Abstract
A molecular feature of Sézary syndrome (SS) is the abnormal expression of T-plastin by malignant T cells. Herein, we investigated the molecular mechanisms involved in T-plastin synthesis and the functions of this actin-binding protein, with a special interest in chemoresistance and migration. We confirm the specific expression of T-plastin in peripheral blood lymphocytes (PBLs) from SS patients and its total absence in PBLs from patients with mycosis fungoides, inflammatory cutaneous or hematologic diseases, and from healthy volunteers. Only 3 of 4 SS patients did constitutively express T-plastin. To assess whether T-plastin expression was inducible, T-plastin–negative PBLs were stimulated by phorbol 12-myristate 13-acetate and ionomycin. Our results demonstrate that T-plastin synthesis was induced in negative PBLs from SS patients, other studied patients, and healthy volunteers. Both constitutive and calcium-induced T-plastin expression was down-regulated by calcineurin inhibitors and involved nuclear factor of activated T cells transcription pathway. Constitutive T-plastin expression in SS was associated with resistance to etoposide-induced apoptosis and cell migration toward chemokines (TARC/CCL17, IP-10). In conclusion, T-plastin is a marker restricted to malignant lymphocytes from SS patients and plays a role for cell survival and migration. This opens new strategies for the treatment of SS advanced stages.
Introduction
Cutaneous T-cell lymphomas (CTCLs) represent a heterogeneous group of non-Hodgkin lymphomas that affect the skin as the primary site, among which mycosis fungoides (MF) and the leukemic variant Sézary syndrome (SS) are the most common subtypes.1 Both MF and SS are characterized by a proliferation of T cells with usually mature phenotype CD3+CD4+CD45RO+. MF presents a limited skin invasion with an indolent clinical course, whereas its leukemic variant has an aggressive evolution associated with poor prognosis.2 SS is characterized by erythroderma, generalized lymphadenopathy, and the presence of atypical T cells with cerebriform nuclei called “Sézary cells”3 in the skin, lymph node, and peripheral blood. In biologic behavior, the tumor cells have abnormal epidermal tropism with expression of CLA (cutaneous lymphocyte antigen) and several chemokine receptors4 (CCR4,5,6 CCR10,7,8 CCR7,9 CXCR3, and CXCR410 ). They present frequent loss of certain cell-surface antigens, such as CD7 and CD26,11,12 monoclonal rearrangement of the T-cell receptor (TCR) gene, and aberrant but nonuniform chromosomal abnormalities.13-16 We reported the expression of NK receptors, such as KIR3DL2/CD158k on SS T cells from most patients who allow distinguishing phenotypically reactive malignant from normal lymphocytes.17,18
Nevertheless, the detection of early blood involvement using cytologic criteria can be difficult, because Sézary-like cells may be found in healthy individuals and unrelated diseases. Among the last decade, several studies including transcriptome analysis reported the identification of specific molecular markers of Sézary cells. Here, we focus on one of them, T-plastin (PLS3), claimed as a specific marker for SS diagnostic.2,19-23
T-plastin is 1 of 3 isoforms of the widely conserved actin-bundling protein plastin (also called fimbrin) found in humans.24,25 Proteins of this family share the presence of an actin-binding domain called “ABD” (30 kDa) composed of 2 calponin-homology (CH) domains. Among the members of this family (actinin, filamin, spectrin, dystrophin, ABP-120), plastin is the only protein to bear 2 ABDs on the same polypeptide chain that forms the core of the molecule which allow crosslinking of actin filaments into tight bundles. There are also emerging evidences that plastin/fimbrin might have additional functions: control of actin dynamics via stabilization of microfilaments with no necessity of crosslink,26,27 participation to signal transduction through integrin signaling,28 and a role during bacterial entry.29 Fimbrin was reported to bind to vimentin, an intermediate filament protein, suggesting that it participates in mechanisms other than the simple crosslinking of actin microfilaments.30 Moreover, T-plastin expression has been reported as increased in tumor cells that were resistant to cisplatin and reducing its expression correlated with increased cell sensitivity to cisplatin.31
T-plastin gene expression is up-regulated in CTCL cells from SS patients and it is usually not expressed in normal T lymphocytes,2,19-23,32 except in a recent study that observed T-plastin transcription in PBLs from less than 5% healthy individuals.33 However, the full biologic and clinical value of such aberrant T-plastin mRNA and protein expression in Sezary malignant cells remains to be clearly defined. To date, the molecular mechanisms leading to the transcription of T-plastin and its functions in tumor lymphocytes of CTCL patients remain unknown.
In this study, we confirmed that more than 75% of the patients with Sézary syndrome expressed T-plastin compared with a wide range of hemopathies and healthy donors that do not at all express this marker in our study. Interestingly, we demonstrated that T-plastin expression was enhanced or induced by calcium entry after phorbol 12-myristate 13-acetate (PMA) and ionomycin stimulation of T lymphocytes from SS patients, as well as from patients with MF, inflammatory cutaneous or hematologic diseases, and from healthy volunteers, although to a lesser extent. It is well known that sustained Ca2+ entry leads to activation of calcineurin/nuclear factor of activated T cells (NFAT) pathways.34,35 The present data showed that down-regulation of NFAT activation in CTCL cell lines and PBLs from SS patients by specific NFAT siRNA transfection or calcineurin A (CaN) inhibitors induced an inhibition of T-plastin synthesis.
We further investigated the role of T-plastin expression in Sézary malignant T cells and focused on 2 functional properties including crosslink of actin filaments24-27 and the ability to confer tumor cell resistance to apoptosis.31 Previous data showed that CTCL cells from patients with SS were resistant to apoptosis induced by antineoplastic agents.36 However, the mechanisms responsible for SS malignant-cell resistance remain not well defined and might involve constitutive activation of transcription factors such as nuclear factor (NF)–κB pathway.37
Our present results clearly show that T-plastin expression confers resistance to drug-induced apoptosis and that reducing T-plastin expression by specific siRNA or CaN inhibitor favors an increase in apoptosis induction.
Evidence is also given that T-plastin may promote migration in response to some chemokines that have been shown to be overexpressed in serum and tumor skin of SS patients,4,38 such as thymus and activation-regulated chemokine (TARC/CCL17) or inducible protein (IP-10/CXCL10).
To ascertain our data, stable T-plastin–expressing clones were established by transfection of PLS3-expressing plasmid in Jurkat T cells that do not express constitutive T-plastin.
Methods
Reagents
The calcineurin inhibitor FK506 was purchased from Calbiochem, ionomycin (Iono), PMA and ethylene glycol tetraacetic acid (EGTA) from Sigma-Aldrich, small interfering RNA (siRNA) oligos for PLS3 from Ambion (Applied Biosystems), siRNA for NFAT1 and NFAT2 from Thermo Scientific Dharmacon, and TARC and IP-10 from PeproTech.
Patients
Peripheral blood samples from a total of 94 patients (37 females, 57 males) affected by SS (mean age, 71.7 ± 13.9) and 34 patients (16 females, 18 males) with MF (69.6 ± 12.5) were from the Dermatology Department (Hôpital Saint-Louis, Paris and Hôpital Avicenne, Bobigny). Diagnosis of SS and MF was based on clinical, histologic, and biologic criteria.1,39 According to the revised staging and classification criteria for MF and SS including TNMB (tumor-node-metastasis-blood) status,39 patients with SS were characterized by a staging II (5/94), III (29/94) or IV (60/94), and patients with MF by a staging I (19/34) or II (15/34).
Routine cell immuno-phenotyping was performed for characterization of tumor cells (Vβ expression, CD3, CD4, CD8, CD45RA, CD7, CD26), completed with clonality analysis by polymerase chain reaction (PCR) VγJγ, diversity of the TCR repertoire (Lab Immunology, Pr Toubert, Hôpital Saint-Louis) and genetic variation in the third complementarity-determining region (CDR3), region of the TCR Vβ chain using immunoscope technology.
For comparative analysis, blood samples were obtained from patients with hyper-eosinophilic syndrome (n = 8, Dermatology Department, Pr Lionel Prin, Lille), undetermined erythroderma (n = 10), or hematopoietic malignancies (3 patients with T-cell lymphoma [1 CD8+, 2 CD30+], 2 mantle cell lymphoma, 4 T-cell acute lymphoblastic leukemia [T-ALL], 5 B-cell chronic lymphoid leukemia [B-CLL; Pole of Hematology, Hôpital Saint-Louis]), autoimmune diseases (6 scleroderma), as well as 78 healthy controls from Etablissement Français du Sang.
All patients analyzed in this study were enrolled in clinical protocols approved by the institutional ethics committee (Comité de Protection des Personnes). Informed consent was provided in accordance with the Declaration of Helsinki.
Cells and cell lines
Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque density gradient centrifugation and resuspended in RPMI1640 Glutamax-I medium, supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, 100 μg/mL streptomycin, and 10mM HEPES (N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid; all from Gibco; complete medium) at a 5 × 105 cells/mL final concentration. Cells were incubated at 37°C in a 5%CO2 humidified atmosphere for 2 hours to allow adhesion of mononuclear cells. The nonadherent lymphocyte fraction (PBL) was collected and maintained in culture at 5 × 105 cells/mL in complete medium at 37°C with 5% CO2.
The Sézary cell line HuT-78 and the leukemia cell line Jurkat (E6.1) were purchased from the European Collection of Animal Cell Cultures (ECACC). SeAx cell line established from a SS patient was kindly provided by Dr Keld Kaltoft (University of Aarhus, Denmark).40 All CTCL cell lines were grown at 37°C with 5% CO2 at 2 × 105 cells/mL in complete medium. In the case of cultures of stably transfected Jurkat cells, complete medium was supplemented with 1 μg/mL G418 (Sigma-Aldrich).
For activation experiments, PBLs and cell lines were cultured in the presence of PMA (10 ng/mL) and/or ionomycin (1μM) during 16 hours. In some experiments, CaN inhibitor FK506 (10μM) or calcium chelator EGTA (200μM) were added during 16 hours at least.
Gene transfer by nucleofection
Stable clones expressing T-plastin were established by transfection of Jurkat cells with PGL3-plasmid expressing PLS3 kindly provided by Pr Wirth33 using the Human T Cell Nucleofector Technology, according to the manufacturer's instructions (Lonza). Immediately after transfection, pcDNA3.1 or PLS3-transfected Jurkat cells were maintained in culture in the presence of 2 μg/mL G418 to select stable clones. After 3 weeks of culture, T-plastin expression was assessed by immunoblotting. Single-cell suspensions were obtained by clonal dilution to further establish specific PLS3-positive or PLS3-negative clones.
Jurkat and CTCL cell lines were transiently transfected with siRNA by electroporation, using the Nucleofector. Briefly, cells were immediately suspended at 5 × 105 cells/mL in prewarmed RPMI complete medium after transfection, and cultured in 24-well microplates at 37°C for 24 hours before etoposide was added (or not). After 24 hours, cells were harvested, viability was determined by trypan blue exclusion and apoptosis was monitored as detailed thereafter.
RNA isolation and cDNA synthesis
RNA was isolated from whole PBMCs using RNeasy Mini Kit according to the manufacturer's instructions with One-Step DNAse I (QIAGEN). For cDNA synthesis, total RNA (1 μg) was used for reverse transcription (RT) with ThermoScript reverse transcriptase (Invitrogen) using oligo-dT primers.
Multiplex and quantitative real time-PCR analysis
The resulting first-strand cDNA was assayed by standard multiplex PCR using the QIAGEN multiplex PCR kit according to the manufacturer's instruction and, by quantitative real-time PCR (qRT-PCR) using SYBR Green PCR Core Reagents (ABI PRISM 7300 Real-time PCR System; Applied Biosystems) according to the manufacturer's instructions. Details concerning primers and qRT-PCR conditions are available in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Microscopic analysis
Cells were cytospined onto glass slides, fixed with paraformaldehyde (PFA) 3% for 20 minutes, permeabilized with 0.5% Triton X-100 for 10 minutes. Nonspecific protein binding was prevented by blocking the cells with 10% fetal bovine serum (FBS) in phosphate-buffered saline (PBS). Cells were stained with the appropriate antibody (1/50) for 1 hour at room temperature. After 3 washes with PBS, the slides were stained with the appropriated secondary antibody (1/100) for 1 hour and washed with PBS. Coverslips were applied with mounting medium with 4,6-diamidino-2-phenylindole (DAPI; Vectashield). The cells were visualized using a scanning “Axiovert”40C microscope (Carl Zeiss). Images were captured with a 60× oil objective using the appropriate filter sets. Digital images were obtained using the manufacturer's AxioVision Version 4.6.3.0 software.
Analysis of apoptosis
PBLs from SS patients, CTCL cell lines or stably pcDNA3.1/PLS3 Jurkat were incubated in the presence of 2 to 10μM etoposide (Dakota Pharm) for 24 hours and analyzed for apoptosis by mitochondrial membrane potential disruption ΔΨm analysis and annexinV/propidium iodide (PI) staining according to previously described protocols, using a FACSCalibur (BD Biosciences) and the Pro-CellQuest Version 5.1 software.37
Chemotaxis assay
In vitro migration assays were performed using modified Boyden chambers in 24-well plates with 8 μm-pore inserts (Becton Dickinson). Cells were starved in RPMI containing 0.5% bovine serum albumin (RPMI 0.5% bovine serum albumin [BSA]) for 16 hours, then 2.5 × 105 cells were plated in the upper chamber in migration medium (RPMI 0.5% BSA). The lower chamber contained migration medium supplemented or not with TARC (10 ng/mL) or IP-10 (50 ng/mL). The number of cells in the lower chamber was evaluated after 4 hours of incubation at 37°C by cell counting in the presence of trypan blue.
Immunoblot analysis
Cells (10 × 106) were lysed for 1 hour at 4°C in RIPA lysis buffer (Rockland Immunochemicals) supplemented with protease inhibitor cocktail (Sigma-Aldrich). After centrifugation (14 000 rpm, 15 minutes, 4°C), whole-cell protein extracts (25-50 μg) were loaded onto 8% polyacrylamide gels containing sodium dodecyl sulfate (SDS), subjected to electrophoresis, and transferred to nitrocellulose membrane. The blot was blocked with 5% nonfat milk for 1 hour and incubated overnight at 4°C with anti-PLS3 (1/500; Santa-Cruz), anti-NFAT1 or NFAT2 (1/500; Santa-Cruz). They were subsequentially exposed to anti–mouse (1/2500) antibody coupled to horseradish peroxidase (Amersham Pharmacia Biotech) for 1 hour. An enhanced chemiluminescent system (Amersham Pharmacia Biotech) was used for detection. Equal protein loading was confirmed by the use of monoclonal GAPDH or β-actin (Sigma-Aldrich). Densitometry was done using Imager FX System and analyzed using ImageJ Version 1.45 software.
Statistical analyses
Statistical significance was assessed using the 2-sided student t test. Differences were considered as significant at P < .05.
Results
T-plastin is a reliable specific marker expressed by malignant T cells from patients with SS
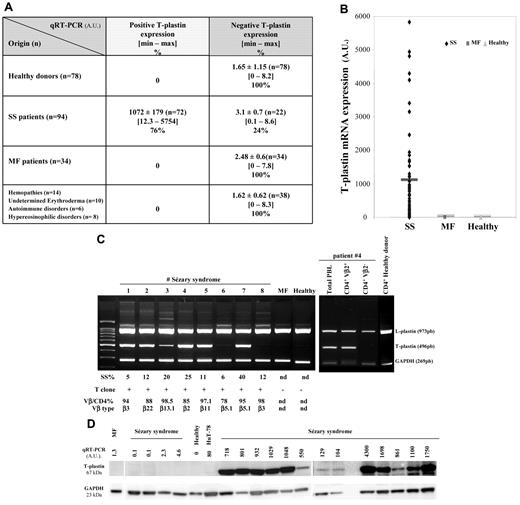
We initially studied the accuracy of T-plastin expression in a large cohort of 138 CTCL patients including 94 SS and 34 MF compared with 38 patients with other T-cell lymphoproliferative disorders (T-cell lymphoma CD8+, CD30+, T-ALL), other hemopathies (B-CLL, mantle cell lymphoma, hypereosinophilic syndrome), inflammatory undetermined erythroderma or autoimmune diseases (scleroderma), as well as to 78 healthy donors. Values of T-plastin mRNA expression from lymphocytes of patients and healthy donors obtained by qRT-PCR are presented in Figure 1A and B in arbitrary units (AU). Very low T-plastin levels, ranging from a minimum 0 to a maximum 8.2, with a mean value of 1.65 ± 1.15 (± SEM, n = 78), were detected in PBLs from the healthy population. A threshold of 95% significance was set at 10 and any value less than 10 was considered negative. The quantification of T-plastin transcript in PBLs from patients with MF, with undetermined erythroderma or with hematologic malignancies showed values equivalent to healthy population and were considered as negative for T-plastin mRNA expression (Figure 1A). Among the 94 patients with SS that were analyzed by qRT-PCR, 72 patients expressed a high rate of T-plastin transcript with a mean value of 1072 ± 179 (± SEM) and 22 patients did not express the transcript (3.1 ± 0.5; Figure 1A-B).
T-plastin mRNA and protein expression in lymphocytes from patients with SS, MF, various hemopathies or undetermined erythroderma and from healthy donors. Total mRNA was extracted from blood lymphocytes of patients with SS (n = 94), MF (n = 34), hemopathies, undetermined erythroderma and scleroderma (n = 38), and of healthy donors (n = 78), purified and used for qRT-PCR (A-D) or multiplex standard PCR (C). Results from qRT-PCR (A-D) are obtained as ΔCt values expressed in arbitrary units (AU) and they are presented as mean ± SEM in panel A, as individual data in panels B through D, and in panel B, with a dark line showing mean values for each set of data. Results in panel C are from multiplex standard PCR of PBLs from 8 SS patients, 1 MF patient, 1 healthy volunteer and for purified CD4+Vβ2+ and CD4+Vβ2− cells from SS patient no. 4 as well as purified CD4+ cells from 1 healthy donor. CD4+ cells were isolated by magnetic-activated cell sorting using the CD4 cell isolation kit (Miltenyi Biotec) and further Vβ2+ isolation using anti-TCR Vβ2 (Clone MPB2D5) from Beckman Coulter and magnetic dynabeads goat anti–mouse IgG. For each CTCL patient are given percentage of Sézary cells (SS%), detection of T cell clone by Vγ-Jγ PCR presented as positive (+) or negative (−), percentage of positive Vβ/CD4 subset and Vβ type obtained by immunophenotyping using flow cytometry (except immunoscope analysis for Vβ6.1). nd indicates not determined. In panel D, under T-plastin mRNA expression obtained by qRT-PCR is shown respective T-plastin protein expression from total extract of PBLs from SS patients (SS, n = 17), 1 MF patient, 1 healthy donor, and HuT-78 cells, as assessed by Western blotting. GAPDH (glyceraldehyde-3-phosphate dehydrogenase) expression is presented as a loading control.
T-plastin mRNA and protein expression in lymphocytes from patients with SS, MF, various hemopathies or undetermined erythroderma and from healthy donors. Total mRNA was extracted from blood lymphocytes of patients with SS (n = 94), MF (n = 34), hemopathies, undetermined erythroderma and scleroderma (n = 38), and of healthy donors (n = 78), purified and used for qRT-PCR (A-D) or multiplex standard PCR (C). Results from qRT-PCR (A-D) are obtained as ΔCt values expressed in arbitrary units (AU) and they are presented as mean ± SEM in panel A, as individual data in panels B through D, and in panel B, with a dark line showing mean values for each set of data. Results in panel C are from multiplex standard PCR of PBLs from 8 SS patients, 1 MF patient, 1 healthy volunteer and for purified CD4+Vβ2+ and CD4+Vβ2− cells from SS patient no. 4 as well as purified CD4+ cells from 1 healthy donor. CD4+ cells were isolated by magnetic-activated cell sorting using the CD4 cell isolation kit (Miltenyi Biotec) and further Vβ2+ isolation using anti-TCR Vβ2 (Clone MPB2D5) from Beckman Coulter and magnetic dynabeads goat anti–mouse IgG. For each CTCL patient are given percentage of Sézary cells (SS%), detection of T cell clone by Vγ-Jγ PCR presented as positive (+) or negative (−), percentage of positive Vβ/CD4 subset and Vβ type obtained by immunophenotyping using flow cytometry (except immunoscope analysis for Vβ6.1). nd indicates not determined. In panel D, under T-plastin mRNA expression obtained by qRT-PCR is shown respective T-plastin protein expression from total extract of PBLs from SS patients (SS, n = 17), 1 MF patient, 1 healthy donor, and HuT-78 cells, as assessed by Western blotting. GAPDH (glyceraldehyde-3-phosphate dehydrogenase) expression is presented as a loading control.
As shown by multiplex PCR analysis from 8 representative patients (Figure 1C), PBLs from 2/8 SS patients did not express T-plastin mRNA, although they did present L-plastin transcripts and exhibited a major circulating T-cell clone that was detected by VγJγ PCR, and the Sézary cell absolute number in the blood and TNMB status of the patients. In contrast to L-plastin, no T-plastin mRNA was detected by multiplex PCR in PBLs from MF patients and healthy donors, as shown for 1 representative respective case. Importantly, when protein expression for T-plastin was assessed by Western blot, it strictly correlated with T-plastin mRNA expression detected by qRT-PCR (Figure 1D).
To further demonstrate whether T-plastin was only expressed by malignant clones from SS patients, the cells were purified using specific Vβ isolation on CD4+ cell population and studied by qRT-PCR and standard multiplex PCR. As shown in Figure 1C for the representative patient no. 4 (right panel), only the clonal CD4+Vβ2+ fraction expressed T-plastin mRNA. This first set of results confirmed previously published data showing that T-plastin transcriptional expression is specific and restricted to tumor lymphocytes from patients with SS.2,19-23,32
Calcium induces T-plastin synthesis and calcineurin/NFAT pathway is involved in T-plastin regulation
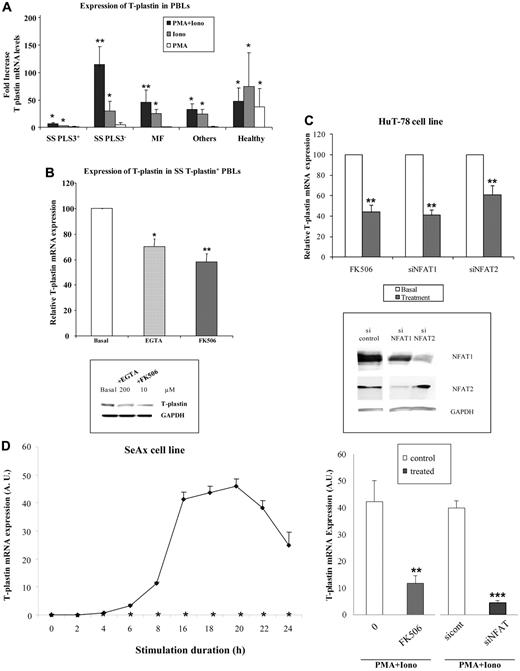
Cutaneous T-cell lymphomas are characterized by infiltration of activated T lymphocytes in the skin. Our present data and literature indicate that T-plastin is a specific marker of tumor T lymphocytes but this is true only for 3/4 patients with SS. To investigate whether T-plastin synthesis could be induced by cell activation, T-plastin– negative PBLs from SS patients, from patients with other hemopathies, and from healthy donors were stimulated with PMA and/or ionomycin to mimic TCR pathway activation. As shown in Figure 2A, activation of negative T-plastin PBLs from SS patients (SS PLS3−) by PMA and ionomycin combination resulted in a synergistic 115-fold increase in the transcription of T-plastin compared with unstimulated cells. Ionomycin, known as a potent calcium ionophore, induced a significant 30-fold increase in mRNA T-plastin levels, whereas PMA alone was not significantly efficient. Lymphocytes from SS patients, that constitutively expressed T-plastin (SS PLS3+), also showed a significant increase in T-plastin mRNA expression under stimulation with PMA and ionomycin, with a mean fold of 6.8 ± 2 (± SEM, n = 24) compared with constitutive levels.
T plastin expression is activated by calcium entry and down-regulated by calcineurin inhibitors and NFAT siRNA. (A) PBLs from patients with SS (n = 40), MF (n = 8), various hemopathies or undetermined erythroderma (others, n = 8), and from healthy donors (healthy, n = 16) were incubated with PMA and ionomycin combination (dark columns), ionomycin (gray columns), PMA (white columns), for 16 hours. For PBLs from patients with SS, 2 sets were distinguished, according to expression of constitutive T-plastin mRNA (SS PLS3+, n = 24) or not (SS PLS3−, n = 16). T-plastin mRNA expression was quantified by qRT-PCR. Results are presented as fold increase of T-plastin mRNA levels detected in stimulated cells relative to basal T-plastin mRNA levels detected in respective unstimulated cells. *P < .05, **P < .01, comparison of T-plastin mRNA levels from stimulated cells to T-plastin mRNA levels from respective nonstimulated cells. (B) Top panel: PBLs from patients with SS (n = 12) that constitutively expressed T-plastin mRNA were treated with EGTA (200μM) or FK-506 (10μM) or not treated (basal) for 16 hours. T-plastin mRNA expression was quantified by qRT-PCR. The constitutive T-plastin mRNA levels measured without treatment was reported to 100 to allow comparison between PBLs from SS patients. Results are presented as mean percentages (± SEM) of T-plastin mRNA levels obtained in the presence of EGTA (light gray bar) or FK506 (dark gray bar) relative to respective T-plastin levels detected in the absence of treatment (white bar). *P < .05, **P < .01, comparison of T-plastin mRNA levels from treated cells to T-plastin mRNA levels from basal nontreated cells. Bottom panel: PBLs from SS patients were not incubated (basal) or incubated with EGTA (200μM) or FK506 (10μM) for 16 hours. T-plastin protein expression was determined by immunoblot analysis with anti–T-plastin antibody and with GAPDH antibody to confirm equal loading. Results are representative of 3 independent experiments. (C) Top panel: HuT-78 cells constitutively expressed T-plastin mRNA. In a set of experiments, cells were preincubated with FK506 (10μM), or not for 16 hours before mRNA extraction. In another set of experiments, HuT-78 cells were transiently transfected with control siRNA (200 or 400nM), siRNA for NFAT1 (200nM), and NFAT2 (400nM) 24 hours before mRNA extraction. T-plastin mRNA expression was quantified by qRT-PCR and results are expressed in relative values of T-plastin expression of treated cells (gray) to respective basal nontreated cells (white) reported as 100 to allow experiment comparison (mean ± SEM, n = 6). **P < .01, comparison of T-plastin mRNA levels from treated cells to T-plastin mRNA levels from respective basal nontreated cells. Bottom panel: HuT-78 cells were transiently transfected with control siRNA (400nM), NFAT1 siRNA (200nM), or NFAT2 siRNA (400nM) 24 hours before total protein extraction was performed. NFAT1 and NFAT2 protein expression was determined by immunoblot analysis with anti-NFAT1 and anti-NFAT2 antibodies and with GAPDH antibody to confirm equal loading. Results are representative of 3 independent experiments. (D) Top panel: SeAx cells (0.2 × 106 cells/mL) were incubated (◊) or not (*) with PMA and ionomycin combination (PMA+Iono) over a 24-hour period (as triplicates) and collected for mRNA extraction at indicated times. T-plastin mRNA expression was quantified by qRT-PCR. Results are expressed in AUs and presented as mean ± SD (n = 3). In the absence of stimulation, SeAx cells did not express T-plastin mRNA (ΔCt values are less than 0.01 AU) whatever the collection time. Bottom panel: Cells were preincubated with FK-506 (10μM) for 1 hour before addition of PMA and ionomycin (PMA+Iono) for further 16 hours. In some experiments, SeAx cells were transiently transfected with control siRNA (200nM) or NFAT1 siRNA (200nM). Eight hours after, transfected cells were stimulated by addition of PMA and ionomycin (PMA+Iono) for 16 hours before mRNA extraction. T-plastin mRNA expression was quantified by qRT-PCR and results are expressed in arbitrary units (AU) and presented as mean ± SEM (n = 6). **P < .01, ***P < .001, comparison of T-plastin mRNA levels from treated cells to T-plastin mRNA levels from respective control cells.
T plastin expression is activated by calcium entry and down-regulated by calcineurin inhibitors and NFAT siRNA. (A) PBLs from patients with SS (n = 40), MF (n = 8), various hemopathies or undetermined erythroderma (others, n = 8), and from healthy donors (healthy, n = 16) were incubated with PMA and ionomycin combination (dark columns), ionomycin (gray columns), PMA (white columns), for 16 hours. For PBLs from patients with SS, 2 sets were distinguished, according to expression of constitutive T-plastin mRNA (SS PLS3+, n = 24) or not (SS PLS3−, n = 16). T-plastin mRNA expression was quantified by qRT-PCR. Results are presented as fold increase of T-plastin mRNA levels detected in stimulated cells relative to basal T-plastin mRNA levels detected in respective unstimulated cells. *P < .05, **P < .01, comparison of T-plastin mRNA levels from stimulated cells to T-plastin mRNA levels from respective nonstimulated cells. (B) Top panel: PBLs from patients with SS (n = 12) that constitutively expressed T-plastin mRNA were treated with EGTA (200μM) or FK-506 (10μM) or not treated (basal) for 16 hours. T-plastin mRNA expression was quantified by qRT-PCR. The constitutive T-plastin mRNA levels measured without treatment was reported to 100 to allow comparison between PBLs from SS patients. Results are presented as mean percentages (± SEM) of T-plastin mRNA levels obtained in the presence of EGTA (light gray bar) or FK506 (dark gray bar) relative to respective T-plastin levels detected in the absence of treatment (white bar). *P < .05, **P < .01, comparison of T-plastin mRNA levels from treated cells to T-plastin mRNA levels from basal nontreated cells. Bottom panel: PBLs from SS patients were not incubated (basal) or incubated with EGTA (200μM) or FK506 (10μM) for 16 hours. T-plastin protein expression was determined by immunoblot analysis with anti–T-plastin antibody and with GAPDH antibody to confirm equal loading. Results are representative of 3 independent experiments. (C) Top panel: HuT-78 cells constitutively expressed T-plastin mRNA. In a set of experiments, cells were preincubated with FK506 (10μM), or not for 16 hours before mRNA extraction. In another set of experiments, HuT-78 cells were transiently transfected with control siRNA (200 or 400nM), siRNA for NFAT1 (200nM), and NFAT2 (400nM) 24 hours before mRNA extraction. T-plastin mRNA expression was quantified by qRT-PCR and results are expressed in relative values of T-plastin expression of treated cells (gray) to respective basal nontreated cells (white) reported as 100 to allow experiment comparison (mean ± SEM, n = 6). **P < .01, comparison of T-plastin mRNA levels from treated cells to T-plastin mRNA levels from respective basal nontreated cells. Bottom panel: HuT-78 cells were transiently transfected with control siRNA (400nM), NFAT1 siRNA (200nM), or NFAT2 siRNA (400nM) 24 hours before total protein extraction was performed. NFAT1 and NFAT2 protein expression was determined by immunoblot analysis with anti-NFAT1 and anti-NFAT2 antibodies and with GAPDH antibody to confirm equal loading. Results are representative of 3 independent experiments. (D) Top panel: SeAx cells (0.2 × 106 cells/mL) were incubated (◊) or not (*) with PMA and ionomycin combination (PMA+Iono) over a 24-hour period (as triplicates) and collected for mRNA extraction at indicated times. T-plastin mRNA expression was quantified by qRT-PCR. Results are expressed in AUs and presented as mean ± SD (n = 3). In the absence of stimulation, SeAx cells did not express T-plastin mRNA (ΔCt values are less than 0.01 AU) whatever the collection time. Bottom panel: Cells were preincubated with FK-506 (10μM) for 1 hour before addition of PMA and ionomycin (PMA+Iono) for further 16 hours. In some experiments, SeAx cells were transiently transfected with control siRNA (200nM) or NFAT1 siRNA (200nM). Eight hours after, transfected cells were stimulated by addition of PMA and ionomycin (PMA+Iono) for 16 hours before mRNA extraction. T-plastin mRNA expression was quantified by qRT-PCR and results are expressed in arbitrary units (AU) and presented as mean ± SEM (n = 6). **P < .01, ***P < .001, comparison of T-plastin mRNA levels from treated cells to T-plastin mRNA levels from respective control cells.
Although with a lesser extent than PBLs from SS patients, PBLs from patients with MF and other hemopathies also exhibited a significant increase in T-plastin transcription under PMA and ionomycin activation. Of interest, PBLs from healthy donors did present significant T-plastin mRNA synthesis after PMA and ionomycin combination as well as after either ionomycin alone or PMA alone. All these inductions were significant compared with the extremely low, almost undetectable, level of T-plastin mRNA detected in respective unstimulated cells. In all cases (MF, hemopathies, healthy donors), T-plastin–induced synthesis was restricted to CD4+ lymphocyte subpopulations (data not shown). Altogether, our results clearly demonstrate that T-plastin expression is not restricted to malignant SS T cells as previously reported, and that activation of any T cells by calcium entry may induce T-plastin transcription in those lymphocytes.
Because ionomycin stimulation induced an increase in intracellular calcium by opening calcium membrane pores and release of calcium ions from endoplasmic reticulum pools, and as calcium acts as a second messenger for several signaling molecules in T-cell activation, including the protein phosphatase calcineurin,34 we assessed the involvement of calcium in T-plastin synthesis using potent Ca2+ chelator (EGTA) and CaN inhibitor (FK506). As shown in Figure 2B, treatment of T-plastin expressing PBLs from SS patients (n = 12) with EGTA or FK506 for 16 hours induced a significant inhibition of T-plastin mRNA transcription compared with basal T-plastin levels (top panel), and a decrease in protein expression as shown by Western blot analysis (bottom panel).
Calcineurin is a calcium/calmodulin-dependent serine/threonine protein phosphatase crucial for the activation of the transcription factor NFAT, and under activation dephosphorylates it among many substrates, leading to its translocation to the nucleus. Involvement of calcineurin-dependent NFAT pathway in T-plastin synthesis was assessed by carrying out NFAT siRNA transfection in HuT-78 and SeAx CTCL cell lines. HuT-78 cells constitutively expressed T-plastin mRNA, with a mean level of 98 ± 10.6 AU (± SEM) as measured by qRT-PCR in 6 independent experiments. This constitutive mRNA level was significantly decreased by FK506 addition as well as by transient transfection with siRNA of the 2 main isoforms NFAT1 or NFAT2 expressed in HuT-78 cells (Figure 2C top panel). Transfection with NFAT siRNA efficiently down-regulated respective NFAT isoform expression as shown by Western blot analysis (Figure 2C bottom panel). Stimulation by PMA and ionomycin of HuT-78 cells induced a 2.25 ± 0.8-fold increase in constitutive basal T-plastin mRNA synthesis, and this increase was totally reversed by FK506 pretreatment (data not shown). SeAx cells, that do not constitutively express T-plastin mRNA (mean level < 0.01 AU as measured by qRT-PCR), showed a potent induction of T-plastin mRNA expression after PMA and ionomycin stimulation as shown for 1 representative time-course experiment (Figure 2D top panel). This mRNA induction was significantly reduced by FK506 preincubation (Figure 2D bottom panel). It was also decreased by transient transfection with siRNA for NFAT1 isoform that is abundantly expressed by SeAx cells.
All these results indicate that calcineurin pathway is involved in the synthesis of T-plastin in T cells from SS.
T-plastin expression confers partial resistance to apoptosis in CTCL
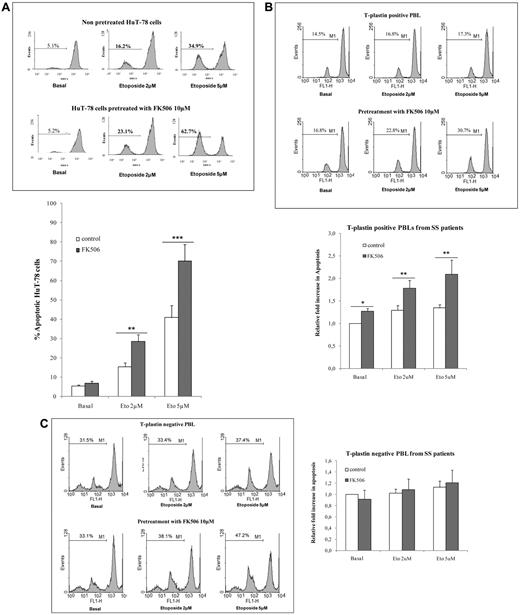
Because increase in T-plastin expression in cells resistant to cisplatin has been reported to be correlated with apoptosis resistance,31 and because CTCL malignant cells are quite resistant to apoptosis,36 we hypothesized that T-plastin expression in CTCL cells might be involved in the molecular mechanisms underlying malignant cell resistance to apoptosis. To assess this hypothesis, HuT-78 cells and T-plastin–positive PBLs from SS patients were incubated with FK506 at 10μM for 24 hours, then exposed to etoposide at 2 and 5μM for further 24 hours before apoptosis was measured by investigating the disruption of mitochondrial potential ΔΨm using flow cytometry analysis. As shown in Figure 3A (HuT-78cells) and B (T-plastin–positive PBLs from SS patients), FK506 alone did not induce apoptosis in HuT-78 cells and slightly in SS PBLs, although it significantly potentiated etoposide-induced apoptosis of both cell types. The mean potentiation of 5μM etoposide-induced apoptosis by FK506 reached 2.10 ± 0.77 (± SD, n = 6) in T-plastin–positive lymphocytes from SS patients. Of interest, FK506 addition did not alter apoptosis induced by etoposide alone in T-plastin–negative PBLs from SS patients (Figure 3C). Similar results were obtained using annexinV/PI staining (data not shown).
T-plastin expression in CTCL cells induces apoptosis resistance to etoposide and FK506 pretreatment potentiates CTCL cell apoptosis to conventional etoposide. HuT-78 cells or PBLs from SS patients were pretreated by 10μM FK506 (FK506) or not (control) for 1 hour, before exposed to etoposide (2 or 5μM) or not (basal) for 24 hours. Apoptosis was determined by percentage of cells exhibiting a loss of mitochondrial transmembrane potential (ΔΨm), as assessed by DiOC6 staining and flow cytometry analysis. (A) Top panel: Results are representative data from 1 experiment with HuT-78 cells. Bottom panel: Results are expressed as mean percentages of apoptotic cells (± SD) in response to etoposide (2 or 5μM) exposure from 3 independent experiments. **P < .01, ***P < .001, comparison of FK506-pretreated HuT-78 cells (gray) with nonpretreated HuT-78 cells (white) exposed to the same respective concentration of etoposide. (B) As in panel A with T-plastin–positive PBLs from patients with SS. Top panel: Results are presented for 1 representative SS patient. Bottom panel: Results are expressed as mean (± SEM) fold increase in apoptosis of FK506-treated cells (gray) and FK506 untreated cells (white) relative to respective apoptosis of cells not exposed to apoptosis (basal) and taken as 1 to allow patient comparison (n = 6). *P < .05, **P < .01, comparison of FK506-pretreated PBLs with nonpretreated PBLs exposed to the same respective concentration of etoposide. (C) As in panel B, with T-plastin–negative PBLs from patients with SS. (D) HuT-78 cells were transiently transfected with control siRNA (400nM), NFAT1 siRNA (200nM) and NFAT2 siRNA (400nM), as well as PLS3 siRNA (200μM) 24 hours before treatment with etoposide (2 or 5μM) or not (basal) for further 24 hours. Results are representative of 3 independent experiments.
T-plastin expression in CTCL cells induces apoptosis resistance to etoposide and FK506 pretreatment potentiates CTCL cell apoptosis to conventional etoposide. HuT-78 cells or PBLs from SS patients were pretreated by 10μM FK506 (FK506) or not (control) for 1 hour, before exposed to etoposide (2 or 5μM) or not (basal) for 24 hours. Apoptosis was determined by percentage of cells exhibiting a loss of mitochondrial transmembrane potential (ΔΨm), as assessed by DiOC6 staining and flow cytometry analysis. (A) Top panel: Results are representative data from 1 experiment with HuT-78 cells. Bottom panel: Results are expressed as mean percentages of apoptotic cells (± SD) in response to etoposide (2 or 5μM) exposure from 3 independent experiments. **P < .01, ***P < .001, comparison of FK506-pretreated HuT-78 cells (gray) with nonpretreated HuT-78 cells (white) exposed to the same respective concentration of etoposide. (B) As in panel A with T-plastin–positive PBLs from patients with SS. Top panel: Results are presented for 1 representative SS patient. Bottom panel: Results are expressed as mean (± SEM) fold increase in apoptosis of FK506-treated cells (gray) and FK506 untreated cells (white) relative to respective apoptosis of cells not exposed to apoptosis (basal) and taken as 1 to allow patient comparison (n = 6). *P < .05, **P < .01, comparison of FK506-pretreated PBLs with nonpretreated PBLs exposed to the same respective concentration of etoposide. (C) As in panel B, with T-plastin–negative PBLs from patients with SS. (D) HuT-78 cells were transiently transfected with control siRNA (400nM), NFAT1 siRNA (200nM) and NFAT2 siRNA (400nM), as well as PLS3 siRNA (200μM) 24 hours before treatment with etoposide (2 or 5μM) or not (basal) for further 24 hours. Results are representative of 3 independent experiments.
To further assess whether NFAT pathway is involved in apoptosis resistance through T-plastin expression and whether T-plastin expression concerns SS cell resistance to apoptosis, transient transfection of NFAT1 and NFAT2 siRNA as well as of PLS3 siRNA was performed in HuT-78 cells 24 hours before etoposide was added. As shown for 1 representative experiment in Figure 3D, etoposide-induced apoptosis of HuT-78 cells transfected with NFAT1 or NFAT2 siRNA was significantly higher than in control siRNA-transfected cells, with a mean fold increase of 1.78 ± 0.3 and 1.75 ± 0.5 (± SD, n = 3) compared with control siRNA apoptosis, respectively, for treatment with 5μM etoposide. Transfection of HuT-78 cells with PLS3 siRNA also increased 5μM etoposide-induced apoptosis of 1.59 ± 0.4 (± SD, n = 3) of control siRNA apoptosis.
Altogether, our results demonstrate that decrease in T-plastin expression by calcineurin/NFAT pathway inhibition significantly potentiates apoptosis to antineoplasic drugs, suggesting that T-plastin contributed to apoptosis resistance of SS malignant cells.
Stable T-plastin expression in Jurkat cells confers partial resistance to apoptosis
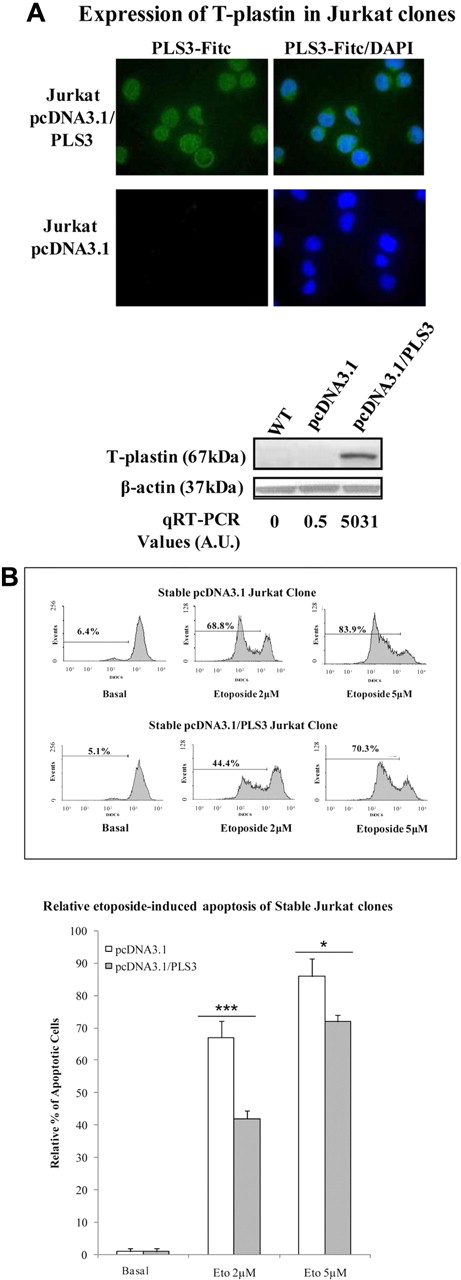
To further confirm the role of T-plastin (PLS3) expression in SS tumor cells, stable transfection with a PLS3-expressing plasmid was carried out in Jurkat E6.1 cells. These cells were used because they neither express T-plastin mRNA nor protein and because T-plastin synthesis was not at all inducible by calcium entry. As shown in Figure 4A, stably transfected clones expressed T-plastin mRNA and protein as depicted by qRT-PCR analysis, immunoblot, and immunohistochemistry.
Stable T-plastin surexpression in Jurkat cells induces apoptosis resistance to etoposide and T-plastin down-regulation by siRNA partially restored apoptosis. (A) Cytoplasmic expression and whole protein expression of T-plastin by mmunostaining (top panel) and Western blotting (bottom panel), respectively, for 1 representative T-plastin–transfected clone (Jurkat pcDNA3.1/PLS3) and 1 sham-transfected clone (Jurkat pcDNA3.1). Immunoblots from wild-type, nontransfected Jurkat cells (WT) are shown in the first column of the bottom panel. The immunoblot membrane was stripped and reprobed for expression of β-actin to control for loading (bottom panel). (B) Stable T-plastin–expressing Jurkat clones (pcDNA3.1/PL3) and vector control Jurkat clone (pcDNA3.1) were exposed to etoposide (2 or 5 μM) or not (Basal) for 24 hours. Apoptosis was determined as in Figure 3A. Top panel: data are presented for 1 representative pcDNA3.1 clone and 1 pcDNA3.1/PLS3 clone. Bottom panel: results are mean relative percentages (± SEM) of etoposide-induced apoptosis from pcDNA3.1 (white) and pcDNA3.1/PLS3 (gray) clones relative to apoptosis of respective clones, nonexposed to etoposide (Basal) and taken as 1 (n = 12). *P < .05, **P < .01, comparison of apoptosis of pcDNA3.1/PLS3 clones with that of pcDNA3.1 clones incubated with the same respective concentration of etoposide.
Stable T-plastin surexpression in Jurkat cells induces apoptosis resistance to etoposide and T-plastin down-regulation by siRNA partially restored apoptosis. (A) Cytoplasmic expression and whole protein expression of T-plastin by mmunostaining (top panel) and Western blotting (bottom panel), respectively, for 1 representative T-plastin–transfected clone (Jurkat pcDNA3.1/PLS3) and 1 sham-transfected clone (Jurkat pcDNA3.1). Immunoblots from wild-type, nontransfected Jurkat cells (WT) are shown in the first column of the bottom panel. The immunoblot membrane was stripped and reprobed for expression of β-actin to control for loading (bottom panel). (B) Stable T-plastin–expressing Jurkat clones (pcDNA3.1/PL3) and vector control Jurkat clone (pcDNA3.1) were exposed to etoposide (2 or 5 μM) or not (Basal) for 24 hours. Apoptosis was determined as in Figure 3A. Top panel: data are presented for 1 representative pcDNA3.1 clone and 1 pcDNA3.1/PLS3 clone. Bottom panel: results are mean relative percentages (± SEM) of etoposide-induced apoptosis from pcDNA3.1 (white) and pcDNA3.1/PLS3 (gray) clones relative to apoptosis of respective clones, nonexposed to etoposide (Basal) and taken as 1 (n = 12). *P < .05, **P < .01, comparison of apoptosis of pcDNA3.1/PLS3 clones with that of pcDNA3.1 clones incubated with the same respective concentration of etoposide.
To determine whether the expression of T-plastin does protect cells from etoposide-induced apoptosis, Jurkat pcDNA3.1/PLS3 and pcDNA3.1 (control vector) clones were treated for 24 hours with etoposide and apoptosis was measured by flow cytometry as described for PBLs from SS patients. Jurkat pcDNA3.1/PLS3 clones exhibited a significantly lower apoptosis induction in response to etoposide than Jurkat pcDNA3.1 control clones, as shown by dose and time-dependent disruption of ΔΨm in response to etoposide for 1 representative pcDNA3.1 control clone and 1 pcDNA3.1/PLS3 clone (Figure 4B top panel). Mean percentages from 12 control and 12 pcDNA3.1/PLS3 clones are shown in Figure 4B (bottom panel).
Involvement of T-plastin in cell resistance to apoptosis was further confirmed using stable Jurkat pcDNA3.1/PLS3 clones in which partial reversion of T-plastin expression was obtained by T-plastin siRNA transfection (data not shown).
These results confirm that T-plastin is partly responsible for the resistance to drug-induced apoptosis of pcDNA3.1/PLS3 Jurkat clones.
T-plastin expression favors cell migration in CTCL
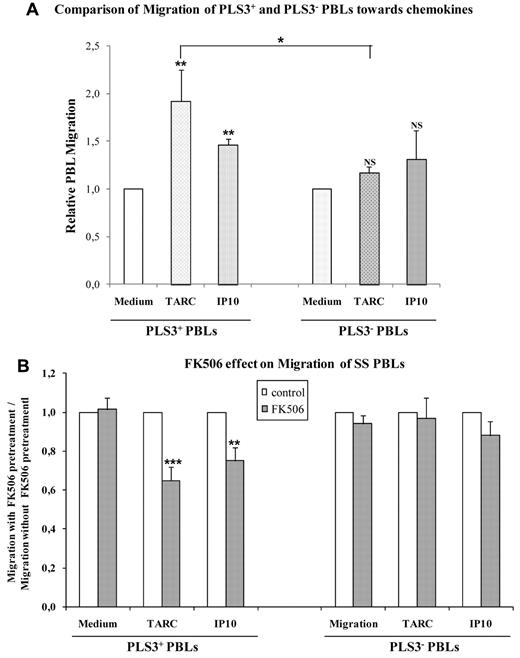
On one hand, T-plastin interacts with cytoskeleton and actin filaments, and consequently, might interfere with cell morphology and motility. On another hand, the diagnosis of SS is established on the identification of the malignant Sézary cell based primarily on characteristic cytologic features (“cerebriform” nucleus and enlarged cytoplasm) as well as by histologic observation of “skin-homing” T cells within the dermis and the epidermis. Because our results showed that T-plastin synthesis by tumor T lymphocytes from SS involved NFAT pathway and because NFAT factors have been clearly implicated in the migration process for different cell types,41-43 we hypothesized that T-plastin might be involved in malignant T-cell migration. To test this hypothesis, a transwell chemotaxis assay was performed using 2 chemokines specifically detected in SS cutaneous tumors, TARC, and IP-10. PBLs from SS patients as well as established Jurkat pcDNA3.1/PLS3 clones were seeded in the upper chamber and further counted in the lower one after 4 hours.
As shown in Figure 5A, T-plastin–positive T cells from SS patients showed a significant increased migration toward both TARC and IP-10 compard with their basal migration to control medium. TARC-induced migration of T-plastin–positive PBLs was significantly higher than that of T-plastin–negative PBLs toward the same chemokine. Pretreatment of T-plastin–positive T cells with 10μM FK506 significantly decreased their migration toward TARC and IP-10, compared with cells that were not pretreated, whereas it did not affect the chemokine-induced migration of T-plastin–negative PBLs (Figure 5B). Therefore, T-plastin expression appears, at least partly, as responsible for the increased migration of T-plastin–positive lymphocytes.
T-plastin expression in CTCL cells favors migration that was inhibited by FK506 pretreatment. (A) T-plastin–positive (PLS3+) PBLs (left) and –negative (PLS3−) PBLs (right) from SS patients were plated in the upper chamber of a modified Boyden chamber. The lower chamber contained migration medium alone or supplemented with TARC (10 ng/mL) or IP-10 (50 ng/mL). Results are mean (± SEM, n = 6) migration of PLS3+ and PLS3− PBLs toward TARC or IP-10 relative to respective migration into medium alone taken as 1. **P < .01, comparison of PLS3+ PBL migration toward chemokines to respective cell migration into medium alone. *P < .05, comparison between relative migration of PLS3+ PBLs and PLS3− PBLs toward TARC. (B) T-plastin positive (PLS3+) and negative (PLS3−) PBLs from CTCL were pretreated by FK506 (10μM) or not for 1 hour before migration was assessed as in panel A. Results are expressed as mean (± SEM, n = 6) ratio of migration of FK506-pretreated PBLs (gray) relative to migration of respective control (non-FK506–pretreated) PBLs (white), taken as 1. **P < .01, ***P < .001, comparison of chemokine-induced migration of FK506-pretreated PBLs with the 1 of respective PBLs non-pretreated by FK506.
T-plastin expression in CTCL cells favors migration that was inhibited by FK506 pretreatment. (A) T-plastin–positive (PLS3+) PBLs (left) and –negative (PLS3−) PBLs (right) from SS patients were plated in the upper chamber of a modified Boyden chamber. The lower chamber contained migration medium alone or supplemented with TARC (10 ng/mL) or IP-10 (50 ng/mL). Results are mean (± SEM, n = 6) migration of PLS3+ and PLS3− PBLs toward TARC or IP-10 relative to respective migration into medium alone taken as 1. **P < .01, comparison of PLS3+ PBL migration toward chemokines to respective cell migration into medium alone. *P < .05, comparison between relative migration of PLS3+ PBLs and PLS3− PBLs toward TARC. (B) T-plastin positive (PLS3+) and negative (PLS3−) PBLs from CTCL were pretreated by FK506 (10μM) or not for 1 hour before migration was assessed as in panel A. Results are expressed as mean (± SEM, n = 6) ratio of migration of FK506-pretreated PBLs (gray) relative to migration of respective control (non-FK506–pretreated) PBLs (white), taken as 1. **P < .01, ***P < .001, comparison of chemokine-induced migration of FK506-pretreated PBLs with the 1 of respective PBLs non-pretreated by FK506.
Stable T-plastin expression in Jurkat cells favors cell migration
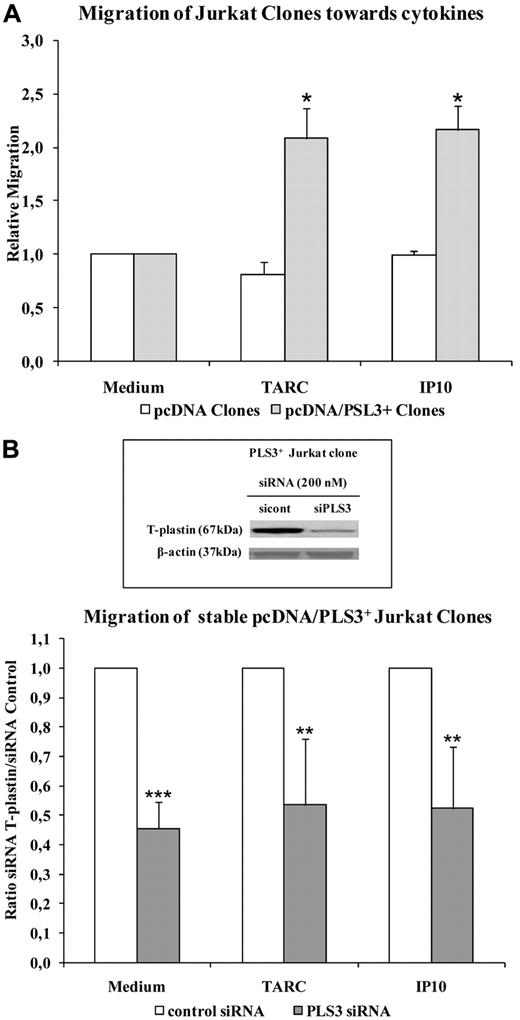
We confirmed the contribution of T-plastin in T-lymphocyte migration using stable T-plastin Jurkat cells. Figure 6A shows that migration of Jurkat pcDNA3.1/PLS3 cells was significantly higher than that of pcDNA3.1 clones toward TARC and IP-10. Down-regulation of T-plastin expression by siRNAs targeting T-plastin (PLS3 siRNA), as depicted by immunoblot (Figure 6B frame), induced a significant decrease in migration of pcDNA3.1/PLS3 cells toward chemotaxis medium, TARC, and IP-10 compared with pcDNA3.1/PLS3 cells transfected with control siRNA (Figure 6B).
T-plastin surexpression in Jurkat cells favors migration that was reversed by T-plastin down-regulation by siRNA transient transfection. (A) Migration of T-plastin–positive (pcDNA3.1/PLS3+, gray) and –negative (pcDNA3.1, white) Jurkat clones was assessed as in Figure 5A. Results are expressed as mean (± SEM) ratio of migration of pcDNA3.1/PLS3+ clones (n = 6) and pcDNA3.1 clones (n = 6) relative to migration into medium alone of respective clones, taken as 1. *P < .05, comparison of cell migration toward chemokines to respective cell migration in control medium. (B) Same assay as in panel A using stable T-plastin– expressing Jurkat clones (PLS3+ clones) transiently transfected with 200nM control siRNA (white) or PLS3 siRNA (gray). Results are expressed as mean (± SD) ratio of chemokine-induced migration of PLS3 siRNA transfected clones (n = 3) to respective chemokine-induced migration of control siRNA clones (n = 3) taken as 1. **P < .01, ***P < .001, comparison of chemokine-induced cell migration of PLS3 siRNA-transfected PLS3+ clones to that of PLS3 clones transfected with control siRNA. In frame is presented the protein expression of T-plastin in 1 representative stable PLS3+ Jurkat clones transiently transfected with control siRNA (sicont) or PLS3 siRNA (siPLS3), assessed by Western blotting and normalized by β-actin.
T-plastin surexpression in Jurkat cells favors migration that was reversed by T-plastin down-regulation by siRNA transient transfection. (A) Migration of T-plastin–positive (pcDNA3.1/PLS3+, gray) and –negative (pcDNA3.1, white) Jurkat clones was assessed as in Figure 5A. Results are expressed as mean (± SEM) ratio of migration of pcDNA3.1/PLS3+ clones (n = 6) and pcDNA3.1 clones (n = 6) relative to migration into medium alone of respective clones, taken as 1. *P < .05, comparison of cell migration toward chemokines to respective cell migration in control medium. (B) Same assay as in panel A using stable T-plastin– expressing Jurkat clones (PLS3+ clones) transiently transfected with 200nM control siRNA (white) or PLS3 siRNA (gray). Results are expressed as mean (± SD) ratio of chemokine-induced migration of PLS3 siRNA transfected clones (n = 3) to respective chemokine-induced migration of control siRNA clones (n = 3) taken as 1. **P < .01, ***P < .001, comparison of chemokine-induced cell migration of PLS3 siRNA-transfected PLS3+ clones to that of PLS3 clones transfected with control siRNA. In frame is presented the protein expression of T-plastin in 1 representative stable PLS3+ Jurkat clones transiently transfected with control siRNA (sicont) or PLS3 siRNA (siPLS3), assessed by Western blotting and normalized by β-actin.
Altogether, these results demonstrate that the expression of T-plastin in T cells does increase their migration toward chemokines known to be present in the blood and the tissue microenvironment of cutaneous lymphoma and that down-regulation of T-plastin expression by CaN inhibitors significantly limits T-cell migration.
Discussion
The present data obtained with a large cohort of CTCL patients confirmed previously published reports showing that T-plastin transcriptional expression is specific and restricted to tumor lymphocytes from patients with SS.2,19-23,32 In accordance with previous studies, we found no significant expression of T-plastin transcript in the 78 healthy volunteers tested, in contrast to the recent data published by Oprea et al, where 5% of healthy donors expressed a T-plastin transcript in PBLs.33 Several reports have previously claimed that T-plastin can serve as a positive genetic marker, and that its expression correlated to blood tumor burden.22 However, our data showed a wide range of T-plastin expression levels among CTCL cases and 1/4 patients did not present T-plastin transcript although they exhibited clinical characteristic of a well-characterized SS.
To better understand T-plastin expression in CTCL, we assessed activation of malignant T cells and demonstrated that T-plastin synthesis was induced, or increased, by calcium entry in all neoplastic cells from CTCL patients. Of interest, T-plastin was also induced in T lymphocytes from healthy donors as well as from MF and other studied hemopathies. Calcium influx triggers the Ser/Thr phosphatase calcineurin; under activation, calcineurin dephosphorylates many substrates, including NFAT, leading to their translocation to the nucleus and binding to promoters of target genes.34,35 Suppression of calcineurin phosphatase activity can be obtained by immunosuppressive drugs, such as FK-506, that lead to a marked reduction of the TCR-induced NFAT,44 as well as JNK45 and NF-κB activity.46,47
Here, involvement of calcium entry and NFAT pathway activity in T-plastin synthesis was clearly demonstrated using calcium chelators, CaN inhibitors and/or specific NFAT siRNA transfection in T-plastin–positive lymphocytes from SS patients as well as in CTCL cell lines. These results were obtained at T-plastin mRNA message and protein levels by qRT-PCR and immunoblotting, respectively. Detection of intracellular T-plastin using a new specific antibody kindly provided by Dr Tracey Mitchell (Skin Tumor Institute, London) and flow cytometry analysis confirmed not only T-plastin expression in CD4+ T cells from SS patients, but also a decrease in mean fluorescence intensity of constitutive T-plastin staining after treatment with EGTA or CaN inhibitors (F.J.-L., L.M., unpublished data, 2011).
Pretreatment with CaN inhibitors totally inhibited the synthesis of T-plastin induced by PMA+ionomycin stimulation although it only partially reduces the constitutive T-plastin expression. This partial inhibition suggests involvement of other molecular events in T-plastin synthesis. Moreover, the degree of inhibition in T-plastin synthesis was almost identical by pretreatment with CaN inhibitors and by siRNA-mediated down-regulation of NFAT. The inhibition by FK506 might be partly efficient because it only impairs the activity of CaN and consequently only partly alters NFAT dephosphorylation. This is also the case of the siRNA-mediated knockdown experiments, which could allow a remaining NFAT signal transcription.
Of interest, T-plastin mRNA synthesis was also induced in PBLs from healthy donors by TCR stimulation using anti-CD3 antibody and soluble CD28 as costimulatory signal but in contrast, no significant modification of T-plastin mRNA expression was obtained by TCR stimulation of PBLs from CTCL patients (E.B., F.J.-L., L.M., unpublished data, 2010-2011).
Previously, T-plastin was reported as increased in cells resistant to cisplatin and expression correlated with apoptosis resistance.31 We show here that constitutive T-plastin expression in CTCL, as well as overexpression of T-plastin in stably transfected Jurkat cells, confers resistance to apoptosis induced by antineoplastic agents, such as etoposide as well as staurosporin and bortezomib (data not shown), and that T-plastin down-regulation restores, in part, drug-sensibility. We previously reported that the CTCL resistance to apoptosis triggered by antineoplastic agents was associated with NF-κB constitutive activation.37 Here, we provide evidence for an additional molecular mechanism that controls malignant cell resistance to drug-induced apoptosis in CTCL and that is not linked with Bcl2 expression (F.J.-L., unpublished data, 2011). This suggests that CaN inhibitors might be combined with other antineoplastics drugs to counterattack resistance of CTCL tumors as well as to down-regulate malignant cell migration within the skin. Topical formulations of immunomodulators, such as FK506 or cyclosporine, were recently investigated for their potential roles in treating early MF and appear as promising both as primary and adjunctive therapy.48
Indeed, our results clearly show that T-plastin promotes migration in response to chemokines that have been shown to be overexpressed in tumor skin of SS patients, such as TARC or IP-10. T lymphocytes from SS patients expressed the chemokine receptors CCR4 and CXCR3, recognized by TARC and IP-10, respectively.4 To determine whether alteration of chemokine receptor expression could explain increase in cell migration, we assessed receptor CCR4 and CXCR3 surface expression on malignant T cells and detected an intense staining for both chemokine receptors, similarly to CD4+ T cells from healthy donors (E.B., F.J.-L., unpublished data, 2010-2011). Therefore, chemokine receptor expression did not explain CTCL migration alteration.
Using a migration assay under agar in vitro, it was previously shown that peripheral blood lymphocytes from SS patients migrated to normal allogeneic skin whereas PBLs from controls did not.49 This migration was organ specific, required the integrity of lymphocyte membrane receptors, and did not seem to involve serum factors. In our experiments, serum collected from SS patients induced a potent migration of malignant SS lymphocytes, whereas human AB serum did not all and a significant difference between migration of T-plastin–positive and T-plastin–negative PBLs toward SS serum was detected (E.B., L.M., unpublished data, 2010-2011). The presence of various factors, including chemokines, in patients' serum may be responsible for this specific T-cell migration and reinforces the pathologic role of T-plastin in CTCL. Further experiments with T cells isolated from cutaneous tumors might bring evidence for T-plastin involvement in lymphocyte migration toward local chemokines. Moreover, such experimental approaches will allow comparative analysis of lymphocytes from MF and SS lesional skin.
T-plastin was reported to bind to vimentin, an intermediate filament protein,30 that we previously reported to be expressed by CTCL cells.50 These molecules, alone or bound, might contribute to cell migration toward chemotactic gradient within tumor skin.
We also detected L-plastin (LCP1) messages and protein in the neoplastic CTCL cells, in accordance with previous reports.2 LCP1's actin-bundling function was shown to be important in signaling pathways associated with activation and migration of T cells51 and might contribute with T-plastin to this function in CTCL cells.
Based on our own and literature data, we conclude that T-plastin expression is a specific biologic marker of neoplastic T cells in SS. An elevation in intracellular calcium incites a signaling cascade that leads to T-plastin synthesis through the activation of malignant and normal T cells but the constitutive expression of T-plastin in CTCL might depend on several pathways that must be further elucidated. A better understanding of these pathways promises to shed light on normal immune function as well as CTCL etiology and to obtain efficient down-regulation of T-plastin contribution to tumor cell resistance and cell migration.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Pr Lionel Prin (Department Dermatology, Hôpital Saint-Joseph, Lille, France) for providing samples from patients with hypereosinophilic syndrome; Pr Antoine Toubert and his team for immunophenotyping (Laboratory of Immunology, Hôpital Saint-Louis, Paris, France); and Dr Niclas Setterblad (Plateforme IUH, Hôpital Saint-Louis, Paris, France) for fluorescent microscopic analysis. They are grateful to Dr Tracey Mitchell (Skin Tumor Institute, London, United Kingdom), Pr Brunhilde Wirth (Institute of Human Genetics, Cologne, Germany), and Dr Keld Kaltoft (University of Aarhus, Denmark) for providing a personal T-plastin monoclonal antibody for flow cytometry analysis, PLS3-expressing plasmid, and the SeAx cell line, respectively.
Authorship
Contribution: E.B. and L.M. designed and conducted research, analyzed data, and performed interpretation; E.B., F.J.-L., and L.M. performed experiments and analysis; M.B., J.-M.C., L.L., N.P., and H.B. provided the study materials and patients; S.J., A.B., and G.C. performed interpretation; S.J. and G.C. contributed to the conception and design; and E.B. and L.M. were responsible for the paper writing; and all authors approved the final paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Laurence Michel, Inserm UMRS 976, Université Paris Diderot, Centre de Recherche sur la Peau, Hôpital Saint-Louis, 1 Ave Claude Vellefaux, 75475 Paris Cedex 10, France; e-mail: laurence.michel@inserm.fr.