Abstract
Multiple myeloma (MM) is an incurable B-cell malignancy in which the marrow microenvironment plays a critical role in our inability to cure MM. Marrow stromal cells in the microenvironment support homing, lodging, and growth of MM cells through activation of multiple signaling pathways in both MM and stromal cells. Recently, we identified annexin II (AXII) as a previously unknown factor produced by stromal cells and osteoclasts (OCL) that is involved in OCL formation, HSC and prostate cancer (PCa) homing to the BM as well as mobilization of HSC and PCa cells. AXII expressed on stromal cells supports PCa cell lodgment via the AXII receptor (AXIIR) on PCa cells, but the role of AXII and AXIIR in MM is unknown. In this study, we show that MM cells express AXIIR, that stromal/osteoblast-derived AXII facilitates adhesion of MM cells to stromal cells via AXIIR, and OCL-derived AXII enhances MM cell growth. Finally, we demonstrate that AXII activates the ERK1/2 and AKT pathways in MM cells to enhance MM cell growth. These results demonstrate that AXII and AXIIR play important roles in MM and that targeting the AXII/AXIIR axis may be a novel therapeutic approach for MM.
Introduction
Multiple myeloma (MM) is an incurable plasma cell malignancy that develops in the BM.1,2 The marrow microenvironment plays a critical role in MM by supporting tumor cell growth, increasing bone destruction, and providing a safe haven for tumor-initiating cells to remain dormant for long periods of time.3 In addition, the tumor microenvironment contributes to the chemoresistance of MM cells and activates the dormant tumor cells to enter the cell cycle and propagate to other sites. Strategies to identify and target the components of the marrow microenvironment that support MM are critically needed to eradicate MM.
We have reported that annexin II (AXII) plays an important role in tumor cell and normal HSC homing to the marrow.4 AXII is a member of a family of proteins that bind to anionic phospholipid surfaces in the presence of calcium,5 and has been implicated in multiple intracellular and extracellular processes.5,6 AXII exists as a monomer, or a heterotetramer composed of 2 p36 AXII molecules and 2 p11 molecules.7 The heterotetramer is the predominant species in all tissues and cells, and appears to be the active form of AXII.7 The p11 subunit serves as the regulatory subunit of the AXII heterotetramer.8 We previously cloned an AXII receptor (AXIIR) from human BM stromal cells (BMSCs) that specifically binds the p11 subunit of AXII.9
Osteoblasts (OBL) and marrow endothelial cells express high levels of AXII,4,10,11 and adhesion of HSCs to OBL derived from AXII−/− mice was significantly reduced compared with OBL from AXII+/+ animals. Moreover, fewer HSCs were present in the marrow of AXII−/− mice.4 Furthermore, short-term engraftment and survival studies of lethally irradiated mice, transplanted with whole marrow and treated with a N-terminal peptide fragment of AXII or anti-AXII Abs, showed decreased HSC lodging and engraftment, and the survival of the irradiated mice was shortened.4 Similarly, long-term competitive repopulation studies revealed that blocking AXII also blocked HSC homing.4 Collectively, these findings demonstrated that AXII regulates HSC homing and lodging in the BM microenvironment.
More recently, we demonstrated that the AXII/AXIIR axis plays a crucial role in establishing prostate cancer (PCa) bone metastasis.12 We found that AXII is chemotactic for PCa cells and that blocking AXII or AXIIR in animal models limited short-term and long-term localization of PCa to the bone.12 AXII facilitated the growth of PCa cells in vitro and in vivo by signaling through ERK1/2, consistent with our previous results showing that AXII signaled through ERK1/2 in stromal cells to induce RANKL.13 We previously found that AXII was also produced and secreted by osteoclasts (OCL) and induced OCL formation.14 However, the role of AXII in MM is just being defined.
Claudio et al found that AXII is one of the most highly expressed genes in primary MM cells, and it was differentially expressed in MM cells compared with B-cell lines.15 More recently, AXII was shown to increase the proliferation of, and had antiapoptotic effects on, human MM cell lines.16
Based on these previous studies, it is our hypothesis that AXII and AXIIR mediate critical interactions between MM cells, stromal cells/OBL, and OCLs in the BM microenvironment that promote MM cell growth. In this study, we show that AXIIR is expressed by MM cell lines and CD138+ cells from MM patients, and that both AXII and AXIIR are involved in adhesion of MM cells to OBL and stromal cells through RhoA. In addition, we show that AXII signals through ERK1/2 and AKT in MM cells, and that OCL-derived AXII enhances the growth of MM cells. These results suggest that the AXII/AXIIR axis may play a critical role in supporting MM cell growth and adhesion to stromal cells/OBL in the BM.
Methods
Materials
Purified bovine lung AXII tetramer (AXII) was purchased from Biodesign International. Agarose, ethidium bromide, BSA, annexin V (AXV), Tris buffered saline containing 0.05% (v/v) Tween 20, pH 8.0 (TBS-T), avidin-FITC were purchased from Sigma-Aldrich. PBS, FCS, FBS, IMDM medium, RPMI 1640 medium, and α-MEM medium were purchased from Invitrogen. Restore Western blot stripping buffer (Pierce) and methanol were purchased from Thermo Fisher Scientific. RANKL, IL-6, and MCSF were purchased from R&D Systems. Antibiotic-antimytotic solution (Ab/Am) was purchased from Cellgro. Draq5 was purchased from Biostatus Limited. Fluorescent mounting medium was purchased from DAKO. Annexin-binding buffer was purchased from Clontech. RIPA lysis buffer was purchased from Santa Cruz Biotechnology. Nitrocellulose transfer membrane, running buffer, transfer buffer, and precast gels were purchased from Bio-Rad. Total cell lysis buffer was purchased from Cell Signaling Technology. Reverse Transcriptase System was purchased from Promega. ECL Plus Western blotting detection system was purchased from GE Healthcare.
Abs
The anti-AXII Abs (anti-p36 and anti-p11) were purchased from BD Transduction Laboratories. Abs to phosphorylated p44/42 MAP kinase (Thr-202/Tyr-204), total p44/42 MAPK, phosphorylated p-AKT (Ser-473), and total-AKT, HRP-conjugated mouse and rabbit secondary Abs were purchased from Cell Signaling Technology. Ab to β-actin was purchased from Sigma-Aldrich. Anti-AXIIR Abs (mouse and rabbit) were purchased from Abcam. RhoA Ab was purchased from ProteinTech Group Inc. Control IgGs (mouse and rabbit) and α-tubulin Abs were purchased from Santa Cruz Biotechnology. Anti–rabbit Alexa Fluor 488 was purchased from Invitrogen.
Cell cultures
The MM1.S human MM cell line was generously provided by Dr Steven Rosen (Northwestern University, Chicago, IL). The ANBL-6 human MM cell line was generously provided by Dr Diane Jelinek (Mayo Clinic, Rochester, MN). The KM101 cell line was generously provided by Dr Joel Greenberg (University of Pittsburgh, Pittsburgh, PA). The H929, U266, RPMI8226, and OPM-2 cell lines were generously provided by Dr Suzanne Lentzch (University of Pittsburgh, Pittsburgh, PA). The MM cells were cultured in RPMI 1640 (Invitrogen) and were supplemented with 10% (v/v) FCS (Invitrogen) and 1% (v/v) Ab/Am (Cellgro). ANBL-6 cells were cultured in RPMI 1640 (Invitrogen) supplemented with 10% (v/v) FCS (Invitrogen), 1% (v/v) Ab/Am (Cellgro), and IL-6 (1 ng/mL). KM101 cells were cultured in IMDM (Invitrogen) supplemented with 10% (v/v) FCS (Invitrogen) and 1% (v/v) Ab/Am (Cellgro). All cells were maintained at 37°C in 5% CO2 air.
Animals
The laboratory of Dr K. A. Hajjar (Weill Medical College of Cornell University, New York, NY) generated the AXII−/− animals used in our study and graciously provided Dr Russell Taichman's laboratory at the University of Michigan, Ann Arbor, with a pair of the homozygous AXII−/− mice for breeding.
Human BM cultures
After obtaining informed consent in accordance with the Declaration of Helsinki, 2 mL of BM was aspirated from the posterior-superior iliac crest of MM patients into a syringe containing 1 mL of α-MEM (Invitrogen) and 100 U of preservative-free heparin. These studies were approved by the Institutional Review Board of the University of Pittsburgh. The marrow mononuclear cells were obtained by density gradient centrifugation using Ficoll-Hypaque (Sigma-Aldrich). From these, the CD138+ primary MM cells were isolated by anti-CD138–coated immunomagnetic microbeads (Miltenyi Biotec). Samples containing at least 95% CD138+ cells were used for the experiments.
Biotinylation of AXII and immunocytochemistry
AXII was biotinylated using the BiotinTag Kit (Sigma-Aldrich) according to the manufacturer's protocol and as previously described, and biotinylation of AXII (bio-AXII) was confirmed by Western blotting.9 MM1.S or CD138+ MM cells (2 × 105) were blocked in 3% (w/v) BSA/PBS for 1 hour. The cells were then incubated with 1 μg/mL bio-AXII in the presence of annexin-binding buffer (Clontech) for 30 minutes on ice. After washing 3 times for 5 minutes with PBS, the cells were incubated with avidin-FITC (Sigma-Aldrich) at 1:100 dilution in PBS for 1 hour on ice. Draq5 (Biostatus Limited) was used as a nuclear stain at 1:1000 dilution and included with avidin-FITC. After washing 3 times for 5 minutes in PBS, the cells were mounted using Fluorescent Mounting Medium (DAKO). Similar experiments were performed in the presence of 100-fold excess of unlabeled AXII or AXV. Staining was visualized using the confocal microscope, Olympus Fluoview FV1000 (VA Pittsburgh Healthcare System, Pittsburgh, PA).
Immunocytochemistry with AXIIR Ab
Cells were fixed in methanol for 10 minutes after which cells were rehydrated in PBS for 5 minutes. Nonspecific sites on MM1.S cells (2 × 105) were blocked with 3% (w/v) BSA/PBS for 1 hour on ice. Polyclonal rabbit AXIIR Ab or control IgG was added to the MM1.S cells at 1:200 dilution in 3% (w/v) BSA/PBS for 1 hour on ice. After washing 3 times with PBS for 5 minutes each, Alexa Fluor 488 (Invitrogen) was used at 1:20 dilution in PBS for 1 hour on ice. Draq5 (Biostatus Limited) was used as a nuclear stain at 1:1000 dilution and included with Alexa Fluor 488 (Invitrogen). After washing 3 times for 5 minutes in PBS, the cells were mounted using Fluorescent Mounting Medium (DAKO). Staining was visualized using the confocal microscope, Olympus Fluoview FV1000 (VA Pittsburgh Healthcare System).
RT-PCR
Total RNA was extracted using the RNeasy kit (QIAGEN) per the manufacturer's protocol. The first-strand cDNA was synthesized using the Reverse Transcriptase System (Promega). The primers used for AXIIR were TTGGGATTCCGCAGAGGTGG (forward) and CAGCACGCCCAGAGTACAGAGAA (reverse) and the primers for GAPDH were ACCACAGTCCATGCCATCAC (forward) and TCCACCACCCTGTTGCTGTA (reverse).9 The PCR was performed using GeneAmp PCR Systems 2700 (Applied Biosystems). The PCR parameters were 94°C for 15 seconds, 68°C for 90 seconds, and 72°C for 1 minute for 35 cycles and 60°C for 7 minutes for the final elongation. PCR products were analyzed by electrophoresis on a 1.2% (w/v) agarose gel and visualized by ethidium bromide staining.
Adhesion assays
Calvarial OBL (104) from AXII+/+ or AXII−/− mice were plated in 96-well plates in 100 μL of α-MEM containing 10% (v/v) FCS and incubated overnight at 37°C. The next day, cells were washed with PBS and fluorescent-labeled MM1.S cells were added to the OBL. The plates were centrifuged at 52g for 5 minutes and placed in the dark for 15 minutes. The background fluorescence was measured with a plate reader. The cells were washed with PBS and read again. Anti-AXII (5 μg/mL) or control IgG (5 μg/mL) was added to a set of calvarial OBL from AXII+/+ mice. Alternatively, unlabeled MM cells were used in adherence assays with KM101 or BMSCs from AXII+/+ and AXII−/− mice. Briefly, KM101 (2 × 105) or BMSCs from AXII+/+ and AXII−/− (1 × 105) mice were plated in 12-well plates in 1 mL of media containing 10% (v/v) FCS and incubated overnight at 37°C. The next day, the cells were rinsed with coculture media. The MM cells (1 × 106) in 1 mL of coculture media were then added to the KM101 cells or BMSCs from AXII+/+ and AXII−/− mice and incubated for either 6 hours at 37°C. At the end of 6 hours, the nonadherent MM cells were removed and counted. The coculture media for adhesion assays between KM101 and MM cells was 50% (v/v) IMDM and 50% (v/v) RPMI 1640 containing 10% (v/v) FCS. The coculture media for adhesion assays between MM cells and BMSCs from AXII+/+ and AXII−/− mice was 50% (v/v) RPMI 1640 and 50% (v/v) α-MEM containing 10% (v/v) FCS. AXIIR Ab (rabbit) was used at 1 μg/mL in the adhesion assay.
Knockdown of AXIIR
MM cells were transfected with either siControl or siAXIIR (Santa Cruz Biotechnology) according to the manufacturer's protocol or with siAXIIR and siControl generated previously12 using a nucleofector II (Program S-20; Amaxa BioSystems). Knockdown of AXIIR was confirmed each time by RT-PCR using GeneAmp PCR Systems 2700 (Applied BioSystems) and by real-time RT-PCR using a Bio-Rad iCycler; the cells were then used for adhesion assays.
Western blotting for AXIIR
Total protein from the MM cell lines was extracted in RIPA lysis buffer (Santa Cruz Biotechnology). Twenty micrograms of total protein was loaded on a 12% precast gel (Bio-Rad) and subjected to SDS-PAGE. Separated proteins were transferred to a 0.45-μm nitrocellulose membranes (Bio-Rad) and the nonspecific sites blocked with 5% (w/v) BSA/TBS-T. Blots were incubated overnight at 4°C with AXIIR Ab (mouse) at a 1:250 dilution in 5% (w/v) BSA/TBS-T, followed by 3 washes of 5 to 7 minutes each in TBS-T. Blots were incubated with HRP-conjugated anti–mouse secondary Ab (Cell Signaling Technology) for 1 hour at room temperature, washed 3 times for 5 to 7 minutes in TBS-T, and incubated with ECL Plus Western blotting detection system (GE Healthcare) for 5 minutes. The blots were visualized using CL-XPosure Film (Thermo Fisher Scientific) and developed using the Kodak X-OMat 2000A processor. The membranes were stripped and reprobed for β-actin (Sigma-Aldrich). Blots were incubated with β-actin at a 1:1000 dilution in 5% (w/v) BSA/TBS-T for 1 hour at room temperature, followed by 3 washes of 5 to 7 minutes each in TBS-T, and then incubated with HRP-conjugated anti–rabbit secondary Ab (Cell Signaling Technology) for 1 hour at room temperature. The blots were washed 3 times for 5 to 7 minutes in TBS-T and analyzed as mentioned for AXIIR Ab.
Phosphorylation experiments: stimulation of MM1.S cells with AXII and Western blotting
MM1.S cells (2 × 106) were plated in 6-well plates, serum starved for 16 hours, and stimulated with 1 μg/mL AXII for the indicated time periods. Total protein was extracted using total cell lysis buffer (Cell Signaling Technology). Twenty micrograms of total protein was loaded on a 10% precast gel (Bio-Rad) and subjected to SDS-PAGE. Separated proteins were transferred to a 0.45-μm nitrocellulose membrane (Bio-Rad) and the nonspecific sites blocked with 5% (w/v) BSA/TBS-T. Blots were incubated overnight at 4°C with the primary signaling Abs (1:1000 dilution) in 5% (w/v) BSA/TBS-T, followed by 3 washes of 5 to 7 minutes each in TBS-T. Blots were then incubated with HRP-conjugated secondary Abs (Cell Signaling) for 1 hour at room temperature. The blots were washed 3 times for 5 to 7 minutes in TBS-T. The blots were then incubated with ECL Plus Western blotting detection system (GE Healthcare) for 5 minutes, visualized using CL-XPosure Film (Thermo Fisher Scientific) and developed using the Kodak X-OMat 2000A Processor. The membranes were stripped and reprobed for α-tubulin (Santa Cruz Biotechnology) to ensure equal loading. Blots were incubated with α-tubulin at a 1:1000 dilution in 5% (w/v) BSA/TBS-T for 1 hour at room temperature, followed by 3 washes of 5 to 7 minutes each in TBS-T, and then incubated with HRP-conjugated anti–mouse secondary Ab (Cell Signaling) for 1 hour at room temperature. The blots were washed 3 times for 5 to 7 minutes in TBS-T and analyzed as mentioned for signaling Abs.
Cell growth assays with OCL-conditioned media
MM1.S cells (5 × 103) were plated in 24-well plates overnight at 37°C. The next day, MM1.S cells were rinsed with media and then growth media containing 50% of conditioned media (CM) obtained from either AXII+/+ and AXII−/− OCL were added to the MM1.S cells. Cells were incubated for 72 hours at 37°C. At the end of 72 hours, the total cell numbers were counted. OCL were prepared as previously described.13
Coculture experiments
BM was flushed from the femurs and tibia of AXII+/+ mice in α-MEM medium containing 2% (v/v) FBS. The adherent BMSCs were cultured in α-MEM containing 10% (v/v) FBS and 1% (v/v) Ab/Am. BMSCs (1 × 105) were plated in 24-well plates overnight at 37°C. The next day, MM1.S cells (1 × 106) were added to the BMSCs and incubated for 48 hours at 37°C after which the MM1.S cells were removed by vigorous pipetting and analyzed for proliferation using a Cell Proliferation kit (G358C) from Promega.
Cell growth assays
MM cells (1 × 105) alone or cells expressing control or AXIIR shRNAs were plated in 24-well plates overnight at 37°C in RPMI 1640 containing 10% (v/v) FCS. The next day, AXII (1 μg/mL) was added to the cells. Cells were incubated for 72 hours at 37°C. At the end of 72 hours, the total cell numbers were counted. For the inhibitor studies, PD98059 and LY294002 were used at 50 μM and were added to the cultures 1 hour before addition of AXII. Alternatively, MM1.S cells (1 × 105) were plated in 96-well plates overnight at 37°C in RPMI 1640 containing 10% (v/v) FCS. The next day, AXII (1 μg/mL) was added to the cells. Cells were incubated for 72 hours at 37°C. At the end of 72 hours, cell growth of MM1.S cells was confirmed using a BrdU labeling kit (Roche).
Statistical analysis
The unpaired Student t test was used for analyzing differences between the 2 groups. Significance was set at α = 0.05. Results were expressed as mean ± SE.
Results
MM cells and CD138+ cells from MM patients express AXIIR
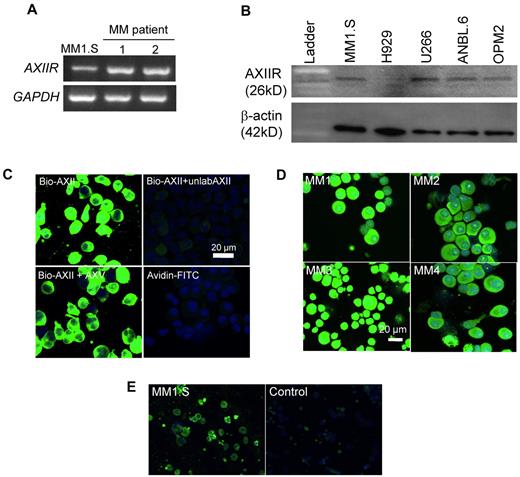
We determined whether MM cells expressed AXIIR by RT-PCR, Western blotting, and immunocytochemistry. Both MM1.S cells and CD138+ cells from MM patients expressed AXIIR mRNA (Figure 1A). To demonstrate that AXIIR protein is present in MM cells, protein extracts from MM1.S, H929, ANBL-6, OPM-2, and U266 were analyzed by Western blotting. All these MM cell lines expressed varying levels of AXIIR protein (Figure 1B). To demonstrate the presence of AXIIR protein on the surface of MM cells, MM1.S cells were incubated with biotinylated AXII (bio-AXII) in the presence or absence of a 100-fold molar excess of unlabeled AXII or annexin V. Bio-AXII bound effectively to the MM1.S cells and binding was competed by a 100-fold molar excess of unlabeled AXII (Figure 1C). In contrast, excess unlabeled annexin V did not compete for AXII binding to MM1.S cells, demonstrating that the binding of bio-AXII to its receptor on MM1.S cells was specific (Figure 1C). Bio-AXII also bound to CD138+ cells obtained from 4 MM patients (Figure 1D). A total of 8 MM patients were included in the study and 90% of CD138+ cells from all patients expressed AXIIR. Furthermore, immunocytochemistry with the AXIIR Ab confirmed that MM1.S cells expressed AXIIR on the cell surface (Figure 1E).
AXIIR is expressed by MM cells and CD138+ cells from MM patients. (A) RNA was extracted from CD138+ cells from MM patients (nos. 1, 2) and the MM1.S cell line. cDNA was reverse-transcribed and RT-PCR was performed with primers specific to AXIIR and GAPDH. (B) Total protein (20 μg) was extracted from MM cell lines and analyzed for AXIIR expression (AXIIR mouse Ab) by Western blotting. β-actin was used as a loading control. (C) MM1.S cells were incubated with 1 μg/mL bio-AXII alone or in the presence of either 100-fold molar excess unlabeled AXII (unlabAXII) or 100-fold excess annexin V (AXV). A secondary only control with avidin-FITC was included in the experiment. (D) CD138+ cells from MM patients (n = 4) were incubated with 1 μg/mL bio-AXII and after extensive washing were incubated with avidin-FITC. Staining was visualized using confocal microscopy. (E) MM1.S cells were incubated with AXIIR Ab (rabbit) at a 1:200 dilution. After extensive washing, the MM1.S cells were then incubated with Alexa Fluor 488. A set of experiments with an IgG control was performed. Staining was visualized using confocal microscopy.
AXIIR is expressed by MM cells and CD138+ cells from MM patients. (A) RNA was extracted from CD138+ cells from MM patients (nos. 1, 2) and the MM1.S cell line. cDNA was reverse-transcribed and RT-PCR was performed with primers specific to AXIIR and GAPDH. (B) Total protein (20 μg) was extracted from MM cell lines and analyzed for AXIIR expression (AXIIR mouse Ab) by Western blotting. β-actin was used as a loading control. (C) MM1.S cells were incubated with 1 μg/mL bio-AXII alone or in the presence of either 100-fold molar excess unlabeled AXII (unlabAXII) or 100-fold excess annexin V (AXV). A secondary only control with avidin-FITC was included in the experiment. (D) CD138+ cells from MM patients (n = 4) were incubated with 1 μg/mL bio-AXII and after extensive washing were incubated with avidin-FITC. Staining was visualized using confocal microscopy. (E) MM1.S cells were incubated with AXIIR Ab (rabbit) at a 1:200 dilution. After extensive washing, the MM1.S cells were then incubated with Alexa Fluor 488. A set of experiments with an IgG control was performed. Staining was visualized using confocal microscopy.
AXII is involved in adhesion of MM cells to BM cells
To determine whether MM cells adhere to AXII, tissue-culture plates were coated with AXII or BSA at different concentrations. The MM1.S cells were then added to the plates. MM1.S cells bound significantly better to AXII than to BSA (Figure 2A). Furthermore, MM1.S, RPMI8226, and H929 MM cells bound calvarial OBL from AXII+/+ mice significantly better compared with calvarial OBL from AXII−/− mice (Figure 2B). In addition, an anti-AXII or -AXIIR Ab blocked adhesion of MM1.S cells to the calvarial OBL (Figure 2C-D). These results demonstrate that AXII and AXIIR support adhesion of MM cells.
AXII and AXIIR are involved in the adhesion of MM cells. (A) Ninety-six–well plates were coated with the indicated concentrations (μg/mL) of AXII or BSA by incubating overnight at 4°C. The next day, MM1.S cells were added to AXII or BSA-coated plates and the percentage of change in binding of MM1.S cells to the AXII or BSA-coated plates was assessed relative to uncoated plates. (B) Calvarial OBL (104) from AXII+/+ or AXII−/− mice were plated in 96-well plates in 100 μL of α-MEM containing 10% FCS and incubated overnight at 37°C. The next day, cells were washed with PBS and fluorescent-labeled MM cells were added to the OBL. The plates were centrifuged at 52g for 5 minutes and placed in the dark for 15 minutes. The background fluorescence was measured with a plate reader. The cells were washed with PBS and read again to determine the percentage of adherence. (C) Calvarial OBL (104) from AXII+/+ mice were plated in 96-well plates in 100 μL of α-MEM containing 10% FCS and incubated overnight at 37°C. The next day, cells were washed with PBS and fluorescent-labeled MM cells were added to the OBL. Control IgG and anti-AXII were added to some of the wells. The plates were centrifuged at 52g for 5 minutes and placed in the dark for 15 minutes. The background fluorescence was measured with a plate reader. The cells were washed with PBS and read again to determine the percentage adherence. (D) Forty-eight– well plates were coated with AXII (1 μg/mL) by incubating plates overnight at 4°C. The next day, MM1.S cells were added to the plate and incubated at 37°C for 2 hours. AXIIR Ab (rabbit) or a control IgG (1 μg/mL) was included in a set of wells. At the end of 2 hours, the nonadherent MM1.S cells were counted. (*P < .05; **P < .01; ***P < .001).
AXII and AXIIR are involved in the adhesion of MM cells. (A) Ninety-six–well plates were coated with the indicated concentrations (μg/mL) of AXII or BSA by incubating overnight at 4°C. The next day, MM1.S cells were added to AXII or BSA-coated plates and the percentage of change in binding of MM1.S cells to the AXII or BSA-coated plates was assessed relative to uncoated plates. (B) Calvarial OBL (104) from AXII+/+ or AXII−/− mice were plated in 96-well plates in 100 μL of α-MEM containing 10% FCS and incubated overnight at 37°C. The next day, cells were washed with PBS and fluorescent-labeled MM cells were added to the OBL. The plates were centrifuged at 52g for 5 minutes and placed in the dark for 15 minutes. The background fluorescence was measured with a plate reader. The cells were washed with PBS and read again to determine the percentage of adherence. (C) Calvarial OBL (104) from AXII+/+ mice were plated in 96-well plates in 100 μL of α-MEM containing 10% FCS and incubated overnight at 37°C. The next day, cells were washed with PBS and fluorescent-labeled MM cells were added to the OBL. Control IgG and anti-AXII were added to some of the wells. The plates were centrifuged at 52g for 5 minutes and placed in the dark for 15 minutes. The background fluorescence was measured with a plate reader. The cells were washed with PBS and read again to determine the percentage adherence. (D) Forty-eight– well plates were coated with AXII (1 μg/mL) by incubating plates overnight at 4°C. The next day, MM1.S cells were added to the plate and incubated at 37°C for 2 hours. AXIIR Ab (rabbit) or a control IgG (1 μg/mL) was included in a set of wells. At the end of 2 hours, the nonadherent MM1.S cells were counted. (*P < .05; **P < .01; ***P < .001).
AXIIR expressed by MM cells is involved in adhesion to BM cells
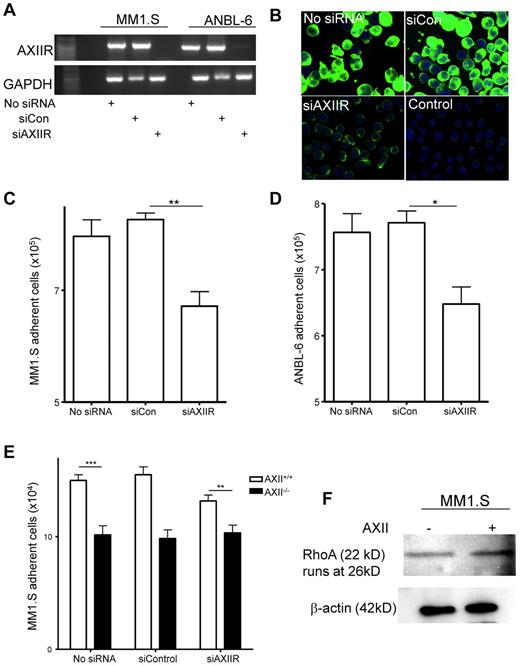
To determine whether AXIIR is involved in adhesion of MM cells to stromal cells, AXIIR was transiently knocked-down in MM1.S cells using siAXIIR RNA. The extent of AXIIR knockdown was determined by conventional PCR (Figure 3A). AXIIR knockdown was confirmed by real-time RT-PCR and ranged between 55% and 63% in the MM1.S and ANBL-6 cells in all experiments (data not shown). To determine whether the siAXIIR also decreased AXIIR surface expression, untreated MM1.S cells or MM1.SsiAXIIR and MM1.SsiControl cells were incubated with bio-AXII. Binding of bio-AXII was dramatically reduced in MM1.SsiAXIIR cells (Figure 3B). Furthermore, the adherence of MM1.SsiAXIIR cells and ANBL.6siAXIIR cells, a stromal cell–dependent MM cell line, to human KM101 stromal cells was significantly decreased (Figure 3C-D) demonstrating MM-expressed AXIIRs role in MM cell adhesion to stromal cells.
AXIIR on MM cells plays a role in adhesion of MM cells to BM stromal cells. (A) MM cells, MM1.S, and ANBL.6 were transfected with either siControl (siCon) or siAXIIR according to the manufacturer's protocol. RNA was prepared from untransfected MM cells (No siRNA) or MM cells transfected with either siControl (siCon) or siAXIIR. cDNA was reverse-transcribed and RT-PCR was performed. We observed between a 55% and 63% reduction in the level of AXIIR mRNA in MMsiAXIIR cells compared with MMsiControl cells in all the following experiments. (B) Untransfected MM1.S, MM1.SsiAXIIR and MM1.SsiControl cells were incubated with 1 μg/mL bio-AXII and after extensive washing were incubated with avidin-FITC. A secondary only control with avidin-FITC was included in the experiment (Control). Staining was visualized using confocal microscopy. (C-D) KM101 cells were seeded in 12-well plates and incubated at 37°C overnight. Untransfected MM cells, MMsiAXIIR, and MMsiControl cells (106) were added to the KM101 cells for 3 hours at 37°C; (C) MM1.S and (D) ANBL.6. Nonadherent MM cells were removed after being washed 3 times with PBS, counted, and used to calculate the number of adherent cells. MM1.SsiAXIIR cells adhered significantly less compared with MMsiControl cells. (E) Stromal cells (5 × 104) from either AXII+/+ or AXII−/− were plated in 24-well plates, and incubated overnight at 37°C. The next day, MM1.S, MM1.SsiAXIIR, and MM1.SsiControl cells (2.5 × 105) were added to the stromal cells and incubated for 6 hours at 37°C. Nonadherent MM cells were removed after washing 3 times with PBS and counted. Adhesion between AXII−/− and MMsiAXIIR cells compared with AXII+/+ and MM1.S cells was reduced significantly. (F) MM1.S cells (1 × 106) were incubated with AXII (1 μg/mL) for 48 hours at 37°C. At the end of 48 hours, total protein was collected and analyzed for RhoA expression by Western blotting. β-actin was used as a loading control. (*P < .05; **P < .01; ***P < .001)
AXIIR on MM cells plays a role in adhesion of MM cells to BM stromal cells. (A) MM cells, MM1.S, and ANBL.6 were transfected with either siControl (siCon) or siAXIIR according to the manufacturer's protocol. RNA was prepared from untransfected MM cells (No siRNA) or MM cells transfected with either siControl (siCon) or siAXIIR. cDNA was reverse-transcribed and RT-PCR was performed. We observed between a 55% and 63% reduction in the level of AXIIR mRNA in MMsiAXIIR cells compared with MMsiControl cells in all the following experiments. (B) Untransfected MM1.S, MM1.SsiAXIIR and MM1.SsiControl cells were incubated with 1 μg/mL bio-AXII and after extensive washing were incubated with avidin-FITC. A secondary only control with avidin-FITC was included in the experiment (Control). Staining was visualized using confocal microscopy. (C-D) KM101 cells were seeded in 12-well plates and incubated at 37°C overnight. Untransfected MM cells, MMsiAXIIR, and MMsiControl cells (106) were added to the KM101 cells for 3 hours at 37°C; (C) MM1.S and (D) ANBL.6. Nonadherent MM cells were removed after being washed 3 times with PBS, counted, and used to calculate the number of adherent cells. MM1.SsiAXIIR cells adhered significantly less compared with MMsiControl cells. (E) Stromal cells (5 × 104) from either AXII+/+ or AXII−/− were plated in 24-well plates, and incubated overnight at 37°C. The next day, MM1.S, MM1.SsiAXIIR, and MM1.SsiControl cells (2.5 × 105) were added to the stromal cells and incubated for 6 hours at 37°C. Nonadherent MM cells were removed after washing 3 times with PBS and counted. Adhesion between AXII−/− and MMsiAXIIR cells compared with AXII+/+ and MM1.S cells was reduced significantly. (F) MM1.S cells (1 × 106) were incubated with AXII (1 μg/mL) for 48 hours at 37°C. At the end of 48 hours, total protein was collected and analyzed for RhoA expression by Western blotting. β-actin was used as a loading control. (*P < .05; **P < .01; ***P < .001)
We then performed adhesion assays using BMSCs from either AXII+/+ or AXII−/− mice with MM1.SsiAXIIR cells. Like the KM101 cells, MM1.SsiAXIIR cell binding to AXII+/+ BMSCs was significantly lower than MM1.SsiControl cells (Figure 3E). As in Figure 2B, MM1.S cell binding to AXII−/− BMSCs was significantly less compared with AXII+/+ BMSCs (Figure 3E). Because AXII and AXIIR mediate adhesion of MM cells to OBL/stromal cells, we determined the adhesion molecules stimulated by AXII in MM1.S cells. RhoA was up-regulated by AXII in MM1.S cells (Figure 3F).
AXII stimulates growth of MM cells
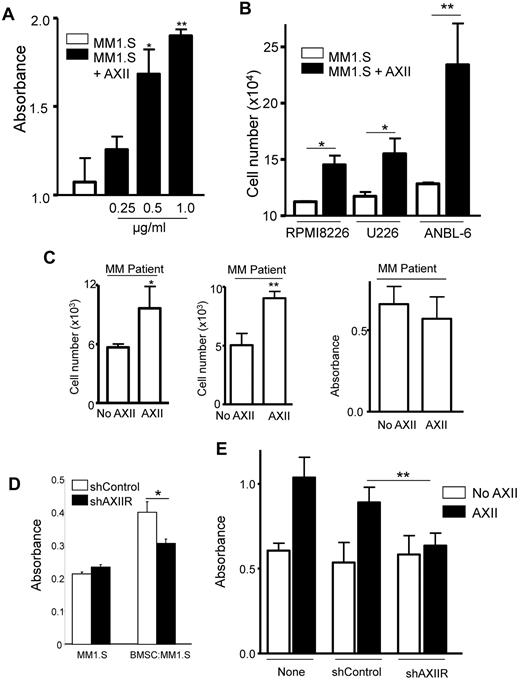
To determine whether AXII stimulated the growth of MM cells, MM1.S cells were incubated with increasing concentrations of AXII. MM1.S cell growth was dose-dependently increased by AXII (Figure 4A). AXII also increased the growth of other MM cell lines, including RPMI8226, U266, and ANBL-6 (Figure 4B). Importantly, AXII significantly stimulated the growth of CD138+ cells from MM patients in 2 of 3 patients (Figure 4C). Furthermore, proliferation of MM1.SshAXIIR cells cocultured with BMSC from AXII+/+ mice was significantly reduced compared with MM1.SshControl cells (Figure 4D). In addition, AXII-mediated proliferation of MM1.SshAXIIR cells was dramatically reduced compared with MM1.SshControl cells (Figure 4E). These results demonstrate that AXII stimulates MM1.S proliferation via AXIIR.
AXII-stimulated ERK1/2 and AKT phosphorylation and growth of MM1.S cells. (A) MM1.S cells were incubated with the indicated concentrations (μg/mL) of AXII as described in “Phosphorylation experiment” and cell growth was analyzed using a BrdU kit (Roche). (B) RPMI8226, U266, and ANBL-6 cells were incubated with the 1 μg/mL AXII as described in “Phosphorylation experiment” and cell growth was analyzed. (C) CD138+ cells (5 × 103) from MM patients were stimulated with AXII (1 μg/mL) and cultured for 72 hours. (D) BMSCs from AXII+/+ mice were cocultured with MM1.S cells for 48 hours. At the end of 48 hours, the MM1.S cells were separated and analyzed for proliferation as described in “Phosphorylation experiment.” (E) AXII was added to either MM1.S cells, MM1.SshAXIIR, or MM1.SshControl cells and cell growth was determined. (*P < .05; **P < .01; ***P < .001).
AXII-stimulated ERK1/2 and AKT phosphorylation and growth of MM1.S cells. (A) MM1.S cells were incubated with the indicated concentrations (μg/mL) of AXII as described in “Phosphorylation experiment” and cell growth was analyzed using a BrdU kit (Roche). (B) RPMI8226, U266, and ANBL-6 cells were incubated with the 1 μg/mL AXII as described in “Phosphorylation experiment” and cell growth was analyzed. (C) CD138+ cells (5 × 103) from MM patients were stimulated with AXII (1 μg/mL) and cultured for 72 hours. (D) BMSCs from AXII+/+ mice were cocultured with MM1.S cells for 48 hours. At the end of 48 hours, the MM1.S cells were separated and analyzed for proliferation as described in “Phosphorylation experiment.” (E) AXII was added to either MM1.S cells, MM1.SshAXIIR, or MM1.SshControl cells and cell growth was determined. (*P < .05; **P < .01; ***P < .001).
AXII activates ERK1/2 and AKT in MM1.S cells
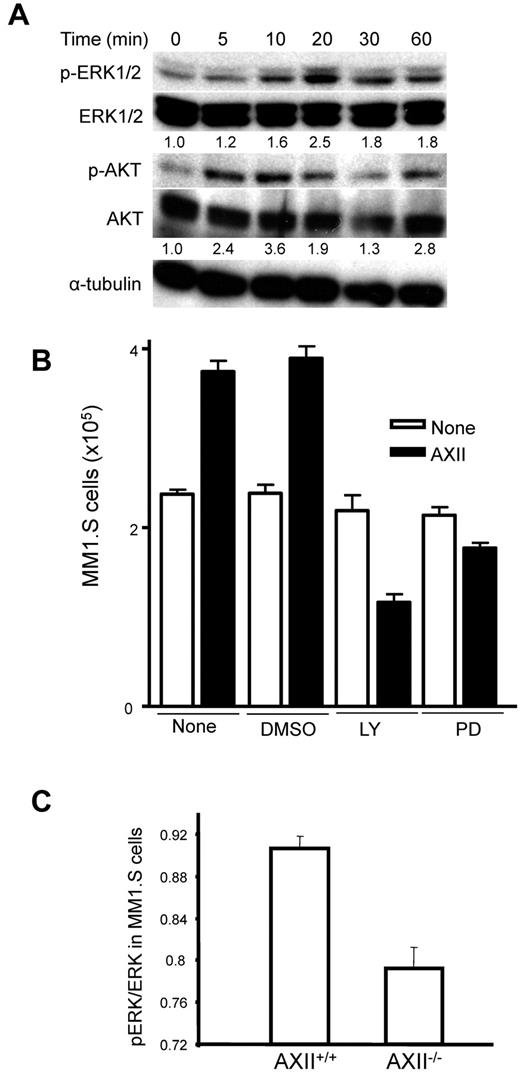
Previous studies demonstrated that AXII signals through ERK1/2 in PCa cells and marrow stromal cells.12,13 To determine whether AXII activated ERK1/2 or any other survival pathways in MM cells, MM1.S were incubated with AXII for different lengths of time, and the phosphorylation status of ERK1/2, p38-MAPK, AKT, and SAPK/JNK determined by Western blotting. AXII rapidly induced phosphorylation of ERK1/2 and AKT (Figure 5A) in MM cells. Furthermore, inhibitors of ERK1/2 or AKT, PD98059, and LY294002 that block phosphorylation of ERK1/2 and AKT, respectively, significantly reduced AXII-stimulated MM cell growth with LY294002 having a greater effect (Figure 5B). Finally, phosphorylation of ERK1/2 was decreased in MM cells cocultured with AXII−/− stromal cells compared with AXII+/+ stromal cells (Figure 5C).
AXII-stimulated growth of MM.1S cells either by AKT or ERK1/2 MAPK pathways. (A) MM1.S cells (2 × 106) were serum-starved for 16 hours, followed by addition of 1 μg/mL AXII for the indicated time periods. Total protein was extracted and analyzed by Western blotting. Ratios of phospho-ERK1/2 to total-ERK1/2 and phospho-AKT to total-AKT were determined by Image J. (B) MM1.S cells (2 × 105) were incubated with either LY294002 (LY) or PD98059 (PD) for 1 hour followed by stimulation with AXII. After 72 hours, the total number of MM1.S cells was counted. Both LY and PD inhibited the growth of AXII-stimulated MM1.S cells. (C) BMSCs from AXII+/+ and AXII−/− mice were cocultured with MM1.S cells for 48 hours. At the end of 48 hours, the MM1.S cells were separated and analyzed for ERK1/2 phosphorylation and total ERK1/2 levels as described in “Phosphorylation experiment.”
AXII-stimulated growth of MM.1S cells either by AKT or ERK1/2 MAPK pathways. (A) MM1.S cells (2 × 106) were serum-starved for 16 hours, followed by addition of 1 μg/mL AXII for the indicated time periods. Total protein was extracted and analyzed by Western blotting. Ratios of phospho-ERK1/2 to total-ERK1/2 and phospho-AKT to total-AKT were determined by Image J. (B) MM1.S cells (2 × 105) were incubated with either LY294002 (LY) or PD98059 (PD) for 1 hour followed by stimulation with AXII. After 72 hours, the total number of MM1.S cells was counted. Both LY and PD inhibited the growth of AXII-stimulated MM1.S cells. (C) BMSCs from AXII+/+ and AXII−/− mice were cocultured with MM1.S cells for 48 hours. At the end of 48 hours, the MM1.S cells were separated and analyzed for ERK1/2 phosphorylation and total ERK1/2 levels as described in “Phosphorylation experiment.”
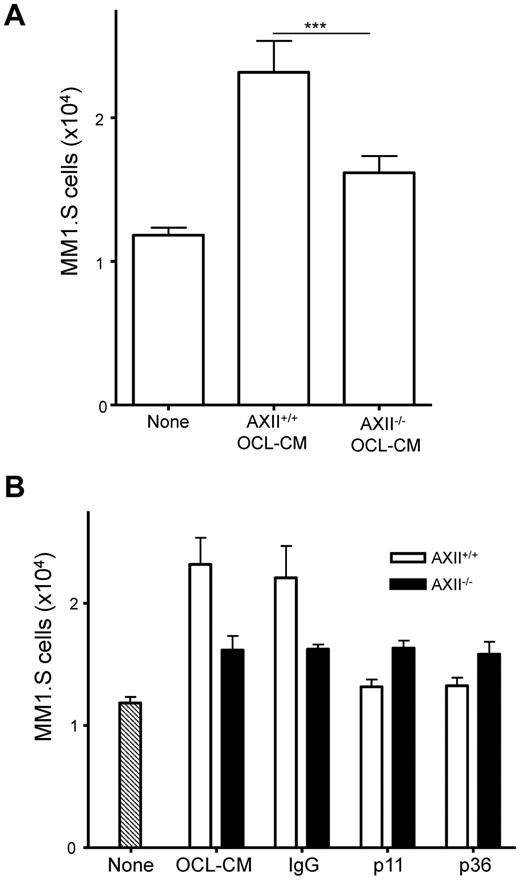
OCL-derived AXII stimulates the growth of MM1.S cells
We previously demonstrated that OCL secrete AXII and can induce OCL formation.13 To determine whether AXII produced by OCL contributed to the growth of MM1.S cells, MM1.S cells were incubated in the presence of either AXII+/+ or AXII−/− OCL-CM. AXII+/+ OCL-CM stimulated the growth of MM1.S cells (Figure 6A). However, stimulation of MM cells growth by AXII−/− OCL-CM was significantly reduced compared with CM from AXII+/+ OCL. Importantly, addition of anti-AXII Abs, either p11 or p36, significantly reduced MM cell growth stimulated by AXII+/+ OCL-CM, but had no effect on MM cell growth stimulated by AXII−/− OCL-CM (Figure 6B). Furthermore, addition of anti-AXII to MM cells alone did not decrease MM cell growth demonstrating anti-AXII was not toxic to MM cell growth (data not shown). These results demonstrate that OCL-derived AXII was stimulating MM cell growth and that the effects of AXII on MM cell growth are paracrine in nature.
MM1.S cell growth stimulated with OCL-CM is blocked by an anti-AXII Ab. MM1.S cells were cultured in the presence of AXII+/+ OCL-CM or AXII−/− OCL-CM. (A) MM1.S cells were cultured for 72 hours. After 72 hours, the number of viable MM1.S cells was counted. (B) Anti-p11 or anti-p36 Abs were added to the MM1.S cells that were stimulated with either AXII+/+ OCL-CM or AXII−/− OCL-CM.
MM1.S cell growth stimulated with OCL-CM is blocked by an anti-AXII Ab. MM1.S cells were cultured in the presence of AXII+/+ OCL-CM or AXII−/− OCL-CM. (A) MM1.S cells were cultured for 72 hours. After 72 hours, the number of viable MM1.S cells was counted. (B) Anti-p11 or anti-p36 Abs were added to the MM1.S cells that were stimulated with either AXII+/+ OCL-CM or AXII−/− OCL-CM.
Discussion
The BM provides a conducive environment for MM cells to adhere, lodge, and grow.3 In this study, we demonstrate that AXIIR is expressed by MM cell lines and CD138+ cells from 8 of 8 MM patients. Furthermore, the interaction between AXIIR and its ligand, AXII, supports MM cell adhesion and growth. Our results demonstrating that AXII expressed by stromal cell/OBL is involved in adhesion of MM cells to stromal cells is consistent with previous reports demonstrating a role for AXII in the adhesion of HSC to the BM OBL.4,12 Furthermore, our study shows that MM expressed AXIIR supports adhesion of MM cells to stromal cells through AXII, similar to the role of AXII/AXIIR axis in supporting adhesion of PCa cells to the BM.12 Expression of RhoA, a molecule that induces adhesion of cancer cells,17 was up-regulated in MM1.S cells in response to AXII stimulation. These results suggest that AXII stimulates adhesion of MM1.S cells through the RhoA pathway, and that MM cells use the AXII/AXIIR axis to localize and grow in the BM.
Functional assays demonstrated that AXII stimulated the growth of MM cell lines and CD138+ cells from MM patients, and that the AXII-mediated growth of MM cells was through the activation of ERK1/2 and AKT. Both ERK1/2 and AKT activation have been previously shown to regulate MM cell growth.18 Stimulation of ERK1/2 by AXII in MM cells is consistent with previous studies that demonstrated that AXII stimulated ERK1/2 phosphorylation.12,13 However, in MM cells, AXII also stimulated AKT, a crucial survival pathway. Thus, MM cell growth in the BM microenvironment could be further augmented by the effects of AXII. Blocking of ERK1/2 and AKT phosphorylation inhibited AXII-stimulated MM cell growth to much lower levels compared with unstimulated MM cells. These results suggest that AXII may also activate a negative feedback pathway, which in the presence of ERK1/2 and AKT inhibitors, has no counterbalance and decreases MM cell survival relative to unstimulated cells.
Previous studies report that AXII is up-regulated in MM,15,16 and that MM-derived AXII increased proliferation of MM cell lines, U266 and RPMI8226, suggesting that in these MM cells, the MM-derived AXII acts in an autocrine manner to support proliferation of the MM cells. However, in our studies, addition of anti-AXII to MM1.S cells did not decrease MM cell growth in the absence of added AXII, even though AXII is expressed by these MM cell lines (supplemental Figure 1). Thus, the predominant effects of AXII and AXIIR interactions on MM cell growth appear to be paracrine.
In preliminary experiments, we injected either MM1.SshAXIIR or MM1.SshControl cells which had a 50% decrease in AXIIR expression into the tibias of SCID mice to assess the effects of blocking the AXII/AXIIR axis in vivo. However, the growth of MM1.SshAXIIR or MM1.SshControl cells in vivo was not changed significantly (data not shown). These results suggests that a 40% knockdown of AXIIR is not sufficient to block the growth-stimulating effects of AXII in vivo or that other MM growth factors can compensate for loss of AXII-AXIIR interactions.
In MM, there is an imbalance in normal bone remodeling with very high OCL activity but very little or no OBL activity. Our previous studies have shown that OCL secrete AXII and that AXII stimulates OCL formation.19 In the current study, we show that OCL-CM from AXII−/− OCL or an anti-AXII Ab blocked the stimulatory effects of OCL-CM on the growth of MM cells. Thus, AXII secreted by OCL and stromal cells may act by 3 mechanisms in MM: (1) AXII enhances the growth of MM cells in the BM by binding to AXIIR on the MM cells and activating the ERK1/2 and AKT pathways; (2) soluble AXII from OCL could disrupt the existing AXII/AXIIR interactions between the stromal cells/OBL and MM cells and potentially mobilize MM cells from the endosteal niche to the peripheral blood; and (3) AXII also can induce stromal cell production of RANKL which stimulates OCL formation, further amplifying the role of AXII in MM. Future studies could determine whether mobilization of MM cells within the BM using an anti-AXII or anti-AXIIR Ab in an in vivo model would provide further insight into the role of the AXII/AXIIR axis in this process.
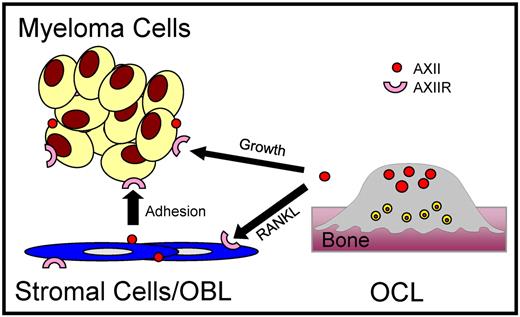
Based on the contribution of AXII expressed by stromal cell/OBL or OCL and its interaction with AXIIR on the MM cells in supporting MM adhesion and growth, we propose that the AXII/AXIIR axis contributes to the vicious cycle of MM cell growth and bone destruction in the BM (Figure 7). In this model, AXII expressed by stromal cells/OBL or secreted by OCL enhances adhesion and growth of MM cells by interacting with AXIIR and activating ERK1/2 and AKT. In turn, MM cells secrete OCL activating factors which increase OCL numbers that can secrete more AXII to further increase MM cell growth. Thus, the interactions between AXII and AXIIR in the myeloma BM microenvironment may potentially regulate localization and growth of MM cells in the BM.
Function of AXII/AXIIR axis in MM cell adhesion and growth in the BM. Interactions between MM-derived AXIIR and OBL/stromal cell–derived AXII MM support the adhesion of MM cells in the BM. As OCL formation increases in response to OCL stimulatory factors produced or induced by MM cells, the OCLs release soluble AXII, which binds AXIIR and induces the growth of MM cells. The AXII/AXIIR axis is a crucial component of the BM microenvironment in supporting MM cells in the BM and could potentially support the homing and lodgment of MM cells in the BM.
Function of AXII/AXIIR axis in MM cell adhesion and growth in the BM. Interactions between MM-derived AXIIR and OBL/stromal cell–derived AXII MM support the adhesion of MM cells in the BM. As OCL formation increases in response to OCL stimulatory factors produced or induced by MM cells, the OCLs release soluble AXII, which binds AXIIR and induces the growth of MM cells. The AXII/AXIIR axis is a crucial component of the BM microenvironment in supporting MM cells in the BM and could potentially support the homing and lodgment of MM cells in the BM.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Judith L. Anderson for excellent technical support and Donna Gaspich for excellent secretarial assistance, Leah Fuhrman for excellent technical assistance, Dr Richard J. Bodnar (Department of Pathology, University of Pittsburgh) for assistance with confocal imaging, and JinKoo Kim and Younghun Jung (University of Michigan) for providing technical assistance. They also thank the VA Pittsburgh Healthcare System, Research and Development for use of the facilities.
This work was supported by National Institutes of Health grant R21 CA141426.
National Institutes of Health
Authorship
Contribution: S.D. designed and performed most of the experiments and wrote the manuscript; N.K. assisted with phosphorylation and OCL experiments; Y.S. performed the adhesion assay between OBL and MM cells; S.D., Y.S., and J.J. performed the in vivo experiments; R.T. provided the AXII−/− mice and the si/shAXIIR and si/shControl RNA; D.L.G. helped with designing experiments and writing the manuscript; and G.D.R. designed experiments and wrote the manuscript.
Conflict-of-interest disclosure: G.D.R. is a consultant for Amgen, Celgene, Onyx, and Millennium. The remaining authors declare no competing financial interests.
Correspondence: G. David Roodman, MD, PhD, Professor of Medicine, UPMC, University of Pittsburgh School of Medicine, VA Healthcare System, R & D 151U, University Dr C, Pittsburgh, PA 15240; e-mail: roodmangd@upmc.edu.