Abstract
Increased fibrinolysis is an important component of acute promyelocytic leukemia (APL) bleeding diathesis. APL blasts overexpress annexin II (ANXII), a receptor for tissue plasminogen activator (tPA), and plasminogen, thereby increasing plasmin generation. Previous studies suggested that ANXII plays a pivotal role in APL coagulopathy. ANXII binding to tPA can be inhibited by homocysteine and hyperhomocysteinemia can be induced by L-methionine supplementation. In the present study, we used an APL mouse model to study ANXII function and the effects of hyperhomocysteinemia in vivo. Leukemic cells expressed higher ANXII and tPA plasma levels (11.95 ng/mL in leukemic vs 10.74 ng/mL in wild-type; P = .004). In leukemic mice, administration of L-methionine significantly increased homocysteine levels (49.0 μmol/mL and < 6.0 μmol/mL in the treated and nontreated groups, respectively) and reduced tPA levels to baseline concentrations. The latter were also decreased after infusion of the LCKLSL peptide, a competitor for the ANXII tPA–binding site (11.07 ng/mL; P = .001). We also expressed and purified the p36 component of ANXII in Pichia methanolica. The infusion of p36 in wild-type mice increased tPA and thrombin-antithrombin levels, and the latter was reversed by L-methionine administration. The results of the present study demonstrate the relevance of ANXII in vivo and suggest that methionine-induced hyperhomocysteinemia may reverse hyperfibrinolysis in APL.
Introduction
Hemorrhage is the main complication of acute promyelocytic leukemia (APL), and approximately 60%-80% of patients present with a coagulation abnormality at diagnosis.1-3 Despite the improvement in APL treatment outcome, early mortality due to bleeding is still prevalent.4 At least 3 components contribute to bleeding due to APL: activation of coagulation, proteolysis, and fibrinolysis. APL blasts express both tissue factor and cancer procoagulant and secrete IL-1β and TNFα,5-7 which leads to excessive fibrin production. Concomitantly, there is proteolytic activity with the generation of D-like fibrin fragments that have an anticoagulant effect.8
Several elements contribute to fibrinolysis in APL. Reduced levels of plasminogen activator inhibitor 1 (PAI1)9 and alpha2-antiplasmin10 are observed in the plasma, and thrombin-activatable fibrinolysis inhibitor activity is markedly below normal.11 Furthermore, urokinase plasminogen activator protein levels are higher in both plasma and leukemic cells.12,13 Using an in vitro assay, Menell et al demonstrated that the APL cell lineage NB4 can up-regulate plasmin synthesis by approximately 28-fold in a medium containing plasminogen and tissue plasminogen activator (tPA).14
Annexin II (ANXII) is thought to play a critical role in APL-associated fibrinolysis. It is a phosphatidylinositol-4,5-bisphosphate–binding protein that is overexpressed in APL cells.14-16 ANXII is a receptor for both tPA and plasminogen and in patients with APL, it increases plasmin generation approximately 60-fold and therefore may participate in the pathogenesis of APL bleeding diathesis.17 Homocysteine competes with tPA for the same site of ANXII through its motif LCKLSL.18 We hypothesized that by inhibiting ANXII activity through the induction of hyperhomocysteinemia or by administering LCKLSL peptide, APL-associated hyperfibrinolysis could be reversed. We took advantage of a transgenic model of APL, hCG-PML-RARα transgenic (TG) mice,19 to test this hypothesis.
Methods
This research project was approved by the Animal Research Ethical Committee of the Medical School of Ribeirão Preto at the University of São Paulo, São Paulo, Brazil (protocol number 175/2008).
APL transgenic model
hCG-PML/RARa TG mice and wild-type littermates were kindly provided by Pier Paolo Pandolfi (Beth Israel Deaconess Medical Center, Harvard Stem Cell Institute, Boston, MA). The mice were bred in a specific pathogen-free animal facility at the Medical School of Ribeirão Preto, University of São Paulo, São Paulo, Brazil. The hematologic counts were monitored monthly, and the following criteria were used for the diagnosis of leukemia: presence of at least 1% of blasts in peripheral blood associated with leukocytosis above 30 000 cells/μL, hemoglobin levels below 10 g/dL, and thrombocytopenia below 500 × 103 cells/μL, as described previously.19 After diagnosis, mice were killed by overdose of 2.5% tribromoethanol and leukemic cells were obtained by flushing BM cavities with RPMI 1640 medium supplemented with 10% FBS.
BM transplantation
Because of the low frequency of leukemia (approximately 10% of the TG mice) and its long latency,19,20 we opted to use a syngeneic BM transplantation model. At 12-16 weeks of age, wild-type littermates were irradiated with 200 cGy during 2 sessions separated by an interval of 3 hours. Four hours after the second session, 2 × 106 BM cells were infused into the retroorbital venous sinus. For the first set of experiments, recipient mice were divided into 2 groups of 8 animals each; animals in the first group were transplanted with BM cells from a wild-type control and those in the second group were injected with hCG-PML/RARa leukemic cells. After 21 days, development of leukemia was assessed by morphology and by determining the percentage of CD117+ cells in the BM by flow cytometry, as described previously.21 All of the animals were killed by overdose of 2.5% tribromoethanol 21 days after the transplantation, and blood was collected from the inferior vena cava after infusion of 180 μL of 3.2% sodium citrate, as described previously.22 The samples were centrifuged and plasma was frozen at −80°C. BM cells were obtained as described in “APL transgenic model” for quantification of ANXII and confirmation of leukemic engraftment.
Quantification of ANXII expression by flow cytometry
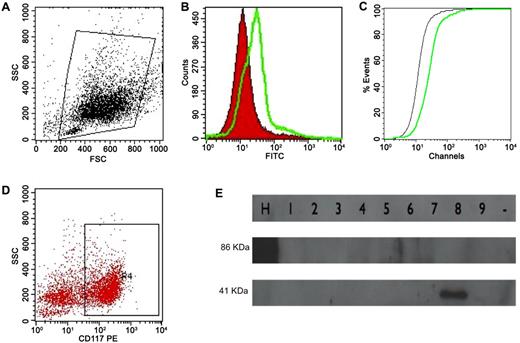
The BM cell suspension was washed in PBS and incubated with anti-ANXII mouse IgG (Santa Cruz Biotechnology) for 30 minutes in the dark. The cells were then washed twice in PBS and incubated with goat anti–mouse IgG conjugated with FITC (BD Biosciences) for 15 minutes. After washing, the cell suspension was incubated with rat anti–mouse IgG2a anti-CD117 conjugated with PE (BD Biosciences) for another 15 minutes. Two control tubes were used, one in which nonspecific mouse IgG replaced the ANXII Ab and another with nonspecific IgG (BD Biosciences) and IgG2a PE Ab (BD Biosciences). All of the samples were acquired using a FACSCalibur flow cytometer (BD Biosciences) and analyzed using CellQuest Version 5.1 software (BD Biosciences). ANXII expression was evaluated in the whole cell population (ungated) and in CD117+ cells and was expressed as the mean fluorescence channel (MFC) ratio (calculated by dividing the MFC for ANXII staining by the MFC for the isotypic control) and as the D value calculated by the Kolmogorov-Smirnov test (Figure 1A-D).
ANXII expression analysis by flow cytometry and cloned ANXII analysis by Western blotting. (A) Initial gating on total cell population. (B) Overlay histogram analyzing ANXII expression (in green) compared with isotype control (in red). (C) Graph illustrating the Kolmogorov-Smirnov test to calculate the D value (the green line is ANXII; the black line is the isotype control). The same strategy was used to analyze ANXII expression in CD117+ cells (D). (E) Western blot to determine ANXII expression in Pichia methanolica. Nine clones were tested for expression, and human serum albumin cloned into P methanolica (lane H) was used as the control (86 kDa). The approximate weight of the ANXII and pMET plasmid backbone was 41 kDa (lane 8).
ANXII expression analysis by flow cytometry and cloned ANXII analysis by Western blotting. (A) Initial gating on total cell population. (B) Overlay histogram analyzing ANXII expression (in green) compared with isotype control (in red). (C) Graph illustrating the Kolmogorov-Smirnov test to calculate the D value (the green line is ANXII; the black line is the isotype control). The same strategy was used to analyze ANXII expression in CD117+ cells (D). (E) Western blot to determine ANXII expression in Pichia methanolica. Nine clones were tested for expression, and human serum albumin cloned into P methanolica (lane H) was used as the control (86 kDa). The approximate weight of the ANXII and pMET plasmid backbone was 41 kDa (lane 8).
Determination of tPA and plasmin plasma levels
Stored plasma was evaluated for tPA (Asserachrom tPA quantitative determination; Diagnostica Stago), plasmin (mouse plasmin activity assay; Molecular Innovations), and thrombin-antithrombin complex (TAT; Enzignostic TAT Micro; Diagnostica Stago) levels by the ELISA method following the directions of the manufacturer.
ANXII p36 cloning and expression
ANXII p36 cDNA was kindly donated by Carl Creutz (University of Virginia, Charlottesville, VA). The sequence was amplified using the following primers: forward (5′-CGGGATCCATGTCTACTGTTCACGAAATCC-3′) and reverse (5′-ATAGTTTAGCGGCCGCGTCATCTCCACCACACAG-3′) to create a 5′ site for BamHI and a 3′ site for NotI. Reactions comprised 1.5μM concentrations of each primer, 2.5 U of Taq DNA polymerase (Invitrogen), 5 ng of original cDNA, 2.5mM MgCl2, and 10% reaction buffer (Invitrogen). Denaturation at 94°C for 2 minutes was followed by 35 cycles with 1 minute of denaturation at 94°C, 1 minute of annealing at 55°C, and 2 minutes of extension at 72°C. The reaction was completed by 7 minutes of extension at 72°C. The PCR products were subjected to electrophoresis on an 8% agarose gel, and DNA was quantified with a GeneQuant Pro (Biochrom). Both PCR products and the pMETB plasmid (Invitrogen) were digested with the endonucleases after a ligation reaction with a 3:1 molar ratio of insert to vector and 0.1 U of T4 ligase (Invitrogen). The plasmid was electoporated at 2.5 V, 200 Ω, and 25 μFD in a gene pulser (Bio-Rad) in an Escherichia coli–eletrocompetent stain. Clones were evaluated by PCR for the presence of the insert and 3 positive clones were DNA sequenced for further confirmation.
Plasmids extracted from one confirmed clone were digested with PstI (New England Biolabs) to separate the expression cassette and then electroporated into the PMAD11 Pichia methanolica strain at 750 V, 25 μF, and ∞ resistance in the gene pulser. Eight colonies, grown in minimum dextrose medium with no adenine added, were analyzed for ANXII expression by Western blotting using an anti-V5 Ab (Invitrogen; epitope added by pMETB plasmid). One positive colony was selected for large-scale expression in BMMY medium followed by protein extraction with glass beads (Sigma-Aldrich) and protein purification using His-TRAP HP columns (GE Health Care Life Sciences). Protein was quantified, confirmed by Western blot analysis with anti-V5 Ab, and frozen at −80°C (Figure 1E).
ANXII infusion experiments
Two groups of 3 wild-type C57BL/6 mice each were infused with 1.0 or 10.0 μg/kg of purified ANXII and killed after 4 hours as described in “APL transgenic model.” Saline-infused C57BL/6 mice (n = 3) were used as controls.
Methionine administration
To assess tPA-ANXII inhibition, 8 mice transplanted with leukemic hCG-PML-RARα cells received 200 mg/kg of L-methionine (Sigma-Aldrich) 3 times in 4-hour sessions using an orogastric tube beginning on day 20 after the cell transplantation. We selected this dose of L-methionine based on previously published data.18 Four hours after the last dose, the animals were killed. The results of this group were compared with untreated transplanted leukemic mice.
Another group of 3 wild-type C57BL/6 mice were administered 10 μg/kg of ANXII via the retroorbital venous sinus and 200 mg/kg of L-methionine via an orogastric tube and killed 4 hours later. ANXII-treated mice killed at the same time point were used as controls.
Treatment with LCKLSL hexapeptide
A group of 5 animals (wild-type) underwent BM transplantation with grafts from a leukemic hCG-PML-RARα mouse. On day 21, they were infused with 5.5 mg of LCKLSL peptide (Inova Scientific do Brasil) through the retroorbital venous sinus and killed 4 hours later. The dose was calculated to produce an approximate serum concentration of 1mM, assuming that the peptide distribution volume was equal to the extracellular volume. Leukemic recipients were used as controls.
Homocysteine levels
Plasma homocysteine levels were measured in the leukemic and in the L-methionine–infused groups using the Immulite 1000 immunoassay system (Homocysteine Kit; Siemens Healthcare Diagnostics)
Statistical analysis
Statistical analysis was carried out with SPSS Version 13.0 software for Windows. Groups were compared using either the Mann-Whitney U test or 1-way ANOVA. Correlations were performed by the Pearson correlation method. P ≤ .05 was considered to be statistically significant.
Results
ANXII expression in leukemic mice
Figure 2 shows that the ANXII expression was significantly higher in the BM of recipients of hCG-PML/RARa leukemic cells compared with controls (mean value of ANXII MFC ratio was 1.038 vs 1.552 in the wild-type and leukemic groups, respectively; P = .012; Figure 2A). The analysis based on D values corroborated this finding (mean D value was 0.339 vs 0.094 in the leukemic and wild-type groups, respectively; P = .001; Figure 2B). ANXII expression assessed by the D value was also higher in the leukemic group when the analysis was restricted to CD117+ cells (0.116 vs 0.355 for the wild-type and leukemic groups, respectively; P = .004). In addition, the percentage of CD117+ cells was correlated with the intensity of ANXII expression regardless of whether the MFC ratio or the D value was considered (Pearson correlation coefficients of 0.834 and 0.964 for MFC ratio and D value, respectively; P < .001).
ANXII expression assessed by flow cytometry. MFC ratio (A) and D values for ANXII expression (B) in BM cells from mice transplanted with leukemic cells from hCG-PML/RARA TG mice (Leukemic) or with normal BM cells from wild-type mice (Wild).
ANXII expression assessed by flow cytometry. MFC ratio (A) and D values for ANXII expression (B) in BM cells from mice transplanted with leukemic cells from hCG-PML/RARA TG mice (Leukemic) or with normal BM cells from wild-type mice (Wild).
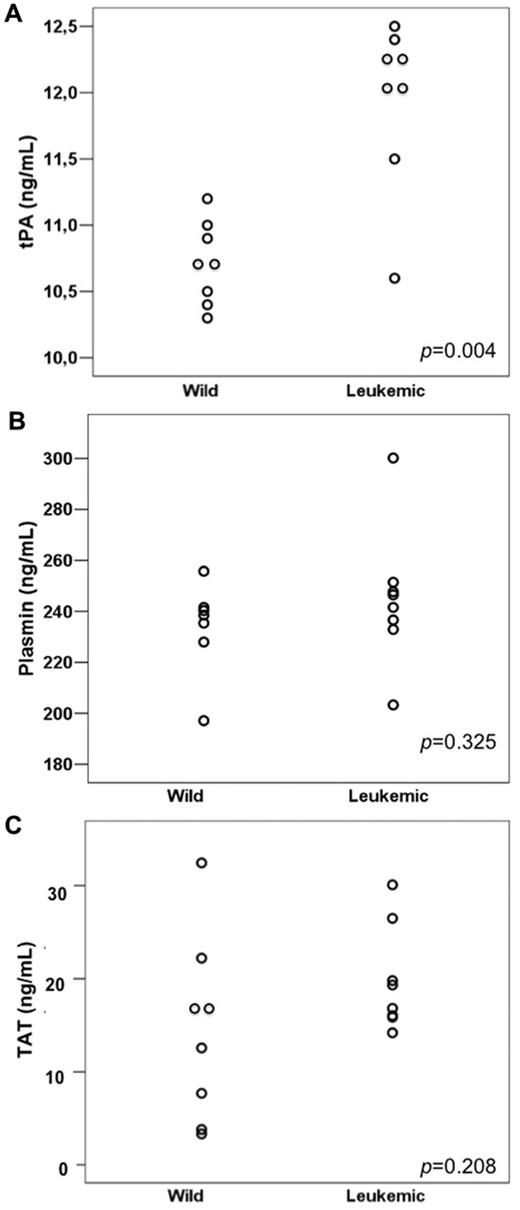
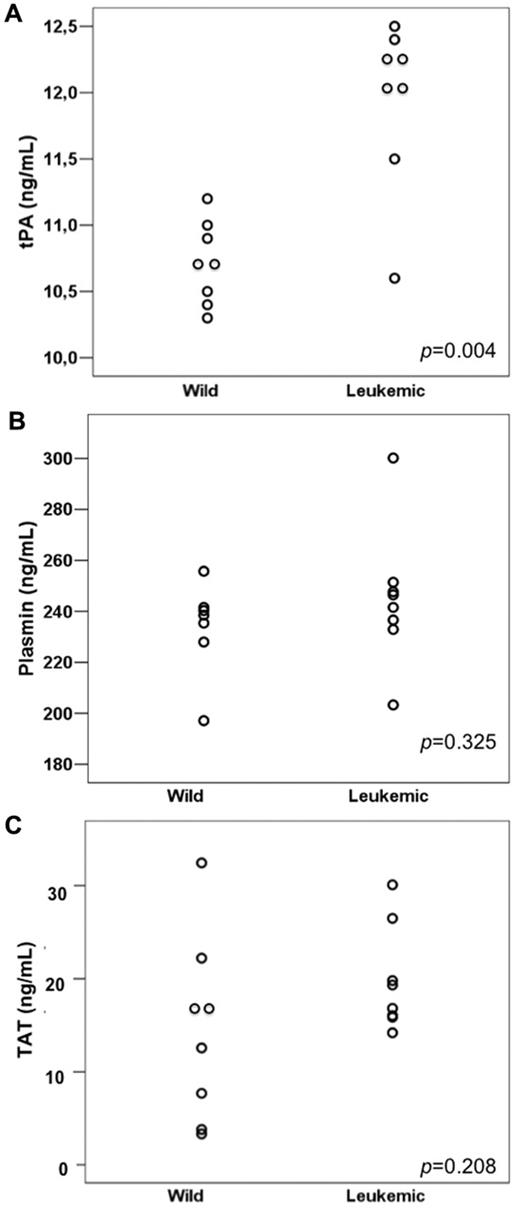
tPA, plasmin, and TAT values
Leukemic mice had significantly higher levels of tPA (the mean tPA was 10.74 and 11.95 ng/mL in the wild-type and leukemic groups, respectively; P = .004) but not of plasmin (233.8 and 237.1 ng/mL in the wild-type and leukemic groups, respectively; P = .325) nor TAT (means, 14.43 and 19.83 ng/mL, respectively; P = .208; Figure 3A-C). We detected a direct correlation between ANXII expression and tPA levels (Pearson correlation coefficient 0.905 [P < .001] for ANXII MFC ratio/tPA levels and 0.896 [P < .001] for ANXII/D value/tPA levels).
Fibrinolysis parameters in wild-type and leukemic recipient mice. (A) tPA levels were higher in mice transplanted with leukemic cells (Leukemic) compared with those transplanted with normal BM cells from wild-type (Wild) donors. No significant difference was observed in plasmin (B) and TAT (C) levels between the 2 groups.
Fibrinolysis parameters in wild-type and leukemic recipient mice. (A) tPA levels were higher in mice transplanted with leukemic cells (Leukemic) compared with those transplanted with normal BM cells from wild-type (Wild) donors. No significant difference was observed in plasmin (B) and TAT (C) levels between the 2 groups.
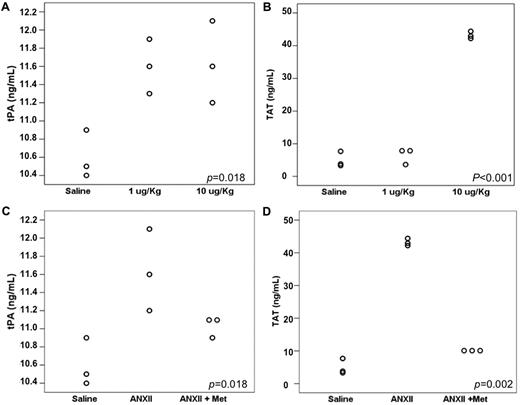
tPA and TAT levels after recombinant ANXII infusion
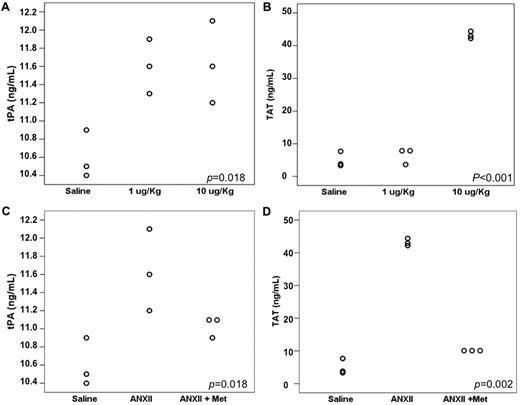
Figure 4A and B show the mean tPA and TAT values in mice injected with either saline (control) or 1 or 10 μg/kg of recombinant p36 component of ANXII. Untreated mice presented a mean tPA level of 10.60 ng/mL, whereas in the 1 μg/kg ANXII group, the mean tPA value was of 11.60 ng/mL and in the 10 μg/kg ANXII group, it was 11.63 ng/mL (P = .018). tPA levels were significantly higher in both groups that received ANXII compared with the control (P = .013 and P = .011, respectively), but they did not differ significantly from each other (P = .910). TAT levels were elevated only in the group that received 10 μg/kg of ANXII (saline, 4.93 ng/mL; 1 μg/kg ANXII, 6.45 ng/mL; 10 μg/kg ANXII, 43.16 ng/mL; P < .001).
Effect of recombinant ANXII infusion and L-methionine supplementation in leukemic mice. (A) tPA levels increased with both 1 and 10 μg/kg doses of ANXII, but no difference was observed between the 2 dosage groups. TAT was higher in mice that received 10 μg/Kg of ANXII. (B). Effect of L-methionine administration on tPA (C) and TAT (D) levels.
Effect of recombinant ANXII infusion and L-methionine supplementation in leukemic mice. (A) tPA levels increased with both 1 and 10 μg/kg doses of ANXII, but no difference was observed between the 2 dosage groups. TAT was higher in mice that received 10 μg/Kg of ANXII. (B). Effect of L-methionine administration on tPA (C) and TAT (D) levels.
Strategies to inhibit binding of tPA to ANXII
The administration of L-methionine to wild-type C57BL/6 mice infused with 10 μg/kg of recombinant ANXII led to similar values as those in saline-infused mice (mean tPA values were 10.60 ng/mL for saline, 11.63 ng/mL for ANXII, and 11.03 ng/mL for ANXII plus L-methionine; P = .018). The observed difference between saline-infused and ANXII-infused mice was significant (P = .006). The administration of L-methionine also reversed the increase in TAT levels observed with ANXII infusion (mean TAT level of 4.93 ng/mL for saline, 43.16 ng/mL for ANXII, and 9.99 ng/mL for ANXII plus L-methionine; P = .050; Figure 4C-D).
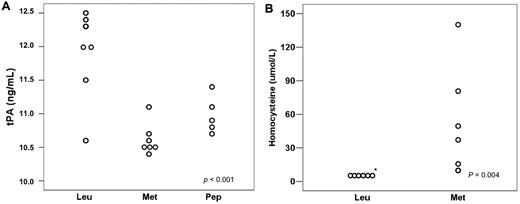
Among the leukemic animals, the group treated with L-methionine had a mean tPA of 10.54 ng/mL, whereas the group treated with the LCKLSL peptide had a mean tPA of 11.07 ng/mL (Figure 5A). When these animals were compared with leukemic, untreated animals, the mean tPA value was significantly different (P < .001) between the leukemic untreated group and the methionine group (P < .001) and also between the leukemic untreated group and the peptide group (P = .001). No significant difference was observed between the 2 treatment groups (P = .182). The mean value of homocysteine levels in the methionine-treated group was 49.0 μmol/mL and no animal in the leukemic group had homocysteine levels above 6.0 μmol/mL (the linearity threshold for detection using a 1:3 dilution; Figure 5B).
Effects of strategies to block tPA binding to ANXII in leukemic mice. (A) tPA plasma levels decreased in mice treated with either L-methionine or the LCKLSL peptide. (B) Homocysteine levels in the leukemic and L-methionine groups.
Effects of strategies to block tPA binding to ANXII in leukemic mice. (A) tPA plasma levels decreased in mice treated with either L-methionine or the LCKLSL peptide. (B) Homocysteine levels in the leukemic and L-methionine groups.
Discussion
Of the oncological diseases, APL is an ideal target for therapy because there is an available drug that is directed against a specific molecular target and the clinical response is frequently excellent. Despite this, coagulation disorders are still frequent and bleeding is the main cause of death in patients with APL. Therefore, new treatment strategies should be pursued to minimize hemostatic abnormalities. ANXII is thought to play an important role in the hyperfibrinolytic state observed in APL because of its action on tPA activity and its reported overexpression by leukemic cells.14-16 In the present study, we took advantage of the transgenic mouse hCG-PML-RARα model to study the effect of ANXII in vivo and to explore the effects of the inhibition of ANXII tPA–binding site by homocysteine.
We found that ANXII expression was higher in APL cell-transplanted mice than in recipients of normal murine BM. Both of the strategies that we used confirmed that ANXII was overexpressed in APL cell–transplanted mice compared with wild-type mice. Interestingly, Menell et al reported an MFC ratio in human APL cells that was 6.9-fold higher than what we observed.14 This could be because of different Ab specificity or a different degree of expression in the transgenic leukemic mice compared with human APL. Furthermore, we found a correlation between the number of CD117 cells and ANXII expression, which suggests that overexpression of ANXII could be because of the higher number of CD117+ cells in leukemic mice. However, when only CD117+ cells were analyzed, we still observed a difference, indicating that leukemic cells express higher levels of ANXII compared with their normal counterparts.
There is little information available about coagulopathy in the mouse model that we used. Studies performed so far showed that higher levels of TAT, TNFα, and PAI1 are present.23 A major concern is that only a small volume of plasma can be collected from each animal. We observed that tPA levels are abnormally higher in leukemic mice, which may indicate activation of fibrinolysis and is consistent with human APL. No alterations were observed in plasmin levels, but this protein has a very short half-life in plasma (only a few seconds) and therefore elevations may not be detected or may be restricted to the site of fibrinolysis activation. Furthermore, TAT levels were higher in leukemic mice, although the difference was of marginal statistical significance.
Menell et al14 demonstrated that transfection of APL-1 cells with ANXII cDNA increased plasmin production by 28-fold in medium with tPA and plasminogen, and strategies used to inactivate ANXII transcription led to abnormal thrombi clearance.23 In the present study, we observed that murine leukemic cells expressed higher levels of ANXII and tPA, but not of plasmin and TAT. Although these findings are not sufficient to demonstrate a direct correlation between ANXII expression and tPA levels, it is worth noting that the administration of 2 competitors of the tPA-binding site of ANXII (homocysteine and the hexapeptide LCKLSL) reduced tPA plasma concentrations in leukemic mice to levels similar to those in controls.
We successfully cloned and expressed the p36 component of ANXII in an expression vector to verify if higher concentrations of ANXII could lead to alterations in tPA or TAT. One previous report of ANXII infusion in rats used a dose of 1 μg/kg24 ; we tested the same dose and a 10 μg/kg dose. Plasma levels of tPA were slightly increased in contrast to TAT levels, which were increased by 8.75-fold. Moreover, we failed to demonstrate in the present study that the changes in tPA and TAT were dose dependent. However, the administration of L-methionine to mice that received ANXII infusion caused the reversal of the changes in tPA and TAT levels. These results are in agreement with those obtained in leukemic animals, supporting the idea that blocking the binding of ANXII to tPA may help to control ANXII-induced fibrinolysis activation.
The changes in tPA levels were not similar in the 2 models evaluated. We hypothesize that leukemic cells were more efficient in increasing tPA levels than the infusion of ANXII p36 because ANXII is a heterotetramer composed of 2 p36 components and 2 S100A10 (p11) components.25 Whereas overexpression of the p36 unit by itself can increase plasminogen cleavage to plasmin by 60-fold,17 the heterotetramer can increase plasmin production up to 340-fold.26 We cloned only the p36 component of the tetramer; it is possible that the effect would be greater if we had used the complete structure that occurs in leukemic cells. Furthermore, in the leukemic mice, the fibrinolysis activation may be concentrated in the BM (among the leukemic animals, none presented with leukocytosis), leading to local plasminogen activation. Moreover, tPA levels did not have a dose-dependent effect, but tPA is not produced directly by fibrinolysis. Instead, its concentration has a compensatory effect during fibrinolysis activation. The administration of L-methionine to leukemic mice or to wild-type mice that received ANXII infusion lowered tPA and TAT levels to normal levels, reinforcing the importance of ANXII in inducing coagulation abnormalities.
A drawback to our study is that our results are based only on an animal model and may not accurately reflect conditions in human APL. However, the difference in the MFC ratio between mice and humans indicates that ANXII may be even more important in human APL than in leukemic hCG-PML-RARα mice.
Historically, the inhibition of fibrinolysis with ϵ-aminocaproic acid or tranexamic acid was associated with a better outcome in patients with APL.27-29 However, only small numbers of patients were studied, and a larger study did not confirm the utility of this approach,30 which was therefore abandoned. Conversely, L-methionine administration per se is not harmful in the short term and its cost is low. Moreover, Mennel et al reported that ANXII overexpression gradually reduces after onset of ATRA treatment and is not detected after 72 hours.14 Therefore, a therapeutic approach aimed at blocking ANXII-mediated hyperfibrinolysis could be restricted to this period of time. Furthermore, the connection between homocysteine and ANXII raises the question of whether patients with lower levels of homocysteine might benefit more from this approach. Finally, using antifibrinolytic drugs in combination with induced hyperhomocysteinemia could potentiate the treatment. A major concern that should be addressed in future studies is thrombosis, which has been related to the use of antifibrinolytics in patients with APL.31
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the staff of Laboratório Sabin (Brasília, Brazil) for help with homocysteine quantification.
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (grant number 573754/2008-0) and the Fundação de Apoio à Pesquisa do Estado de São Paulo (grant number 1998/14247-6 and a fellowship to R.H.J. through grant number 02/11086-9).
Authorship
Contribution: R.H.J. and E.M.R. designed the research and wrote the manuscript; R.H.J., B.A.S.-L., A.S.G.L., P.A., A.P.A.L., L.L.F.-P., L.O.O., S.C.B., M.T.L.B., M.S.B., A.B.G., and R.P.F. performed the experiments; and R.H.J. analyzed the results and produced the figures.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Eduardo Magalhães Rego, Division of Oncology/Hematology, Department of Internal Medicine; Medical School of Ribeirão Preto, University of São Paulo, Av. Bandeirantes 3900, CEP 14049-900, Ribeirão Preto, SP, Brazil; e-mail: emrego@hcrp.fmrp.usp.br.