterized by an accumulation of DCs in the colon and the mesenteric LNs. Such inflammatory DCs contain a monocyte-derived subset that expressed E-cadherin and produced colitogenic cytokines on activation.81 Considering that adoptive transfer of E-cadherin+ BMDCs also exacerbated T cell–mediated colitis, those data suggest that, in the gut-associated lymphoid tissue and colon of colitic mice, E-cadherin marks DCs endowed with pro-inflammatory properties. However, the role played by E-cadherin in the generation and function of those colitogenic DCs, as well as the β-catenin activity in these cells, remain to be established.
E-cadherin in macrophages and osteoclasts
Macrophage heterogeneity
In contrast to the strict immunologic commitment of DCs, macrophages play a much wider role in physiology. In their capacity of professional phagocytic cells (macrophages = “large eaters”) and producers of trophic and immunoregulatory mediators, these cells contribute to tissue development, reorganization, and homeostasis (trophic role of macrophages), as well as the recognition, engulfment, and destruction of pathogens.82 In terms of ontogeny, tissue macrophages are strictly derived from precursor blood monocytes, whereas subsets of DCs can be either derived from a committed DC precursor or from monocytes.83 In mammals, specialized subsets of macrophages are found in various quantities in all tissues, both lymphoid and nonlymphoid, after birth. As prominent members of the innate immune system, macrophages express a wide variety of receptors for the recognition of self versus nonself, resulting in silencing or triggering an immune response, respectively.84 In this respect, macrophages are implicated in both initiation and dampening of inflammatory responses. Accordingly, depending on the environment, they are able to adopt diverse activation states testifying of the remarkable plasticity of these cells. Classically activated macrophages (M1), induced by Th1 cytokines and microbial or endogenous danger signals, are pro-inflammatory and are crucial for pathogen clearance. On the other hand, macrophages can be activated by the prototypical Th2 cytokines IL-4 and IL-13 (inducing alternatively activated macrophages [AAMs], also referred to as M2), IL-10, TGF-β, glucocorticoids, immune complexes, and apoptotic cells (grouped under the generic term M2-like macrophages), which has led to different macrophage classification systems.85-87 AAMs dampen Th1 cytokine-driven inflammation, contribute to wound healing, and are implicated in Th2-driven pathologies, such as helminth infections and asthma.86
E-cadherin is a marker for IL-4/IL-13–exposed alternatively activated macrophages
Rehli et al were the first to report the in vitro induction of Cdh1 gene expression by IL-4 in mouse bone marrow–derived macrophages.88 Next, E-cadherin was shown to be member of a common gene signature for in vivo–induced M2 populations, purified from various sites (spleen, peritoneum) in mice bearing distinct parasitic infections or tumors.89 A follow-up study confirmed that IL-4 and IL-13 are crucial to induce E-cadherin in mouse and human macrophages, both in vitro and in vivo during various Th2-driven diseases, such as Taenia crassiceps helminth infection and allergic asthma90 (Figure 3C). Of note, though E-cadherin surface expression is clearly detected on in vitro– or in vivo–elicited AAMs, it never reaches the levels found on normal epithelial cells. Although IL-10 and TGF-β cooperate with IL-4 to maximize Cdh1 expression, the simultaneous presence of a Th1 cytokine or a TLR ligand, either in vitro or in vivo, abolishes its expression, establishing this membrane marker as a reporter of polarized Th2 responses and AAMs. A notable exception to this rule could be the higher, IL-4/IL-13–independent (as shown in IL-4Rα–deficient mice) E-cadherin expression level on M1-like MHC IIhigh tumor-associated macrophages (TAMs) compared with their M2-like MHC IIlow counterparts91,92 (J.V.d.B. unpublished observations), suggesting that other, as yet undefined E-cadherin-inducing stimuli might exist in the tumor microenvironment. Moreover, MHC IIlow TAMs are predominantly located in hypoxic tumor regions. Hypoxia has been reported to down-modulate E-cadherin expression,93 and hence might explain the lower E-cadherin levels on this TAM subset, a finding that could be more generally applicable to macrophages functioning under hypoxia (eg in ischemic tissues).
Similar to other IL-4–regulated genes, the induction of Cdh1 by IL-4 essentially depends on JAK/STAT6 activation downstream of IL-4Rα90 (Figure 3C). However, whereas the enhancers/promoters of highly IL-4–inducible genes, such as arginase-1, contain at least one typical STAT6 binding site, this is not the case for E-cadherin. Up until now, it is unclear which STAT6-regulated transcription factor(s) mediate Cdh1 gene expression, but IL-4–induced intracellular polyamines (putrescine, spermidine and spermine) act as crucial cofactors for maximal Cdh1 induction.90 Indeed, the depletion of polyamines in macrophages significantly lowers the IL-4–inducibility of most, but not all, IL-4–regulated genes in macrophages (J.V.d.B., W. H. Lamers, E. S. Koehler, J. M. C. Geuns, L. Alhonen, A. Uimari, S. Pirness-Karhu, E. Van Overmeire, Y. Morias, L. Brys, L. Vereecke, P. D. B., J. A. V. G, Arginase-1–independent polyamine production stimulates the expression of IL-4–induced alternatively activated macrophage markers while inhibiting LPS-induced expression of inflammatory genes, under revision), unveiling a novel mechanism for IL-4–dependent gene transcription.
E-cadherin contributes to macrophage fusion and osteoclastogenesis
In naive macrophages, the β-catenin protein is almost undetectable in the absence of E-cadherin but is stabilized in the E-cadherin/catenin complex after IL-4 stimulation.90 Coimmunoprecipitation experiments and immunofluorescence microscopy further confirm the existence of a fully operational E-cadherin/p120-catenin/β-catenin/α-catenin complex in IL-4–exposed AAMs. These complexes accumulate at AAM-AAM contact sites,90 allowing these cells to engage in IL-4–driven fusion, important for physiologic and pathologic processes. For example, fusion of macrophages results in the formation of multinucleated giant cells (MNGs), which is a typical feature of granulomatous inflammation and foreign body reactions.94-98 Moreno et al found that IL-4 induces MNG generation in a STAT6-dependent manner by regulating the expression of at least 2 fusion mediators, E-cadherin and DC-STAMP, resulting in homotypic macrophage fusion and engulfment of large foreign bodies.99 In addition TREM-2 and its signaling association partner DAP12 are required for IL-4–induced E-cadherin and DC-STAMP expression, and giant cell formation in vitro and in vivo.94 E-cadherin is indeed involved in the development of MNGs as anti–E-cadherin antibodies impaired the formation of large MNGs.99 These data were confirmed using macrophage-specific E-cadherin–deficient mice in which, on IL-4–triggered fusion, the volume of each MNG and the average number of nuclei per MNG are significantly lower compared with control macrophages.90 In addition, anti–E-cadherin antibodies inhibited human macrophage fusion elicited by Burkholderia pseudomallei, the causative agent of melioidosis.100
Osteoclasts are bone-resorbing multinucleated cells that differentiate from hematopoietic mononuclear precursors under the influence of factors, such as vitamin D3 and RANKL. Similar to MNG, osteoclast formation was shown to depend on DAP12, DC-STAMP, and E-cadherin.101-103 However, although IL-4 promotes macrophage fusion, it rather inhibits RANKL-induced osteoclast formation.99
Alternatively activated macrophages interact with CD103+ and KLRG1+ cells through E-cadherin
Next to its role during macrophage fusion and osteoclast formation, E-cadherin on AAMs provokes KLRG1 signaling in reporter cells, but AAM E-cadherin levels are below the threshold needed to trigger KLRG1-mediated T-cell inhibition.90 Interestingly, KLRG1 might induce reverse signaling leading to E-cadherin phosphorylation and reduced inflammatory cytokine production in BMDCs, suggesting that a similar mechanism might exist in AAM.104 In addition, E-cadherin on macrophages heterophilically interacts with CD103, promoting the adhesion between AAMs and CD103+ T cells in vitro.90 Hence, E-cadherin might serve to bring these T cells in closer contact with anti-inflammatory AAMs, thereby potentially influencing their retention in tissues and phenotype during polarized Th2 responses.
Future perspectives and potential clinical implications
Although E-cadherin is clearly expressed in mononuclear phagocyte subtypes, its in vivo role in normal physiology and pathology remains poorly defined. Further in vivo assessment of E-cadherin functions will require the conditional deletion of the Cdh1 gene in macrophages and DCs. Of particular interest will be the evaluation of the E-cadherin/catenin complex as a possible regulator of the macrophage and DC inflammatory status, based on its potential influence on NF-κB, PI3K, and Wnt/β-catenin signaling pathways.
In this context, β-catenin is suggested by (a relatively limited number of) recent groundbreaking publications as a central regulator, determining the balance between DC tolerogenicity and immunogenicity, at least in the mouse. These findings should stimulate a systematic assessment of β-catenin functionality, and in relation to this also E-cadherin expression, in lymphoid and nonlymphoid tissue DC subsets in steady state and during inflammation, both in mouse and humans. In the clinic, tolerogenic DCs would be desirable for the treatment of autoimmune diseases, graft rejection, and allergy, whereas strongly immunogenic DCs are needed to fight tumors and infections. Manipulating the Wnt/β-catenin signaling pathway, either during in vitro DC preparation or in vivo, might be a valid strategy to alter DC properties for therapeutic use. Several innovative approaches have been proposed to inhibit Wnt/β-catenin function and could be expected to increase DC immunogenicity, including the use of GSK-3β activators, and small molecule β-catenin/TCF interaction antagonists or transcriptional coactivator antagonists.105 Of note, because Wnt/β-catenin is often aberrantly activated in carcinoma cells, the use of Wnt/β-catenin inhibitors in cancer therapy could serve a dual role: (1) stopping β-catenin-mediated pro-invasive and pro-metastatic genetic programs in cancer cells; and (2) stimulating antitumor adaptive immunity. Interestingly, E-cadherin down-regulation has been associated with a poor prognosis and dissemination in myelodysplastic syndrome, acute myeloid leukemia, and LC histiocytosis, suggesting that β-catenin activation could also play an active role in the severity of myeloid neoplasms106,107 and that Wnt/β-catenin inhibitors could be a novel class of compounds to treat these malignancies. Conversely, a stimulation of β-catenin activity, and hence the induction of tolerogenic DCs, could be achieved by the application of GSK-3β inhibitors, which are under development for the treatment of nervous system disorders and type 2 diabetes.108 Data discussed in this review suggest a potential applicability of GSK-3β inhibitors for the treatment of autoimmunity and graft rejection.
In addition, in macrophages, which are modulators rather than initiators of adaptive immunity, Wnt/β-catenin signaling confers an anti-inflammatory phenotype to the cells,35 further establishing this pathway as a potential therapeutic target in inflammatory diseases. In macrophages, however, the E-cadherin/catenin complex has mainly been studied as adhesive moiety, enabling these cells to fuse or to interact with E-cadherin-, KLRG1-, and αE(CD103)β7 integrin-expressing cells. In this context, chronic inflammation and granuloma formation are characterized by the presence of macrophages with an epithelial morphology and by macrophage homotypic fusion with the formation of MNG. Defining the molecular basis of MNG formation, including the role of E-cadherin in this process, may help the dissection of the actual significance of granulomas. Indeed, up to now, it is unclear whether granulomas constitute a strategy to contain the pathogen or rather provide the pathogen with an immune privileged site. In addition, E-cadherin might mediate fusion between distinct cell types. In this context, heterotypic fusion of tumor-associated myelomonocytic cells and cancer cells has been suggested to give rise to more metastatic variants.109 E-cadherin expression may well underlie the propensity of TAM to fuse with tumor cells.
Besides its fusogenic properties, E-cadherin might serve to anchor macrophage and DC subsets in epithelial layers, in a similar way as has been described for the LC-keratinocyte interaction. For example, dermal macrophages show signs of alternative activation under steady state110 and, hence, might express and use E-cadherin. The same might hold true for wound macrophages, another type of M2-like cell. Interestingly, cutaneous infections by Leishmania major, and possibly other pathogens, are regulated by Tregs that are trapped in the lesions via CD103,26 and it will be of importance to evaluate the contribution of E-cadherin+ mononuclear phagocytes to this phenomenon.
Overall, a discrepancy still exists between the firmly established impact of E-cadherin on intracellular signaling pathways (exclusively established in epithelial cells) and its interaction with KLRG1 and αE(CD103)β7 on the one hand, and the relative paucity of data describing a function for this molecule in DCs and macrophages on the other hand. However, recent data, presented in this review, hint to an immunoregulatory role of E-cadherin and β-catenin and warrant further research on this topic.
Acknowledgments
This work was supported by FWO-Vlaanderen (doctoral grant, J.V.d.B.) and Stichting tegen Kanker (research grant, J.A.V.G. and P.D.B.).
Authorship
Contribution: J.V.d.B., B.M., A.M., P.D.B., and J.A.V.G. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jo A. Van Ginderachter, Lab of Cellular and Molecular Immunology, Vrije Universiteit Brussel, Building E8, Pleinlaan 2, B-1050 Brussels, Belgium; e-mail: jvangind@vub.ac.be.