Abstract
Second-generation tyrosine kinase inhibitors (2G-TKIs) are effective at inducing complete cytogenetic responses (CCyRs) in approximately half of chronic myeloid leukemia patients treated while still in the chronic phase and after failing imatinib. It is less clear whether these responses are durable. In the present study, we report the clinical outcome of 119 patients who received a 2G-TKI as second-line treatment while still in the chronic phase. In an intention-to-treat analysis, the 4-year probabilities of overall and event-free survival were 81.9% and 35.3%, respectively. Sixty-two patients discontinued the initial 2G-TKI because of resistance or intolerance. To further explore the durability of cytogenetic responses, irrespective of the need for a third-line TKI, we used the concept of “current CCyR-survival” (c-CCyRS). The c-CCyRS at 4 years was 54.4%. After introduction of a 2G-TKI, 77 patients had a 3-month BCR-ABL1/ABL1 transcript ratio of ≤ 10% and had significantly superior overall survival (91.3% vs 72.1%, P = .02), event-free survival (49.3% vs 13.0%, P < .001), and c-CCyRS (67.2% vs 11.2%, P = .0001) compared with the 33 patients with ratios > 10%. The 3-month molecular response was the only independent predictor for overall survival. Using an intention-to-treat analysis, we have shown that the responses to second-line therapies are durable. Patients destined to fare poorly can be identified early during therapy.
Introduction
Second-generation tyrosine kinase inhibitors (2G-TKIs) such as dasatinib or nilotinib are effective at inducing complete cytogenetic responses (CCyRs) in approximately half of chronic myeloid leukemia (CML) patients treated while still in the chronic phase (CP) and after imatinib failure.1-5 It is not clear whether these responses are durable or if 2G-TKI therapy can be maintained long-term. Most of the information that we have about the efficacy of these drugs comes from phase 2 commercially sponsored registration studies in which patients were censored when they discontinued the study drug.4,5 This practice is common in such clinical studies, but is a major limitation when trying to define the long-term outcomes of patients, because some of those who discontinue the study drug may still obtain excellent responses on alternative therapy, whereas unresponsive patients are frequently removed from the study (and censored) before progression to advanced phase or death takes place. We report the clinical outcome of 119 CML patients who received a 2G-TKI after imatinib failure in an intention-to-treat analysis.
Methods
Patients
Between March 2005 and March 2011, 119 consecutive patients with CML in CP resistant to imatinib were treated with dasatinib (n = 91), nilotinib (n = 25), or bosutinib (n = 3) at the Hammersmith Hospital (Imperial College, London). The definitions of resistance to imatinib changed with time1,6 ; initially they coincided with the inclusion criteria for the phase 2 registration studies of dasatinib and nilotinib, but were later modified to resemble those of the European LeukemiaNet.7-9 Written informed consent for the use of clinical data were obtained from all patients. The characteristics of the patients were typical of those with imatinib-treated “late” CP1,10 (Table 1). The median follow-up for the surviving patients after starting 2G-TKIs was 36.3 months (range, 6-73) and 30.1% of the living patients were followed up by more than 48 months. Dasatinib, nilotinib, and bosutinib were administered as described previously.1,7,8,11-13 Briefly, patients receiving bosutinib were started at a dose of 500 mg once daily, patients receiving nilotinib were started at a dose of 400 mg every 12 hours, and patients receiving dasatinib started at a dose of either 70 mg every 12 hours (n = 23) or 100 mg once daily (n = 68). Doses were adjusted according to tolerance.7,8 Eleven patients received an allogeneic stem cell transplantation after having failed second- or third-line TKIs.
CP and complete hematologic responses were defined by conventional criteria.9 Primary cytogenetic resistance described patients who never achieved a cytogenetic response; acquired cytogenetic resistance applied to patients who responded initially but then lost a CCyR; CCyR was defined by the failure to detect any Philadelphia chromosome–positive (Ph+) BM metaphases in 2 consecutive BM examinations.
Detection of BCR-ABL1 transcripts and ABL KD mutation analysis
BCR-ABL1 transcripts were measured in the blood at 6- to 12-week intervals using real-time quantitative PCR, as described previously.14-17 Results were expressed as percent ratios relative to an ABL1 internal control, with original laboratory values converted to the international scale.17 Major molecular response (MMR) was defined as a transcript level ≤ 0.1% on the international scale. Complete molecular response (CMR) was defined by the finding of 2 consecutive samples with no detectable transcripts with an ABL1 control > 40 000 copies. Samples obtained for real-time quantitative PCR were also analyzed for kinase domain (KD) mutations before starting therapy with a 2G-TKI using direct sequencing18 and again when resistance to therapy was suspected.19,20
Statistical methods
Probabilities of overall survival (OS), progression-free survival (PFS), and event-free survival (EFS) were calculated using the Kaplan-Meier method. An event was defined as the loss of a CCyR or complete hematologic response, progression to advanced phase, death, or discontinuation of first-choice 2G-TKI therapy. The probabilities of cytogenetic and molecular responses were calculated using the cumulative incidence (CI) procedure, in which cytogenetic or molecular responses were the events of interest, and death or first-choice 2G-TKI therapy discontinuation were the competitors. Univariate and multivariate analyses were performed with standard statistical methodology, and variables found to be significant at the < 0.1 level were included in the multivariate analysis. Current CCyR survival (c-CCyRS) was defined as the probability of being alive and in CCyR at a given time point.21 The c-CCyRS is the analog of “current leukemia free-survival,” a concept that we developed to describe the behavior of patients after allogeneic stem cell transplantation. This designation recognizes the fact that patients may relapse but regain remission with alternative therapy. The c-CCyRS was calculated as described previously.21,22 OS, PFS, and EFS were compared using the log-rank text or a Cox regression model. CI and c-CCyRS were examined using Fine-Gray regression or a modified Cox regression model for recurrent events, respectively.23-26
Results
Responses and outcomes
The 4-year probabilities of OS, PFS, and EFS were 81.9%, 80.6%, and 35.3%, respectively. The 4-year CI of CCyR was 50.0% (in accordance with previous studies).1-5 The 4-year CI of MMR and CMR were 32.0% and 6.8%, respectively. Table 1 shows the probabilities of OS and EFS according to baseline patient characteristics. High Sokal risk group (RR = 3.0, P = .04) and up-front cytogenetic resistance to imatinib (RR = 3.1, P = .03) were the only pre–second-line therapy independent predictors for OS.
BCR-ABL1 transcript levels after 3 months on first-choice 2G-TKI therapy strongly predict for the most relevant clinical outcomes
The 77 patients who after 3 months of second-line therapy had a BCR-ABL1/ABL1 ratio of ≤ 10% had a significantly superior OS (91.3% vs 72.1%, P = .02), PFS (91.0% vs 67.9%, P = .007), EFS (49.3% vs 13.0%, P < .001), CI of CCyR (75.6% vs 4.2%, P < .001), CI of MMR (45.3% vs 0%, P < .001), and CI of CMR (8.8% vs 0%, P = .001) compared with the 30 patients with values > 10% (10 patients had missing data and 2 were already receiving third-line TKI therapy; Figure 1). The 64 patients who were in MCyR at 3 months also had significantly superior OS (92.2% vs 72.4%, P = .04); there was also a trend toward superior OS for the 47 patients who were in CCyR at 3 months (93.1 vs 72.8, P = .13). We performed a multivariate analysis for OS including the molecular and cytogenetic responses at 3 months and the baseline variables found to be significant in the univariate analysis. A transcript level > 10% (relative risk [RR] = 5.6, P = .01) was the only independent predictor for OS.
OS, EFS, CI of CCyR, and c-CCyRS according to the molecular response to first-choice 2G-TKI at 3 months. The 77 patients who after 3 months of second-line therapy had BCR-ABL1/ABL1 ratios of ≤ 10% (solid line) had a significantly superior OS (A; 91.3% vs 72.1%, P = .02), EFS (B; 49.3% vs 13.0%, P < .001), and CI of CCyR (C; 76% vs 4%, P < .001) compared with the 30 patients with transcript ratios > 10% (broken line). Whiskers represent 95% confidence intervals. Ten patients had missing quantitative real-time PCR data and 2 were already receiving third-line TKI therapy and therefore were not included in the analysis. The patients with a transcript ratio ≤ 10% had a significantly higher probability of c-CCyRS (D; defined as the probability of being alive and in CCyR at a given time point): 67.2% for patients with a transcript level ≤ 10% (blue line) and 11.2% (P = .001) for patients with a higher transcript level (red line).
OS, EFS, CI of CCyR, and c-CCyRS according to the molecular response to first-choice 2G-TKI at 3 months. The 77 patients who after 3 months of second-line therapy had BCR-ABL1/ABL1 ratios of ≤ 10% (solid line) had a significantly superior OS (A; 91.3% vs 72.1%, P = .02), EFS (B; 49.3% vs 13.0%, P < .001), and CI of CCyR (C; 76% vs 4%, P < .001) compared with the 30 patients with transcript ratios > 10% (broken line). Whiskers represent 95% confidence intervals. Ten patients had missing quantitative real-time PCR data and 2 were already receiving third-line TKI therapy and therefore were not included in the analysis. The patients with a transcript ratio ≤ 10% had a significantly higher probability of c-CCyRS (D; defined as the probability of being alive and in CCyR at a given time point): 67.2% for patients with a transcript level ≤ 10% (blue line) and 11.2% (P = .001) for patients with a higher transcript level (red line).
We also examined the predictive value of the transcript level at 6 months. The 59 patients with a BCR-ABL1/ABL1 ratio of ≤ 1% had a significantly superior EFS (58.5% vs 18.3%, P < .001), but not OS (90.8% vs 77.0%, P = .1) or PFS (91.5% vs 74.2%, P = .06). We then used a receiver operating characteristic curve to identify the optimal transcript level cutoff at 6 months that would be predictive for OS with the maximal sensitivity and specificity,21 which was 1.1%. The use of this cutoff did not improve the predictive power of the transcript level on OS or PFS.
Responses obtained on second-line therapy are durable
Sixty-two patients (52%) discontinued the first-choice 2G-TKI because of primary resistance (n = 23), acquired resistance (n = 8), or intolerance (n = 31), whereas 57 patients were still on first-choice 2G-TKI therapy at last follow-up. ABL1 KD mutations were identified in 26 patients before 2G-TKI therapy, and 16 of these (62%) developed further mutations on subsequent therapy, in contrast to only 10 (11%) of the 93 patients without mutations at the start of 2G-TKI (P < .001). Seventy-four patients achieved CCyR on first-choice 2G-TKI therapy. Of these, 8 subsequently lost their CCyR (3 patients regained CCyR on alternative therapy). The 4-year CI of loss of CCyR was 14.8%. Of the remaining 66 patients, 14 changed 2G-TKI as a result of intolerance, and 52 continued on first-choice 2G-TKI. Forty-five patients did not achieve CCyR on the first-choice 2G-TKI, of whom 32 then received third-line therapy (alternative TKI, allogeneic stem cell transplantation, and others).
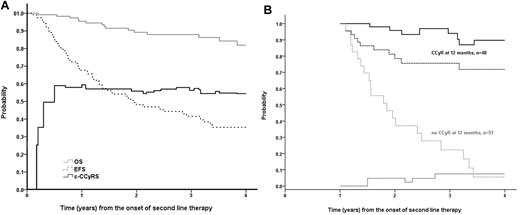
As we have shown, the discontinuation rate of the first-choice 2G-TKI was relatively high. Therefore, to explore the durability of CCyR irrespective of the need of an alternative TKI, we used the concept of c-CCyRS. The c-CCyRS at 4 years was 54% (Figure 2A). The c-CCyRS reaches a plateau after the first year. Furthermore, in a landmark analysis of patients in CCyR at 12 months (Figure 2B), the c-CCyRS at 4 years was 90%. However, 3 of these patients died of causes not related to CML, and when these patients were excluded, the 4-year probability of c-CCyRS was 100%. This shows a remarkable stability of the responses.
OS, EFS, and c-CCyRS in the whole population and according to the cytogenetic response at 12 months. (A) Probabilities of OS (gray solid line), EFS (black broken line), and c-CCyRS (black solid line) in the whole population. The 4-year probability of OS, EFS, and c-CCyRS were 81.9%, 35.3%, and 54.4%, respectively. The figure shows how the EFS continues to decline over time, whereas the c-CCyRS plateaued at approximately 55% after the first year of therapy, reflecting that the responses are stable once they have been achieved. (B) EFS (broken line) and c-CCyRS (solid line) according to the cytogenetic response achieved at 12 months. The 48 patients who were in CCyR at 12 months, represented in black, had a 4-year probability of EFS of 71.8%; conversely, these patients had an excellent 4-year probability of c-CCyRS of 90%. In contrast, the 51 patients who were not in CCyR at 12 months, represented in gray, had a EFS of 5.6% (P < .001) and a c-CCyRS of 7.5% (P = .008)
OS, EFS, and c-CCyRS in the whole population and according to the cytogenetic response at 12 months. (A) Probabilities of OS (gray solid line), EFS (black broken line), and c-CCyRS (black solid line) in the whole population. The 4-year probability of OS, EFS, and c-CCyRS were 81.9%, 35.3%, and 54.4%, respectively. The figure shows how the EFS continues to decline over time, whereas the c-CCyRS plateaued at approximately 55% after the first year of therapy, reflecting that the responses are stable once they have been achieved. (B) EFS (broken line) and c-CCyRS (solid line) according to the cytogenetic response achieved at 12 months. The 48 patients who were in CCyR at 12 months, represented in black, had a 4-year probability of EFS of 71.8%; conversely, these patients had an excellent 4-year probability of c-CCyRS of 90%. In contrast, the 51 patients who were not in CCyR at 12 months, represented in gray, had a EFS of 5.6% (P < .001) and a c-CCyRS of 7.5% (P = .008)
The transcript level of patients with a ratio > 10% at 3 months of first-choice 2G-TKI remains high during follow-up despite changing therapy
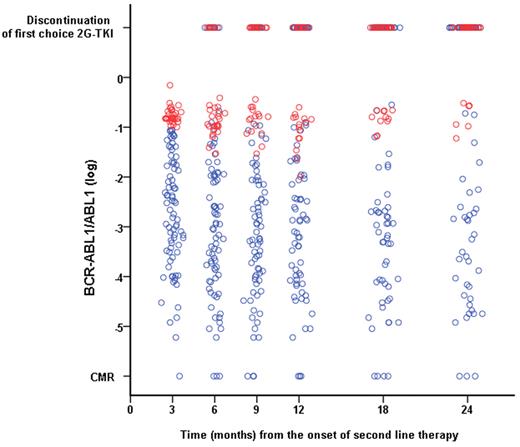
Figure 3 shows the evolution of the transcript levels over time. The 30 patients who had a BCR-ABL1/ABL1 ratio > 10% at 3 months only gained a very modest reduction in their transcript levels during follow-up. The 4-year CI of CCyR for these patients was only 4.2% (Figure 1C). These patients obtained a limited benefit from changing therapy, as shown by the fact that 4-year c-CCyRS for this cohort was only 11.2% compared with 67.2% for the rest of the population (P = .0001, Figure 1D).
Evolution in the BCR-ABL1 transcript level on first-choice second-line 2G-TKI according to the molecular response achieved after 3 months on second-line therapy. Patients who had a BCR-ABL1/ABL1 ratio > 10% after 3 months of therapy (red circles) had a minimal reduction in the transcript level during subsequent follow-up, remaining always above the 1% mark (equivalent to a 2 log reduction commonly identified with CCyR). Patients with a transcript level at 3 months of ≤ 10% are represented by blue circles. The top row represents patients who discontinued their first-choice 2G-TKI at the various time points according to their transcript levels at 3 months.
Evolution in the BCR-ABL1 transcript level on first-choice second-line 2G-TKI according to the molecular response achieved after 3 months on second-line therapy. Patients who had a BCR-ABL1/ABL1 ratio > 10% after 3 months of therapy (red circles) had a minimal reduction in the transcript level during subsequent follow-up, remaining always above the 1% mark (equivalent to a 2 log reduction commonly identified with CCyR). Patients with a transcript level at 3 months of ≤ 10% are represented by blue circles. The top row represents patients who discontinued their first-choice 2G-TKI at the various time points according to their transcript levels at 3 months.
The BCR-ABL1 transcript kinetics are influenced by the early dose intensity of 2G-TKI therapy
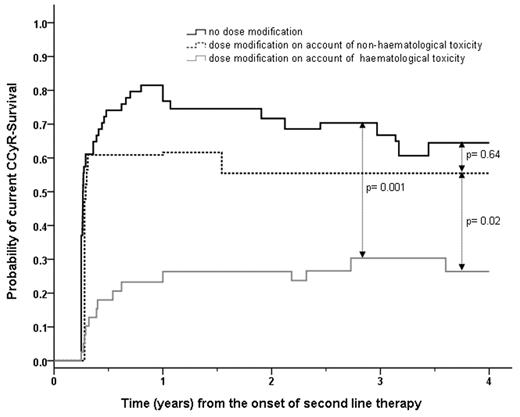
Sixty-two patients required dose reduction or dose interruption during the first 3 months of second-line therapy. These patients had significantly lower 4-year CI of CCyR and EFS, namely 29.3% vs 76.0% (P < .0001) and 29.5% vs 44.4% (P = .03), respectively, compared with the 54 patients who received the full dose (2 patients had already permanently discontinued the first-choice 2G-TKI). Again, these poor risk patients only obtained a limited benefit from changing therapy, with a 4-year c-CCyRS of 32.4% versus 64.5% for the rest of the patients (P = .005). Furthermore, 25 of 30 patients with a BCR-ABL1/ABL1 ratio > 10% at 3 months received a dose intensity lower than 100 mg daily of dasatinib, 400 mg twice daily of nilotinib, or 500 mg daily of bosutinib. To further investigate the effect on outcome of low dose intensity during the first 3 months of therapy, we subclassified the poor tolerance as hematologic (n = 39) or nonhematologic (n = 23). We found that patients with hematologic intolerance fared the worst, whereas the patients with nonhematologic toxicity had a 4-year c-CCyRS comparable to that of patients with good tolerance to therapy (Figure 4). Therefore, the c-CCyRS for patients with hematologic intolerance was 26.4% (P = .001 compared with patients with good tolerance), whereas the c-CCyRS for patients with nonhematologic intolerance was 55.5% (P = .64 compared with patients with good tolerance).
c-CCyRS according to the dose intensity received by the patients during the first 3 months of therapy and reason for dose adjustment. The 39 patients (gray line) who had their therapy with the first-choice 2G-TKI temporarily discontinued or dose reduced because of hematologic toxicity during the first 3 months of treatment had a significantly worse 4-year probability of c-CCyRS than the 54 patients who did not require dose modification, represented by the black line, (26.4% vs 64.5%, P = .001). Conversely, the 23 patients who had their dose of 2G-TKI modified because of nonhematologic toxicity (broken black line) had a 4-year c-CCyRS comparable to that of patients with good tolerance to therapy (55.5% vs 64.5%, P = .64), which was significantly higher than that of patients with hematological intolerance (P = .02).
c-CCyRS according to the dose intensity received by the patients during the first 3 months of therapy and reason for dose adjustment. The 39 patients (gray line) who had their therapy with the first-choice 2G-TKI temporarily discontinued or dose reduced because of hematologic toxicity during the first 3 months of treatment had a significantly worse 4-year probability of c-CCyRS than the 54 patients who did not require dose modification, represented by the black line, (26.4% vs 64.5%, P = .001). Conversely, the 23 patients who had their dose of 2G-TKI modified because of nonhematologic toxicity (broken black line) had a 4-year c-CCyRS comparable to that of patients with good tolerance to therapy (55.5% vs 64.5%, P = .64), which was significantly higher than that of patients with hematological intolerance (P = .02).
Discussion
We and others1-6,27 have shown that 50% of the CP-CML patients who start second-line therapy will achieve CCyR at some point during follow-up. However, to date, there are no data about the durability of those responses. This is particularly relevant, because patients who have failed imatinib are more likely to have acquired resistance or transformed clones that may be responsible for early relapse. Furthermore, a considerable number of the patients who do eventually respond fail the first-choice 2G-TKI because of toxicity and then require alternative therapy. To study these issues, we exploited the concept of current leukemia-free survival, which we developed originally for analysis of results after allogeneic stem cell transplantation for CML. Therefore, the 4-year c-CCyRS, which reflects the probability of being alive and in CCyR at a given time point, was 54.4% (Figure 2A) for the whole population and 90% (Figure 2B) for the patients who were in CCyR after 12 months of second-line therapy (100% if the nonleukemic-related deaths are excluded), showing that the responses were remarkably stable over time. Interestingly, both curves reach a plateau after the initial 12 months despite the fact that patients continue to have “events,” which indicates that patients who have to discontinue their second-line therapy because of side effects fared very well on third line therapy, as reported previously.28
Primary cytogenetic resistance to imatinib and a high Sokal risk score were the only pre–second-line therapy independent predictors for OS. This is consistent with previous reports by us and others in which patients with upfront cytogenetic resistance are less likely to respond to second-line therapy.1,6,29 As for patients on front-line therapy,21 BCR-ABL1 transcript levels measured at 3 months on second-line therapy accurately predicted outcome (Figure 1). The 30% of patients who had a transcript level > 10% had a very low probability of achieving CCyR (4.2%) and a significantly lower OS (72.1% vs 91.3%, P = .02). The transcript levels of patients with a poor 3-month molecular response remained comparatively high during the subsequent follow-up (Figure 3). In fact, further assessments did not contribute to a better definition of poor-risk patients. We reported previously that poor responders to second-line 2G-TKIs obtain very limited benefit from a third-line TKI,28 and indeed, patients who had a BCR-ABL1/ABL1 ratio > 10% at 3 months after starting first-choice 2G-TKI had a very low 4-year c-CCyRS of 11.2% compared with 67.2% for the rest of the population (P = .0001, Figure 1D). Clearly, a high 3-month transcript level identifies patients with intrinsic resistance to TKI therapy, which appears not to be improved by changing to an alternative TKI.
Patients who had difficulty tolerating the first-choice 2G-TKI during the first 3 months (and also during the initial 6 months, data not shown) because of side effects fared worse, but the deleterious effect of a low dose intensity during the beginning of the second-line therapy was mainly limited to patients with hematologic toxicity. Patients with nonhematologic side effects responded well to an alternative TKI and had a 4-year c-CCyRS comparable to that of patients who received the full dose intensity during the first 3 months of therapy. Conversely, patients who suffered hematologic toxicity had a low 4-year c-CCyRS (Figure 4). Again, changing to an alternative therapy had little effect on the long-term outcome of these patients. One possible explanation is that hematologic toxicity is linked in some cases to an intrinsic characteristic of the leukemia that prevents a given patient from responding satisfactorily to TKI therapy, such as the lack of an expandable Ph− stem cell population that can restore effective Ph− hematopoiesis.10,20
In summary, in the present study, we have shown that responses to second-line therapies are durable, although CML patients with side effects may need to be treated with more than one 2G-TKI after failing imatinib to achieve an optimal outcome. Patients destined to fare poorly can be identified early on during therapy. These results are preliminary and should be confirmed by future studies.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful for support from the National Institute for Health Research Biomedical Research Centre Funding Scheme, London, United Kingdom.
Authorship
Contribution: D. Milojkovic provided patient care and wrote the manuscript; J.F.A., M.B., and K.R. provided patient care and commented on the manuscript; G.G. performed the molecular studies and assembled the molecular data; A.R.I. collected patient data, performed the statistical analysis, and wrote the manuscript; R.S. reviewed the statistical analysis and commented on the manuscript; A.R. performed the cytogenetic analysis and commented on the manuscript; L.F. supervised the day-to-day running of the Minimal Residual Disease service and commented on the manuscript; J.G. wrote the manuscript; and D. Marin provided patient care, performed the statistical analysis, and wrote the manuscript.
Conflict-of-interest disclosure: D. Milojkovic, J.F.A., J.G., and D. Marin received research support from Novartis and Bristol Myers-Squibb. The remaining authors declare no competing financial interests.
Correspondence: Dr David Marin, Department of Haematology, Imperial College London, Du Cane Road, London W12 0NN, United Kingdom; e-mail: d.marin@imperial.ac.uk.