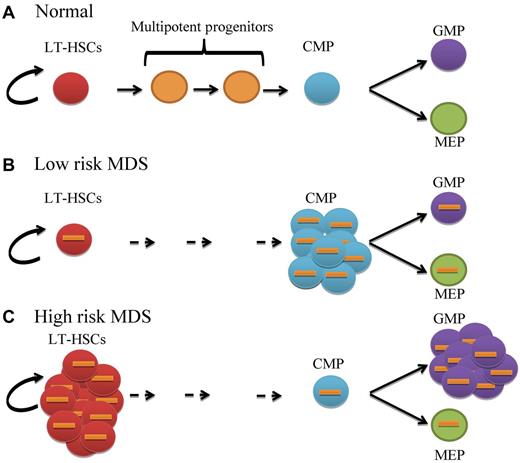
In this issue of Blood, Will et al identify aberrant expansion of distinct stem and progenitors in myelodysplastic syndromes (MDS) and demonstrate that MDS stem cells are functionally abnormal and harbor genetic and epigenetic alterations.1
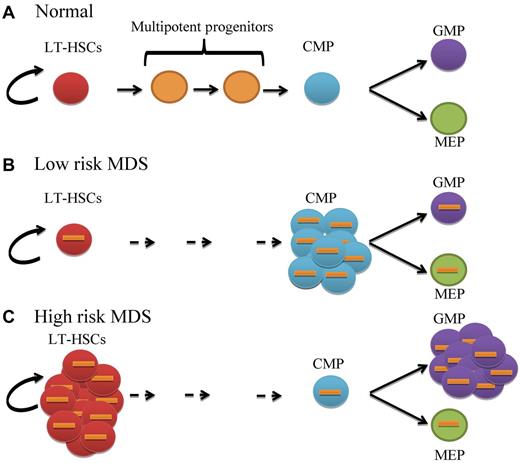
MDS is thought to be a disorder of hematopoietic stem cells (HSCs).2 Chromosomal abnormalities, mutations, and epigenetic changes have been reported in MDS progenitors.3,4 However, the earliest stage at which these molecular and pathogenic events occur and the functional consequences of these events has not been clearly established. In the present study, Will and colleagues functionally and molecularly characterize highly purified primitive stem cells and progenitors using technically challenging techniques in a variety of MDS subtypes. They show that the primitive long-term stem cell (LT-HSC) pool is significantly increased in high-risk MDS subtypes compared with healthy controls (see figure). Further analysis of committed progenitor populations shows that lower-risk MDS patients have skewed expansion of common myeloid progenitors (CMPs). In contrast, analysis of high-risk patients reveals expansion of the granulocyte-monocyte progenitors (GMPs) with a relative decrease in the megakaryocyte-erythrocyte progenitor (MEP) population. Notably, this concept of differential arrest is not unique to MDS; GMP expansion has recently been reported in acute myeloid leukemia (AML)5 and block in myeloid differentiation is a well-characterized feature of the blastic phase of chronic myeloid leukemia (CML-BP).6
Stage-specific aberrant expansion or differentiation block of distinct stem and progenitor populations in MDS. (A) Normal primitive long-term hematopoietic stem cells (LT-HSCs; red circles) have the capacity for self-renewal and give rise to a balanced number of multipotent progenitors (orange circles), common myeloid progenitors (CMPs; blue circles), granulocyte-monocyte progenitors (GMPs; purple circles) and megakaryocyte-erythrocyte progenitors (MEPs; green circles). (B) Low risk MDS LT-HSCs are cytogenetically abnormal (orange bars) with expansion of CMPs but normal GMP and MEP distribution. (C) High-risk MDS LT-HSCs are significantly expanded with increased GMP and slightly reduced MEP compartments. Status of multipotent progenitors in low- and high-risk patients has not been evaluated in this study.
Stage-specific aberrant expansion or differentiation block of distinct stem and progenitor populations in MDS. (A) Normal primitive long-term hematopoietic stem cells (LT-HSCs; red circles) have the capacity for self-renewal and give rise to a balanced number of multipotent progenitors (orange circles), common myeloid progenitors (CMPs; blue circles), granulocyte-monocyte progenitors (GMPs; purple circles) and megakaryocyte-erythrocyte progenitors (MEPs; green circles). (B) Low risk MDS LT-HSCs are cytogenetically abnormal (orange bars) with expansion of CMPs but normal GMP and MEP distribution. (C) High-risk MDS LT-HSCs are significantly expanded with increased GMP and slightly reduced MEP compartments. Status of multipotent progenitors in low- and high-risk patients has not been evaluated in this study.
Will and colleagues further investigate the functional and molecular signatures of primitive MDS HSCs. Karyotypic abnormalities are identified in the majority of immature HSCs from patients representing the various MDS subtypes. Importantly, the authors find that these cytogenetic abnormalities persist even in the expanded CMP and GMP populations. Further, they show MDS-HSCs are functionally deficient in their clonogenic potential and give rise to dysplastic colonies. The authors perform additional genome-wide methylomic and transcriptomic analysis of these rare stem cells and demonstrate that primitive MDS-HSCs are not only karyotypically and functionally abnormal but also exhibit widespread alteration in DNA methylation and gene expression. Although epigenetic alterations including hypermethylation have earlier been investigated in CD34+ cells7 and bone marrow cells8 from MDS patients, Will and colleagues are the first to characterize such patterns in primitive stem cells. In addition to identifying a subset of genes that are hypermethylated, they also describe a novel signature of hypomethylated genes in these cells, implicating a potential previously unrecognized role for altered hypomethylation in MDS pathogenesis. To validate these findings functionally the authors perform additional studies for STAT3, which they find to be significantly hypomethylated and overexpressed in HSCs from all tested subsets of MDS. They demonstrate that MDS HSCs are sensitive to pharmacologic inhibition of STAT3 in clonogenic assays, showing reduced colony formation compared with normal HSC controls. Taken together, these findings describe a list of candidate genes (including STAT3) that are dysregulated in primitive MDS stem cells that may serve as a target for stem cell–directed therapies for the disease.
Clinically, the most relevant finding of this study is that this cytogenetically abnormal stem cell pool persisted in a patient with complete morphologic remission after epigenetic therapy with azacytidine and vorinostat. Furthermore, through careful examination of serial samples, the authors demonstrate that morphologic relapse in this patient was preceded by expansion of the HSC compartment. This is consistent with another recent report that found persistence of rare and malignant HSCs in MDS with the 5q− abnormality in remission and in subsequent progression on lenalidomide therapy.3 These observations suggest that such analysis of the HSC compartment could be performed on MDS patients as both a metric for monitoring response and as a biomarker for development of targeted therapeutics. This further raises the possibility of whether the reason current treatment strategies are unable to cure these patients is the direct result of their inability to eliminate the residual, clonally abnormal HSC pool.
The big remaining question is whether MDS LT-HSCs, CMPs, and GMPs have capability to self-renew in vitro and in vivo, and if so, by what molecular mechanisms. A few studies to date suggest that phenotypic stem and progenitor cell compartments contain leukemia initiating cells in MDS.4,9 It will also be worth evaluating whether MDS GMPs may serve as a reservoir for disease progression to AML, because a recent study found that AML can arise from GMP-like stem cells.5,10 Lastly, interrogation of the precise molecular switches that control this lineage-dependent differentiation block in low- versus high-risk MDS patients will be of great interest and could represent a turning point in therapeutic strategies for the disease. It would also be interesting to know whether such changes occur before disease onset, potentially predisposing to low- versus high-risk MDS, or arises as part of disease progression.
The observation that a small pool of cancer-initiating stem cells cannot be readily eliminated by conventional cytotoxic therapies appears to be something of a common theme among a variety of cancers. In this regard, MDS is another in the queue where efforts are needed for targeting stem cells. Consequently, ongoing efforts to better understand the molecular pathways that regulate disease-initiating cells will potentially have further implications for the development of future targeted therapies in a variety of cancers. Overall, the study by Will and colleagues provides the impetus for defining the genetic and epigenetic events governing HSC and progenitor-cell resistance to therapy and their role in disease progression.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■