Abstract
Regulatory T (Treg) cells have broad suppressive activity on host immunity, but the fate and function of suppressed responder T cells remains largely unknown. In the present study, we report that human Treg cells can induce senescence in responder naive and effector T cells in vitro and in vivo. Senescent responder T cells induced by human Treg cells changed their phenotypes and cytokine profiles and had potent suppressive function. Furthermore, Treg-mediated molecular control of senescence in responder T cells was associated with selective modulation of p38 and ERK1/2 signaling and cell-cycle–regulatory molecules p16, p21, and p53. We further revealed that human Treg-induced senescence and suppressor function could be blocked by TLR8 signaling and/or by specific ERK1/2 and p38 inhibition in vitro and in vivo in animal models. The results of the present study identify a novel mechanism of human Treg cell suppression that induces targeted responder T-cell senescence and provide new insights relevant for the development of strategies capable of preventing and/or reversing Treg-induced immune suppression.
Introduction
Regulatory T (Treg) cells play a central role in controlling immune tolerance and homeostasis of the immune system, preventing autoimmune diseases, and limiting chronic inflammatory diseases.1,2 However, Treg cells can also inhibit effective immune responses against cancer and various pathogen infections.3-5 Therefore, it is critical to better define the suppressive mechanisms used by Treg cells in order to develop effective approaches for their clinical manipulation for therapeutic intervention. Significant progress has been made in delineating the molecules and mechanisms that Treg cells use to mediate suppression.6-8 These mechanisms include suppression by inhibitory cytokines and secreted molecules,9 by cytolysis or apoptosis of target cells,10-12 by consumption of limiting growth factors and metabolic disruption,12-14 and/or by affecting dendritic cell functions.15 The majority of previous studies were performed in animal models, so whether these mechanisms are also used by human Treg cells is still under investigation. In addition, the fate and function of responder T cells suppressed by Treg cells is unclear.
Cellular senescence was described initially more than 40 years ago in human fibroblasts with limited passages in cell culture.16 It is now well known that senescent cells have permanent cell-cycle arrest but remain viable and metabolically active and possess unique transcriptional profiles and gene-regulation signatures.17 There are 2 major categories of cellular senescence: replicative senescence (also known as telomere-dependent senescence)18-20 and premature senescence (also known as extrinsic senescence or telomere-independent senescence).17,21-23 Recent studies suggest that replicative senescence also occurs in the human immune system. Accumulation of senescent CD8+ T cells has been found in persons during normal aging, in younger persons with chronic viral infections, and in patients with certain types of cancers.24-27 Senescent CD8+ T cells show functional changes and have defective killing abilities due to the loss of perforin and granzyme or have defects in signaling of granule exocytosis.28,29 Furthermore, senescent CD8+ T cells have negative regulatory functions that reduce the effects of immunization and vaccinations and prolong the survival of allografts.26,30 Improved understanding of the molecular mechanisms used in the generation of senescent T cells and their functional alterations will open new avenues to restoring T-cell function and will help in the design of novel vaccines for infectious diseases and cancers.
In the present study, we explored the suppressive mechanisms used by human Treg cells and investigated the fate of Treg-treated responder T cells and found that treatment with CD4+CD25hi naturally occurring Treg cells can induce naive/effector T-cell senescence. We further identified the molecular signaling that controls the process of T-cell senescence and characterized these senescent T cells. In addition, our studies revealed that Treg-induced conversion of normal T cells into senescent cells with suppressive function can be blocked by the manipulation of TLR8 signaling and/or by specific MAPK signaling pathway inhibition in vitro and in vivo in animal models.
Methods
T cells and other cell lines
Buffy coats from healthy donors were obtained from the Gulf Coast Regional Blood Center at Houston. These studies were approved by the institutional review board. PBMCs were purified from buffy coats using Ficoll-Paque. Human naive and memory T cells were purified from PBMCs of healthy donors with EasySep enrichment kits (StemCell Technologies). The purity of naive and memory T cells was > 97%, as confirmed by flow cytometry. CD4+CD25hi Treg cells were purified from CD4+ T cells by FACS sorting after staining with anti-CD25–PE (BD Biosciences).
Senescence associated SA-β-Gal staining
Senescence associated β-galactosidase (SA-β-Gal) activity in senescent T cells was detected as described previously.31 Naive CD4+ or CD8+ T cells were labeled with CFSE (4.5μM), and cocultured with or without CD4+CD25hiFoxP3+ Treg cells or control T cells at different ratios of 10:1 to 1:1 in anti-CD3–coated 24-well plates for 3 or 5 days. Naive T cells were separated from cocultures using FACS sorting gated on CFSE+ populations or using Ab-coated microbeads (some experiments) and then cultured for an additional 3-5 days. The purified T cells were stained with SA-β-Gal and examined with a computerized image system composed of a Leica ICC50 camera system equipped on a Leica DM750 microscope (North Central Instruments). For some experiments, the cocultured T cells were determined to be SA-β-Gal+ populations in the presence of the various TLR ligands, cytokines, or MAPK inhibitors. Concentrations of TLR ligands were used as described previously.32,33 Cytokines included: IL-2 (300 and 600 IU/mL), IL-6 (2, 5, and 20 ng/mL), IL-7 (20 and 40 ng/mL), and IL-15 (20 ng/mL; R&D Systems). Neutralizing Abs included: anti–human LAP (TGF-β1; 1-10 μg/mL; R&D Systems), and anti–IL-10 (10-30 μg/mL; clone JES3-19F1; BD Biosciences). MAPK inhibitors included U0126 (1, 5, and 10μM), SB203580 (1, 5, and 10μM), and SP600125 (1, 5, and 10μM; Calbiochem).
Cell-cycle and apoptosis assays
Naive CD4+ T cells were cocultured with CFSE-labeled CD4+CD25hiFoxP3+ Treg cells in the presence of plate-bound anti-CD3 Ab (2 μg/mL) for 72 hours. After separation from cocultured Treg cells, apoptosis in naive CD4+ T cells was analyzed after staining with PE-labeled annexin V and 7-amino-actinomycin D (BD Biosciences) gating on CFSE− cell populations. For cell-cycle analysis, cocultured naive T cells were fixed with 70% ethanol overnight, washed with PBS, and incubated with propidium iodide (10 μg/mL) and RNase A (100 μg/mL). Naive CD4+ or CD8+ T cells cocultured with or without the CD4+CD25− effector T cells served as controls. All of the stained cells were analyzed on a FACSCalibur (BD Biosciences) and the data were analyzed with FlowJo software (TreeStar).
Functional proliferation assays
Proliferation assays were performed either by a CFSE dilution assay or a [3H]-thymidine incorporation assay, as described previously.32,33 To determine whether the T cell–suppressive activity could be blocked by specific Abs, suppressive function assays were performed in the absence or presence of various neutralizing Abs, including anti–IL-10 (10 and 30 μg/mL) or anti–TGF-β (1 and 10 μg/mL; R&D Systems), as described previously.32
Western blot analysis
CFSE-labeled naive CD4+ T cells were cocultured with CD4+CD25+ Treg cells or control T cells at a ratio of 5:1 in assay medium (5% human serum with no IL-2) in the presence of plate-bound anti-CD3 Ab (2 μg/mL) for 0, 1, 3, and 5 days. Naive CD4+ T cells were then sorted by FACS gating on CFSE+ T cells and cultured for an additional 3 days. Whole-cell lysates of the purified naive CD4+ T cells were prepared for Western blotting. The Abs used in Western blotting were as follows: anti-ERK, anti–phospho-ERK, anti-p38, anti–phospho-p38, anti-JNK, anti–phospho-JNK, anti–phospho-p53 (ser15), and rabbit antiactin polyclonal Abs (all Cell Signaling Technology); and anti-p53 goat polyclonal Ab, anti-p21 (C-19), and anti-p16 (C-2) rabbit polyclonal Abs (all Santa Cruz Biotechnology). In some experiments, MAPK inhibitors U0126 (10μM), SB203580 (10μM), and SP600125 (10μM; Calbiochem), or poly-G3 (3 μg/mL) were present in the cocultures and the treated T cells were then analyzed by Western blotting.
ELISA
Naive CD4+ or CD8+ T cells (1 × 106/mL) were cocultured with CD4+CD25hiFoxP3+ Treg cells or control CD4+CD25− T cells at a ratio of 5:1 in the presence of plate-bound anti-CD3 Ab (2 μg/mL) for 3 or 5 days. Naive T cells were then separated from cocultured Treg cells, followed by an additional 3- to 5-day culture. Cytokines in the culture supernatants were measured by ELISA (R&D Systems or eBiosciences) according to the manufacturer's instructions.
Flow cytometric analysis
The expression markers on Treg-treated T cells were determined by FACS analysis after surface staining or intracellular staining with anti–human specific Abs conjugated with either PE or FITC. These human Abs included: anti-CD4, anti-CD8, anti-CD25, anti-CD27, anti-CD28, anti-CD56, anti-CD80, anti–CTLA-4, anti–PD-1, anti–granzyme A and B, anti-perforin, anti–IFN-γ, anti–TNF-α, anti–IL-10, and anti-FoxP3, all of which were from BD Biosciences or eBiosciences. All stained cells were analyzed on a FACSCalibur flow cytometer (BD Biosciences) and data analyzed with FlowJo Version 7.6.1 software (TreeStar).
In vivo studies
Rag1−/− mice (lacking T and B cells) were originally purchased from the National Cancer Institute (National Institutes of Health, Bethesda, MD) and maintained in the institutional animal facility. All animal studies were approved by the institutional animal care committee. Naive CD8+ T cells (5 × 106/mouse), CD4+CD25hiFoxP3+ Treg cells (3 × 106/mouse), and CD4+CD25− effector T cells (3 × 106/mouse) were preactivated with anti-CD3 (2 μg/mL) and adoptively cotransferred into Rag1−/− mice through IV injection as follows: naive CD8+ T cells alone, naive CD8+ T cells plus CD4+CD25hiFoxP3+ Treg cells, and naive CD8+ T cells plus CD4+CD25− effector T cells. Five to 10 mice were included in each group. In a parallel experiment, anti-CD3–activated naive CD8+ T cells were pretreated with or without MAPK inhibitors (SB203580 10μM plus U0126 10μM) for 48 hours before adoptive transfer into the mice. In addition, Treg cells were pretreated with TLR8 ligand (poly-G3, 3 μg/mL) or control poly-T3 (3 μg/mL) for 48 hours before injection. Blood, lymph nodes, and spleens were harvested 12 days after injection and mononuclear cells were purified over Ficoll. The transferred human CD4+ and CD8+ T cells were isolated by Ab-coated microbeads for subsequent phenotypic and functional analyses in vitro. SA-β-Gal staining and 3H-thymidine incorporation assays were performed.
Statistical analysis
Unless indicated otherwise, data are expressed as means ± SD. The significance of difference between groups was determined by a 2-tailed Student t test or 1-way ANOVA.
Please refer to supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article) for information on TLR ligands, FoxP3 methylation–specific real-time quantitative PCR, lentivirus-shRNA transduction of T cells, and transcriptome analyses of Treg-induced senescent T cells. All microarray data are available at the Gene Expression Omnibus under accession number GSE38765.
Results
CD4+CD25hiFoxP3+ Treg cells induce cell-cycle arrest but not apoptosis or cytolysis in responder T cells
To explore the suppressive mechanisms used by human Treg cells, we purified naturally occurring CD4+ Treg cells with the highest expression of CD25 from healthy donors. We further confirmed that these CD4+CD25hi populations were almost all FoxP3+ T cells (Figure 1A). Recent studies have shown that human FoxP3 contains several highly conserved demethylation regions that are exclusive for Treg cells.34,35 We also observed that the Treg-specific demethylated region within the FoxP3 locus of CD4+CD25hiFoxp3+ T cells was almost completely demethylated compared with that of CD4+CD25− T cells (Figure 1B). We then investigated the suppressive capacity of CD4+CD25hiFoxP3+ Treg cells using functional proliferation assays. As shown in Figure 1C, CD4+CD25hiFoxP3+ Treg cells strongly inhibited the proliferation of naive CD4+ T cells in the presence of anti-CD3 Ab using the [3H]-thymidine incorporation assays. We further confirmed the suppression mediated by human CD4+CD25hiFoxP3+ naturally occurring Treg cells using CFSE dilution assays (Figure 1D).33 Theses results suggest that the human CD4+CD25hiFoxP3+ T-cell populations are Treg cells with potent suppressive activity.
The suppression of responder T cells mediated by CD4+CD25hiFoxP3+ Treg cells is due to the induction of G0/G1 cell-cycle arrest. (A) Isolation of CD4+CD25hiFoxP3+ Treg cells and CD4+CD25− effector T cells from PBMCs of healthy donors by FACS sorting. FoxP3 expression in the isolated T cells was further confirmed by FACS analyses. (B) Relative FoxP3 methylation levels of different T cells were determined by real-time quantitative PCR with methylation-specific primers, normalized to β-actin expression, and compared with the expression level of methylated FoxP3 in CD4+CD25− T cells. CD4+CD25− and anti-CD3–activated CD4+ T cells were included as controls. All experiments were performed in triplicate. (C-D) Suppression of naive T-cell proliferation by CD4+CD25hiFoxp3+ Treg cells. CD4+CD25− effector T cells served as a negative control displaying no suppressive activity. Naive CD4+ T cells were cocultured with Treg cells or control T cells at a ratio of 10:1. The proliferation of naive CD4+ T cells in the presence of anti-CD3 Ab was determined by [3H]-thymidine incorporation assays (C) or CFSE dilution assays (D). (E) Suppression of naive CD4+ T-cell proliferation mediated by CD4+CD25hiFoxp3+ Treg cells is not due to the induction of apoptosis. Naive CD4+ T cells were cocultured with CFSE-labeled Treg cells or CD4+CD25− cells in the presence of plate-bound anti-CD3 Ab. Apoptosis in naive CD4+ T cells was analyzed after staining with PE-labeled annexin V and 7-amino-actinomycin D gating on CFSE− cell populations. (F) CD4+CD25hiFoxP3+ Treg cells promoted the accumulation of naive CD4+ T cells in G0/G1 cell-cycle arrest. Cell treatment was the same as in panel E. Cell-cycle distribution in naive CD4+ T cells was analyzed after incubation with 1 μg/mL of propidium iodide and 100 μg/mL of RNase A. Naive CD4+ T cells cocultured with or without CD4+CD25− T cells served as controls. Data are representative of 3 independent experiments with similar results.
The suppression of responder T cells mediated by CD4+CD25hiFoxP3+ Treg cells is due to the induction of G0/G1 cell-cycle arrest. (A) Isolation of CD4+CD25hiFoxP3+ Treg cells and CD4+CD25− effector T cells from PBMCs of healthy donors by FACS sorting. FoxP3 expression in the isolated T cells was further confirmed by FACS analyses. (B) Relative FoxP3 methylation levels of different T cells were determined by real-time quantitative PCR with methylation-specific primers, normalized to β-actin expression, and compared with the expression level of methylated FoxP3 in CD4+CD25− T cells. CD4+CD25− and anti-CD3–activated CD4+ T cells were included as controls. All experiments were performed in triplicate. (C-D) Suppression of naive T-cell proliferation by CD4+CD25hiFoxp3+ Treg cells. CD4+CD25− effector T cells served as a negative control displaying no suppressive activity. Naive CD4+ T cells were cocultured with Treg cells or control T cells at a ratio of 10:1. The proliferation of naive CD4+ T cells in the presence of anti-CD3 Ab was determined by [3H]-thymidine incorporation assays (C) or CFSE dilution assays (D). (E) Suppression of naive CD4+ T-cell proliferation mediated by CD4+CD25hiFoxp3+ Treg cells is not due to the induction of apoptosis. Naive CD4+ T cells were cocultured with CFSE-labeled Treg cells or CD4+CD25− cells in the presence of plate-bound anti-CD3 Ab. Apoptosis in naive CD4+ T cells was analyzed after staining with PE-labeled annexin V and 7-amino-actinomycin D gating on CFSE− cell populations. (F) CD4+CD25hiFoxP3+ Treg cells promoted the accumulation of naive CD4+ T cells in G0/G1 cell-cycle arrest. Cell treatment was the same as in panel E. Cell-cycle distribution in naive CD4+ T cells was analyzed after incubation with 1 μg/mL of propidium iodide and 100 μg/mL of RNase A. Naive CD4+ T cells cocultured with or without CD4+CD25− T cells served as controls. Data are representative of 3 independent experiments with similar results.
Suppression of naive T-cell proliferation mediated by CD4+CD25hiFoxP3+ Treg cells could be due to the induction of apoptosis or cytolysis in the Treg-treated cells.6-8,12 To test these possibilities, we measured apoptosis and cell death in naive CD4+ T-cell populations cocultured with Treg cells. We found that naive CD4+ T cells in medium alone or treated with control CD4+CD25− T cells contained 9%-13% apoptotic T cells after stimulation with anti-CD3 Ab. Unexpectedly, CD4+CD25hiFoxP3+ Treg cells did not induce increased apoptosis or cell death in CD4+ T cells (Figure 1E). In parallel, we studied the cell-cycle distribution of the naive CD4+ T cells treated with CD4+CD25hiFoxP3+ Treg cells. We found that 55%-60% of anti-CD3–activated naive CD4+ T cells remained in G0/G1 in the medium alone and the control CD4+CD25− treatment groups. In contrast, more than 80% of naive CD4+ T cells treated with CD4+CD25hiFoxP3+ Treg cells remained in G0/G1, indicating that human Treg cell treatment promotes the accumulation of naive T cells in cell-cycle arrest (Figure 1F). These data suggest that CD4+CD25hiFoxP3+ Treg cells may use an unknown mechanism independent of apoptosis or cytolysis to exert their suppressive function on naive CD4+ T cells, resulting in cell-cycle arrest of treated responder T cells.
The suppression of responder naive/effector T cells mediated by human Treg cells is due to the induction of T-cell senescence
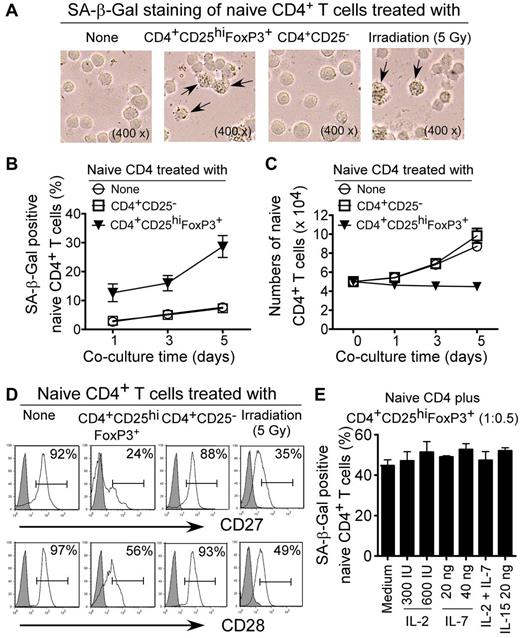
Senescent human cells have permanent cell-cycle arrest, but remain viable and metabolically active.17 Therefore, we reasoned that those Treg cells performing their suppressive function on naive T cells may act through induction of T-cell senescence. In addition to cell-cycle arrest and morphologic characteristics, SA-β-Gal was the first biomarker used to identify senescent human cells.31 As shown in Figure 2A, naive CD4+ T cells treated with ionizing radiation (5 Gy X-ray), which served as a positive control, induced cell senescence significantly, resulting in SA-β-Gal expression.36 However, naive CD4+ T cells cultured in medium only or cocultured with CD4+CD25− effector T cells did not induce SA-β-Gal expression. In contrast, we found significantly increased SA-β-Gal+ T-cell populations in naive CD4+ T cells after coculture with CD4+CD25hiFoxP3+ Treg cells, indicating that Treg cells can induce naive CD4+ T-cell senescence. We observed that the percentages of the SA-β-Gal+ cell populations in naive CD4+ T cells increased dramatically with longer times of coculture with CD4+CD25hiFoxP3+ Treg cells (Figure 2B). Furthermore, treatment with CD4+CD25hiFoxP3+ Treg cells inhibited naive T-cell growth and resulted in decreased cell numbers with increasing coculture time (Figure 2C). In addition, CD4+CD25hiFoxP3+ Treg cell treatment similarly induced senescence in naive CD8+ and memory CD4+ T cells (supplemental Figures 1 and 2). Accumulating evidence suggests that permanent loss of CD28 expression is the most consistent biologic indicator of aging in senescent T cells in elderly people and in patients with chronic viral infections.24,29 We found that both naive and memory T cells cocultured with CD4+CD25hiFoxP3+ Treg cells down-regulated the expression of the costimulatory molecules CD27 and CD28 dramatically, further suggesting immune dysfunction in these Treg-induced senescent responder T cells (Figure 2D and supplemental Figures 1B and 2B). We further confirmed the Treg-mediated down-regulation of CD28 expression at the mRNA level in responder naive CD4+ T cells (supplemental Figure 3). These data provide the first evidence that human CD4+CD25hiFoxP3+ Treg cells suppress T-cell proliferation through the induction of responder T-cell senescence.
Human CD4+CD25hiFoxP3+ Treg cells induce senescence in naive CD4+ T cells. (A) CD4+CD25hi FoxP3+ Treg cell treatment increased SA-β-Gal+ T-cell populations in naive CD4+ T cells significantly. Naive CD4+ T cells cultured in medium only or cocultured with CD4+CD25− effector T cells had little or no SA-β-Gal expression. Naive CD4+ T cells treated with ionizing radiation (5 Gy X-ray) served as positive controls exhibiting SA-β-Gal expression. CFSE-labeled naive CD4+ T cells were incubated alone or were cocultured with Treg cells or CD4+CD25− T cells at a ratio of 5:1 in the presence of plate-bound anti-CD3 (2 μg/mL) for 5 days. The treated naive CD4+ T cells were purified by FACS and stained with SA-β-Gal staining reagents after an additional 3-day culture. The SA-β-Gal+ T cells were identified with dark blue granules, as indicated by the arrows. (B) Time course of the induction of the SA-β-Gal+ T-cell populations in Treg-treated naive CD4+ T cells. The percentages of SA-β-Gal+ cells in naive CD4+ T cells treated with CD4+CD25hi FoxP3+ Treg cells were increased dramatically with progressive coculture time. Cell treatment and procedure were the same as in panel A. (C) Treatment with CD4+CD25hi FoxP3+ Treg cells inhibited naive T-cell growth and resulted in decreased cell numbers with increasing coculture time. Cell treatment and procedure were the same as in panel A and cell numbers were counted by FACS gated on the CFSE+ population. (D) Decreased expression of CD27 and CD28 in naive CD4+ T cells treated by CD4+CD25hi FoxP3+ Treg cells. Cell treatment and procedure were the same as in panel A. Naive CD4+ T cells treated with ionizing radiation (5 Gy X-ray) served as positive controls. CD27 and CD28 expression in treated naive CD4+ T cells were analyzed by FACS gating on CFSE+ populations. (E) The cytokines IL-2, IL-7, and IL-15 cannot prevent senescence identified by SA-β-Gal expression in naive CD4+ T-cell populations induced by CD4+CD25hi FoxP3+ Treg cells. Naive CD4+ T cells were cocultured with CD4+CD25hiFoxP3+ Treg cells for 3 days in the presence of various concentrations of the indicated cytokines. The treated naive CD4+ T cells were purified and SA-β-Gal expression was determined.
Human CD4+CD25hiFoxP3+ Treg cells induce senescence in naive CD4+ T cells. (A) CD4+CD25hi FoxP3+ Treg cell treatment increased SA-β-Gal+ T-cell populations in naive CD4+ T cells significantly. Naive CD4+ T cells cultured in medium only or cocultured with CD4+CD25− effector T cells had little or no SA-β-Gal expression. Naive CD4+ T cells treated with ionizing radiation (5 Gy X-ray) served as positive controls exhibiting SA-β-Gal expression. CFSE-labeled naive CD4+ T cells were incubated alone or were cocultured with Treg cells or CD4+CD25− T cells at a ratio of 5:1 in the presence of plate-bound anti-CD3 (2 μg/mL) for 5 days. The treated naive CD4+ T cells were purified by FACS and stained with SA-β-Gal staining reagents after an additional 3-day culture. The SA-β-Gal+ T cells were identified with dark blue granules, as indicated by the arrows. (B) Time course of the induction of the SA-β-Gal+ T-cell populations in Treg-treated naive CD4+ T cells. The percentages of SA-β-Gal+ cells in naive CD4+ T cells treated with CD4+CD25hi FoxP3+ Treg cells were increased dramatically with progressive coculture time. Cell treatment and procedure were the same as in panel A. (C) Treatment with CD4+CD25hi FoxP3+ Treg cells inhibited naive T-cell growth and resulted in decreased cell numbers with increasing coculture time. Cell treatment and procedure were the same as in panel A and cell numbers were counted by FACS gated on the CFSE+ population. (D) Decreased expression of CD27 and CD28 in naive CD4+ T cells treated by CD4+CD25hi FoxP3+ Treg cells. Cell treatment and procedure were the same as in panel A. Naive CD4+ T cells treated with ionizing radiation (5 Gy X-ray) served as positive controls. CD27 and CD28 expression in treated naive CD4+ T cells were analyzed by FACS gating on CFSE+ populations. (E) The cytokines IL-2, IL-7, and IL-15 cannot prevent senescence identified by SA-β-Gal expression in naive CD4+ T-cell populations induced by CD4+CD25hi FoxP3+ Treg cells. Naive CD4+ T cells were cocultured with CD4+CD25hiFoxP3+ Treg cells for 3 days in the presence of various concentrations of the indicated cytokines. The treated naive CD4+ T cells were purified and SA-β-Gal expression was determined.
Because the proliferation assays used to measure Treg suppressive activity involve anti-CD3 Ab stimulation, we reasoned that TCR activation in responder T cells was required for the induction of senescence. Our data indicated that naive T-cell senescence was induced by human Treg cells rather than by anti-CD3 stimulation, but that TCR activation is required and augmented this process (supplemental Figure 4). IL-2 consumption is one of the major suppressive mechanisms used by Treg cells.12,13 Furthermore, a recent study reported that mouse CD4+CD25+FoxP3+ Treg cells can induce cytokine deprivation–mediated apoptosis in effector CD4+ T cells.12 However, we did not observe any alterations of senescent T-cell populations induced by CD4+CD25hiFoxP3+ Treg cells with the addition of the prosurvival cytokines, including IL-2, IL-7, and IL-15 into the cocultures (Figure 2E). These results indicate that human Treg-cell–induced T-cell senescence is not due to cytokine deprivation.
Human Treg cells induce the selective modulation of MAPK p38 and ERK1/2 signaling pathways in responder T cells that control the molecular process of Treg-induced senescence
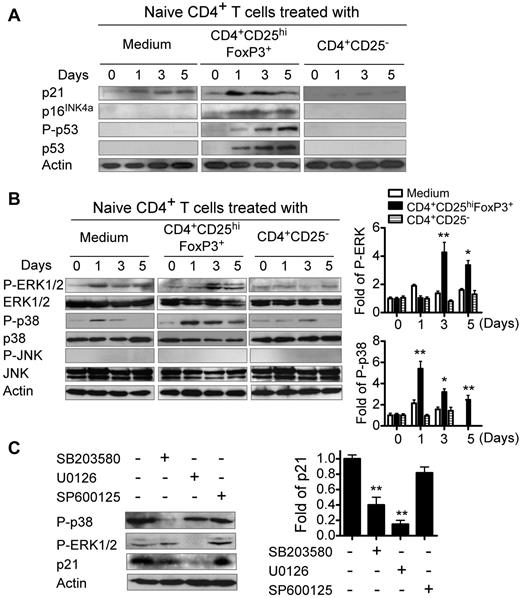
Previous studies have shown that senescent growth arrest is established and maintained by the p53/p21 and/or p16/pRB tumor-suppressor pathways.17,37,38 We reasoned that cell-cycle–regulatory molecules including p53, p21, and p16 might be involved in Treg-induced conversion of T cells into senescent cells. As shown in Figure 3A, we found significantly increased p53, p21, and p16 expression in naive CD4+ T cells after treatment with CD4+CD25hiFoxP3+ Treg cells. In addition, we observed a dramatic increase in p53 phosphorylation in senescent CD4+ T cells induced by Treg cells. These data indicate that cell-cycle–regulatory molecules are involved in human Treg-induced T-cell senescence.
Cell-cycle–regulatory molecules and phosphorylated activation of MAPKs are involved in Treg-induced senescence. (A) Cell-cycle–regulatory molecules p21, p16, and p53 are involved in human Treg-induced T-cell senescence. (B) CD4+CD25hiFoxP3+ Treg cells induced phosphorylation of ERK and p38 in senescent CD4+ T cells. Results from Western blotting analyses of phosphorylated activation of MAPKs are shown in the left panel. Phosphorylated ERK and p38 protein levels shown in the right histogram were analyzed quantitatively and compared against the β-actin expression level with a densitometer. Results shown in the histogram are means ± SD from 3 independent experiments. *P < .05 and **P < .01 compared with the medium and CD4+CD25− control groups. (C) ERK and p38 signaling regulated cell-cycle–regulatory molecule p21 expression in senescent CD4+ T cells. The left panel shows the Western blot analysis results. The right panel shows p21 expression analyzed quantitatively and compared with β-actin expression with a densitometer. Results shown in the histogram are means ± SD from 3 independent experiments. **P < .01 compared with the group not treated with inhibitor. CFSE-labeled naive CD4+ T cells were cocultured with CD4+CD25hiFoxp3+ Treg cells at a ratio of 5:1 in anti-CD3 Ab (2 μg/mL)–precoated plates in the presence or absence of different inhibitors (10μM) SB203580 (p38 inhibitor), U0126 (ERK1/2 inhibitor), or SP600125 (JNK inhibitor) for 0, 1, 3, and 5 days. Cocultured naive CD4+ T cells were purified by FACS and then lysates were prepared for Western blot analyses. Data shown in panel C are the results from day 3 cocultured CD4+ T cells. Data shown in panels A through C are representative of 3 independent experiments with similar results.
Cell-cycle–regulatory molecules and phosphorylated activation of MAPKs are involved in Treg-induced senescence. (A) Cell-cycle–regulatory molecules p21, p16, and p53 are involved in human Treg-induced T-cell senescence. (B) CD4+CD25hiFoxP3+ Treg cells induced phosphorylation of ERK and p38 in senescent CD4+ T cells. Results from Western blotting analyses of phosphorylated activation of MAPKs are shown in the left panel. Phosphorylated ERK and p38 protein levels shown in the right histogram were analyzed quantitatively and compared against the β-actin expression level with a densitometer. Results shown in the histogram are means ± SD from 3 independent experiments. *P < .05 and **P < .01 compared with the medium and CD4+CD25− control groups. (C) ERK and p38 signaling regulated cell-cycle–regulatory molecule p21 expression in senescent CD4+ T cells. The left panel shows the Western blot analysis results. The right panel shows p21 expression analyzed quantitatively and compared with β-actin expression with a densitometer. Results shown in the histogram are means ± SD from 3 independent experiments. **P < .01 compared with the group not treated with inhibitor. CFSE-labeled naive CD4+ T cells were cocultured with CD4+CD25hiFoxp3+ Treg cells at a ratio of 5:1 in anti-CD3 Ab (2 μg/mL)–precoated plates in the presence or absence of different inhibitors (10μM) SB203580 (p38 inhibitor), U0126 (ERK1/2 inhibitor), or SP600125 (JNK inhibitor) for 0, 1, 3, and 5 days. Cocultured naive CD4+ T cells were purified by FACS and then lysates were prepared for Western blot analyses. Data shown in panel C are the results from day 3 cocultured CD4+ T cells. Data shown in panels A through C are representative of 3 independent experiments with similar results.
MAPK signaling pathways play a major role in regulating cell-cycle reentry and oncogenic ras-induced senescence.39,40 It has been reported that ERK1/2 and p38 activation can induce p21-dependent G1 cell-cycle arrest.41 Therefore, in the present study, we investigated whether human Treg-induced naive T-cell cycle G0/G1 arrest and conversion of naive CD4+ T cells into senescent T cells involved MAPK signaling modulation. We first conducted transcriptome analyses of Treg-induced senescent CD8+ T cells using Illumina whole-genome HumanHT-12 BeadChips. Our transcriptome analyses demonstrated that Treg-induced senescent CD8+ T cells induced significant alterations in genes involved in MAPK signaling pathways (supplemental Figure 5). We then confirmed the activation of MAPKs, including ERK1/2, p38, and JNK in naive CD4+ T cells treated with CD4+CD25hiFoxP3+ Treg cells using Western blot analyses. We found that Treg-treated naive CD4+ T cells activated ERK1/2 and p38, but not JNK, selectively, resulting in significantly enhanced phosphorylation of ERK1/2 and p38 (Figure 3B). To further determine the role of ERK1/2 and p38 signaling in regulating the cell cycle of Treg-induced senescent T cells, we used specific pharmacologic inhibitors to block ERK1/2 and p38 activities in the senescent T cells. The optimal concentrations (10μM) of different inhibitors used for our experiments, including SP600125 (a JNK inhibitor), SB203580 (a p38 inhibitor), and U0126 (an ERK1/2 inhibitor), were selected based on their toxic effects on responder T-cell viability and proliferation (supplemental Figure 6). As shown in Figure 3C, we found that the inhibitors SB203580 and U0126 almost completely blocked the phosphorylation of p38 and ERK1/2 in Treg-induced senescent T cells, respectively. Furthermore, we observed that both SB203580 and U0126 decreased p21 expression in senescent T cells markedly. However, SP600125 did not affect the phosphorylation of p38, ERK1/2, or p21 expression in senescent T cells. These results suggest that Treg cell treatment may modulate the MAPK pathways in responder T cells selectively, resulting in differential induction of T-cell senescence.
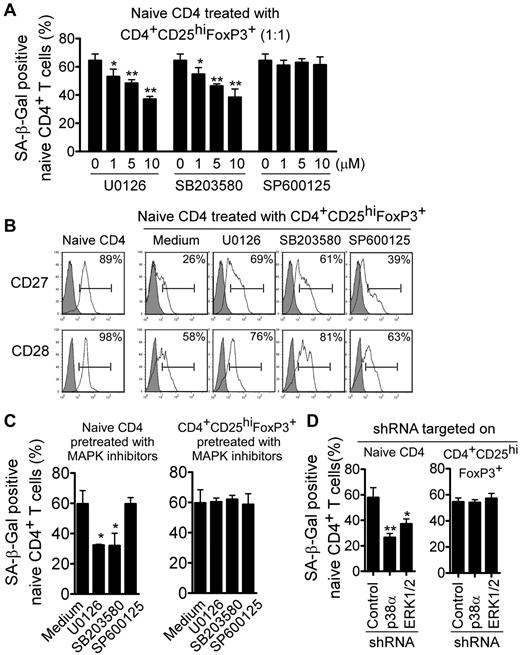
We also investigated whether ERK1/2 and p38 signaling can control the process of Treg-induced senescence. We determined the alteration of senescent cell populations in responder T cells cocultured with Treg cells after blockage of MAPK signaling. We found that the inhibitors U0126 and SB203580, but not SP600125, reduced Treg-induced senescence in naive CD4+ T cells significantly and in a dose-dependent manner (Figure 4A). In addition, we found that the expression of CD27 and CD28 in Treg-induced senescent T cells was restored markedly after treatment with inhibitors specific for ERK1/2 and p38 signaling, but not for JNK signaling (Figure 4B). To exclude the possibilities that the MAPK inhibitors might also target Treg cells or both Treg and responder T cells, naive CD4+ T cells and Treg cells were pretreated with these MAPK inhibitors. We found that only pretreatment of naive CD4+ T cells, but not Treg cells, with p38 and ERK inhibitors decreased the induction of senescent T-cell populations significantly in Treg-treated responder T cells (Figure 4C). We then used siRNA to specifically knock down p38 and ERK1/2 genes in naive CD4+ T cells or Treg cells and measured the effects on Treg-induced responder T-cell senescence.32,33 Consistent with the results obtained in the inhibitor experiments described in Figure 4C, we found that knockdown of p38 and ERK1/2 in naive CD4+ T cells, but not in Treg cells, decreased the senescent T-cell populations significantly (Figure 4D). Our results indicate that human CD4+CD25hiFoxP3+ Treg cells induce selective modulation of specific MAPK p38 and ERK1/2 signaling pathways in responder T cells that control the molecular process of Treg-induced T-cell senescence.
Blockage of ERK1/2 and p38 signaling in responder T cells prevents Treg-induced T-cell senescence. (A-B) Inhibition of ERK1/2 or p38 signaling pathways by specific pharmacologic inhibitors reversed the T-cell senescence induced by CD4+CD25hiFoxP3+ Treg cells significantly, resulting in decreased SA-β-Gal expression (A) and restored CD27 and CD28 expression in Treg-treated naive T cells (B). CFSE-labeled naive CD4+ T cells were cocultured with Treg cells in anti-CD3–coated (2 μg/mL) plates in the presence or absence of different dosages of the inhibitors U0126, SB203580, or SP600125 (1, 5, or 10μM) for 5 days. The treated naive CD4+ T cells were purified by FACS and then stained with SA-β-Gal staining reagents or analyzed for CD27 and CD28 expression. *P < .05 and **P < .01 compared with the group not treated with inhibitor. (C) Pretreatment with inhibitors in naive CD4+ T cells, but not in Treg cells, decreased the SA-β-Gal+ CD4+ T-cell populations. Naive CD4+ T cells or CD4+CD25hiFoxp3+ Treg cells were pretreated with each MAPK inhibitor (10μM) for 2 days and cocultured with untreated Treg cells or naive CD4+ T cells, respectively, for 5 days. The number of SA-β-Gal+–naive CD4+ T cells was then determined. *P < .05 compared with the group not treated with inhibitor. (D) Knockdown of ERK1/2 and p38 genes by shRNA in naive CD4+ T cells but not in CD4+CD25hi FoxP3+ Treg cells reversed Treg-induced T-cell senescence dramatically. Naive CD4+ T cells or Treg cells were transfected with lentiviral shRNAs specific for ERK1/2 or p38 molecules. Transduced (green fluorescent protein–positive) naive CD4+ T cells or Treg cells were purified by FACS sorting and then cocultured with untransduced Treg cells or naive CD4+ T cells, respectively, for 5 days. The number of SA-β-Gal+–naive CD4+ T cells was then determined. *P < .05 and **P < .01 compared with the group transduced with control shRNA. Data shown are representative of 3 independent experiments with similar results.
Blockage of ERK1/2 and p38 signaling in responder T cells prevents Treg-induced T-cell senescence. (A-B) Inhibition of ERK1/2 or p38 signaling pathways by specific pharmacologic inhibitors reversed the T-cell senescence induced by CD4+CD25hiFoxP3+ Treg cells significantly, resulting in decreased SA-β-Gal expression (A) and restored CD27 and CD28 expression in Treg-treated naive T cells (B). CFSE-labeled naive CD4+ T cells were cocultured with Treg cells in anti-CD3–coated (2 μg/mL) plates in the presence or absence of different dosages of the inhibitors U0126, SB203580, or SP600125 (1, 5, or 10μM) for 5 days. The treated naive CD4+ T cells were purified by FACS and then stained with SA-β-Gal staining reagents or analyzed for CD27 and CD28 expression. *P < .05 and **P < .01 compared with the group not treated with inhibitor. (C) Pretreatment with inhibitors in naive CD4+ T cells, but not in Treg cells, decreased the SA-β-Gal+ CD4+ T-cell populations. Naive CD4+ T cells or CD4+CD25hiFoxp3+ Treg cells were pretreated with each MAPK inhibitor (10μM) for 2 days and cocultured with untreated Treg cells or naive CD4+ T cells, respectively, for 5 days. The number of SA-β-Gal+–naive CD4+ T cells was then determined. *P < .05 compared with the group not treated with inhibitor. (D) Knockdown of ERK1/2 and p38 genes by shRNA in naive CD4+ T cells but not in CD4+CD25hi FoxP3+ Treg cells reversed Treg-induced T-cell senescence dramatically. Naive CD4+ T cells or Treg cells were transfected with lentiviral shRNAs specific for ERK1/2 or p38 molecules. Transduced (green fluorescent protein–positive) naive CD4+ T cells or Treg cells were purified by FACS sorting and then cocultured with untransduced Treg cells or naive CD4+ T cells, respectively, for 5 days. The number of SA-β-Gal+–naive CD4+ T cells was then determined. *P < .05 and **P < .01 compared with the group transduced with control shRNA. Data shown are representative of 3 independent experiments with similar results.
Phenotypic and functional features of human Treg-induced senescent T cells
We next sought to investigate whether Treg-induced senescent T cells have significant phenotypic and functional changes after separation from cocultured Treg cells and after an additional 5-day culture. We first examined the phenotypes of Treg-induced senescent T cells using flow cytometric analyses. As shown in Figure 5A and supplemental Figure 7A, both senescent CD4+ and CD8+ T cells induced by CD4+CD25hiFoxP3+ Treg cells down-regulated costimulatory molecules CD27 and CD28 dramatically, which is consistent with our previous results. Furthermore, we found that Treg-induced senescent T cells had minimal or no expression of CD25 and FoxP3 and did not express increased CTLA-4, indicating that Treg-induced senescent T cells do not exhibit the characteristics of CD4+CD25+ naturally occurring Treg cells. In addition, we found that senescent CD4+ and CD8+ T cells also expressed the cytotoxicity-associated markers CD56, granzyme A, and FasL, as well as the inhibitory molecule PD-1, in a manner similar to anti-CD3–stimulated naive T cells (data not shown). However, we did not find any expression alterations among these molecules in responder T cells during the process of senescence, except that Treg cells induced markedly decreased PD-1 expression in senescent responder CD8+ T cells.
Characterization of human Treg-induced senescent T cells. (A) CD27 and CD28, but not other markers in naive CD4+ T cells, were decreased significantly by treatment with CD4+CD25hiFoxP3+ Treg cells. The expression markers on Treg-treated T cells were determined by the FACS analysis. (B) Cytokine profile of Treg-induced senescent CD4+ T cells. CD4+ T cells treated with CD4+CD25− effector T cells served as a control. Treated CD4+ T cells were purified and cytokine secretion evaluated using ELISA. Cytokine concentrations (pg/mL) are shown as the means ± SD from 3 independent experiments. (C) Suppressive function of Treg-induced senescent T cells. Both senescent CD4+ and CD8+ T cells induced by CD4+CD25hiFoxp3+ Treg cells inhibited the proliferation of responding CD4+ T cells strongly. In contrast, naive T cells treated or untreated with control CD4+CD25− effector T cells did not affect the proliferation of responding CD4+ T cells. CFSE-labeled naive CD4+ and CD8+ T cells were cocultured with CD4+CD25hiFoxP3+ Treg cells or control CD4+CD25− effector T cells for 3 days. Treated CD4+ and CD8+ T cells were purified and the suppressive activities on CD4+ T-cell proliferation were evaluated using [3H]-thymidine incorporation assays. Data are representative of 3 independent experiments with similar results. (D) Neutralizing Abs against IL-10 and TGF-β failed to block the suppressive activity mediated by Treg-induced senescent T cells. The suppression mediated by senescent T cells on the proliferative activity of additional naive CD4+ T cells was determined using [3H]-thymidine incorporation assays in the presence of mouse anti–human neutralizing Abs against IL-10 and TGF-β. Results shown are means ± SD from 3 independent experiments.
Characterization of human Treg-induced senescent T cells. (A) CD27 and CD28, but not other markers in naive CD4+ T cells, were decreased significantly by treatment with CD4+CD25hiFoxP3+ Treg cells. The expression markers on Treg-treated T cells were determined by the FACS analysis. (B) Cytokine profile of Treg-induced senescent CD4+ T cells. CD4+ T cells treated with CD4+CD25− effector T cells served as a control. Treated CD4+ T cells were purified and cytokine secretion evaluated using ELISA. Cytokine concentrations (pg/mL) are shown as the means ± SD from 3 independent experiments. (C) Suppressive function of Treg-induced senescent T cells. Both senescent CD4+ and CD8+ T cells induced by CD4+CD25hiFoxp3+ Treg cells inhibited the proliferation of responding CD4+ T cells strongly. In contrast, naive T cells treated or untreated with control CD4+CD25− effector T cells did not affect the proliferation of responding CD4+ T cells. CFSE-labeled naive CD4+ and CD8+ T cells were cocultured with CD4+CD25hiFoxP3+ Treg cells or control CD4+CD25− effector T cells for 3 days. Treated CD4+ and CD8+ T cells were purified and the suppressive activities on CD4+ T-cell proliferation were evaluated using [3H]-thymidine incorporation assays. Data are representative of 3 independent experiments with similar results. (D) Neutralizing Abs against IL-10 and TGF-β failed to block the suppressive activity mediated by Treg-induced senescent T cells. The suppression mediated by senescent T cells on the proliferative activity of additional naive CD4+ T cells was determined using [3H]-thymidine incorporation assays in the presence of mouse anti–human neutralizing Abs against IL-10 and TGF-β. Results shown are means ± SD from 3 independent experiments.
We then evaluated cytokine profiles elaborated by the Treg-induced senescent T cells stimulated with anti-CD3 Ab using ELISA. Senescent CD4+ T cells secreted large amounts of the proinflammatory cytokines IL-6 and TNF-α, but not other cytokines, including IL-1β, IL-2, or IL-4 (Figure 5B). Recent studies have demonstrated that inflammatory cytokines and chemokines such as IL-6 and IL-8 also play a key role in the induction of premature senescence.22,23 In addition, we observed high amounts of IFN-γ secreted by Treg-induced senescent responder T cells, which differs from the findings in senescent T cells obtained from older people.24 These Treg-induced senescent CD4+ T cells secreted large amounts of IL-10 and moderate amounts of TGF-β1, whereas naive CD4+ T cells treated with control CD4+CD25− T cells did not secrete any IL-10 or TGF-β1 (Figure 5B), suggesting that these senescent T cells may have a negative regulatory function. We confirmed the cytokine secretion by Treg-treated CD4+ T cells using intracellular staining with FACS analyses (supplemental Figure 8). In addition, we determined the cytokine profiles elaborated by Treg-induced senescent CD8+ T cells and obtained similar results as shown in Treg-induced CD4+ T cells (supplemental Figure 7B).
We next investigated whether human Treg-induced senescent T cells also have negative regulatory functions. As shown in Figure 5C, we found that both senescent CD4+ and CD8+ T cells induced by CD4+CD25hiFoxP3+ Treg cells inhibited the proliferation of responding CD4+ T cells strongly. In contrast, naive CD4+ and CD8+ T cells treated with or without control CD4+CD25− T cells had no suppressive effect on the proliferation of responding CD4+ T cells. These results suggest that by inducing cell senescence in naive T cells, human Treg cells can convert them into suppressive T cells. Because we found that IL-10 and TGF-β were produced by these senescent T cells, we investigated whether these 2 cytokines were involved in the suppressive function mediated by senescent T cells. However, neither anti–IL-10– nor anti–TGF-β–neutralizing Ab could block the suppressive effect of the senescent T cells, suggesting that immune suppression mediated by senescent T cells is not through the suppressive cytokines IL-10 and TGF-β (Figure 5D). Our results indicate that human Treg cells can convert naive and/or effector T cells into senescent cells that have unique phenotypes, secrete proinflammatory and suppressive cytokines, and possess potent suppressive activity.
Reversal of human Treg-induced responder T-cell senescence through TLR8 signaling
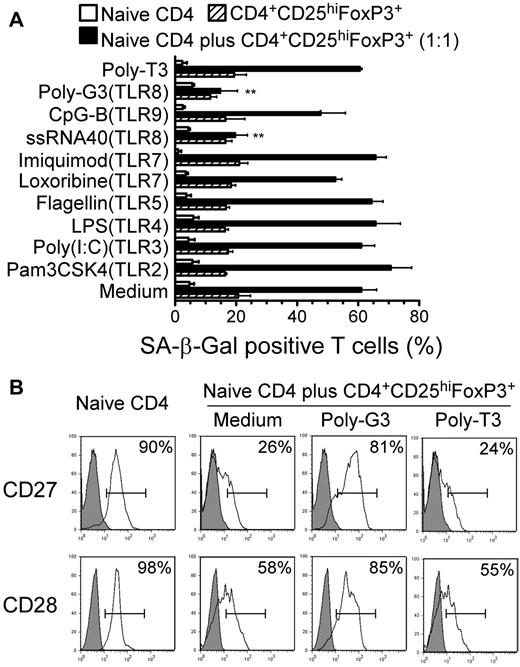
We demonstrated recently that TLR8 signaling reversed the suppressive function mediated by different subsets of Treg cells, including CD4+, CD8+, and γδ Treg cells.32,33 Therefore, in the present study, we investigated whether TLR8 signaling can also inhibit the process of Treg-induced senescence. We cocultured naive CD4+ T cells with CD4+CD25hiFoxP3+ Treg cells in the presence or absence of a panel of TLR ligands and control poly-T3 and tested for their ability to block the induction of T-cell senescence. We found that only the TLR8 ligands poly-G3 and ssRNA40 blocked the induction of responder T-cell senescence induced by CD4+CD25hiFoxP3+ Treg cells significantly, as shown by SA-β-Gal expression (Figure 6A). Furthermore, the TLR8 ligand poly-G3 restored the lost expression of CD27 and CD28 molecules markedly and inhibited ERK1/2 and p38 signaling activation in CD4+CD25hiFoxP3+ Treg-treated responder naive CD4+ T cells significantly (Figure 6B and supplemental Figure 9). To exclude the possibility that TLR8 ligands inhibited Treg-induced senescence through direct effects on responder T cells, we pretreated Treg cells or responder naive CD4+ T cells with TLR ligands. We observed that pretreatment of Treg cells with poly-G3 decreased the senescent responder CD4+ T-cell population dramatically. However, pretreatment of naive CD4+ T cells with the TLR8 ligand poly-G3 did not prevent Treg-induced T-cell senescence (supplemental Figure 10). These results clearly indicate that TLR8 signaling can reverse human Treg-induced responder T-cell senescence by abrogating Treg-suppressive activity.
TLR8 ligands reverse Treg-induced T-cell senescence. (A) The TLR8 ligands poly-G3 and ssRNA40, but not ligands for other TLRs, reversed markedly the ability of human CD4+CD25hiFoxP3+ Treg cells to induce naive CD4+ T-cell senescence. CFSE-labeled naive CD4+ T cells were incubated alone or cocultured with Treg cells in anti-CD3–coated plates in the presence of the indicated TLR ligands for 5 days. The treated naive CD4+ T cells were purified by FACS sorting and the SA-β-Gal+ T-cell populations in the different groups were determined. Poly-T3 (3 μg/mL) served as a control. **P < .01 compared with the groups treated with medium or other TLR ligands. Data shown are representative of 3 independent experiments with similar results. (B) Poly-G3 restored the expression of CD27 and CD28 in naive CD4+ T cells induced by human CD4+CD25hiFoxp3+ Treg cells. Naive CD4+ T cells were cultured with CD4+CD25hiFoxP3+ Treg at a ratio of 1:1 in the presence of poly-G3 or control poly-T3 for 5 days. Treated naive CD4+ T cells were separated and CD27 or CD28 expression was analyzed by FACS.
TLR8 ligands reverse Treg-induced T-cell senescence. (A) The TLR8 ligands poly-G3 and ssRNA40, but not ligands for other TLRs, reversed markedly the ability of human CD4+CD25hiFoxP3+ Treg cells to induce naive CD4+ T-cell senescence. CFSE-labeled naive CD4+ T cells were incubated alone or cocultured with Treg cells in anti-CD3–coated plates in the presence of the indicated TLR ligands for 5 days. The treated naive CD4+ T cells were purified by FACS sorting and the SA-β-Gal+ T-cell populations in the different groups were determined. Poly-T3 (3 μg/mL) served as a control. **P < .01 compared with the groups treated with medium or other TLR ligands. Data shown are representative of 3 independent experiments with similar results. (B) Poly-G3 restored the expression of CD27 and CD28 in naive CD4+ T cells induced by human CD4+CD25hiFoxp3+ Treg cells. Naive CD4+ T cells were cultured with CD4+CD25hiFoxP3+ Treg at a ratio of 1:1 in the presence of poly-G3 or control poly-T3 for 5 days. Treated naive CD4+ T cells were separated and CD27 or CD28 expression was analyzed by FACS.
Control of human Treg-induced conversion of responder T cells into senescent T cells by the manipulation of TLR8 signaling and/or by specific MAPK signaling pathway inhibition in vivo
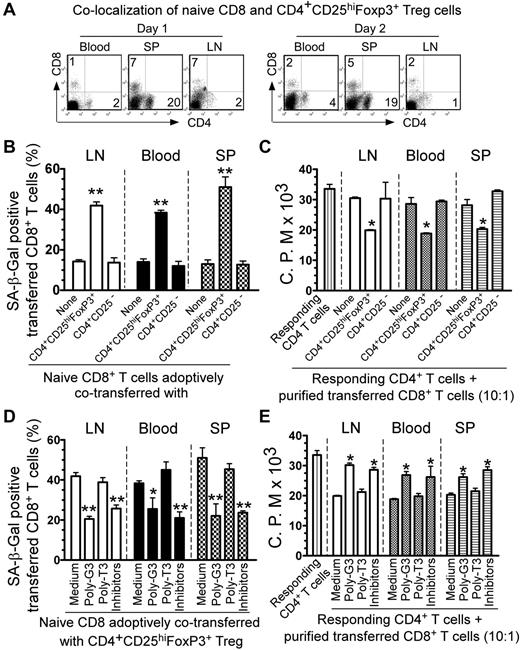
We next explored whether human CD4+CD25hiFoxP3+ Treg cells can convert naive CD4+ and CD8+ T cells into senescent T cells with potent suppressive activity in vivo using our previously established adoptive transfer model.32,33 We showed that adoptively transferred human Treg cells can colocalize with cotransferred responder CD8+ T cells in vivo in a Rag1−/− mouse model (Figure 7A). We then preactivated naive CD8+ T cells and CD4+CD25hiFoxP3+ Treg cells with anti-CD3 Ab and adoptively cotransferred these cells into Rag1−/− mice in different combinations. Transferred human CD8+ T cells were isolated from blood, lymph nodes, and spleens to determine their senescence and suppressive activity. As shown in Figure 7B, we found that approximately 10%-15% of adoptively transferred, preactivated naive CD8+ T cells became senescent T cells in Rag1−/− mice at 12 days after injection. However, we observed that significantly increased senescent T-cell populations were induced in preactivated naive CD8+ T cells when cotransferred with CD4+CD25hiFoxP3+ Treg cells (over 40%). In contrast, cotransfer with CD4+CD25− T cells did not promote naive T-cell senescence. These results indicate that human Treg cells can induce responder T-cell senescence in vivo. The percentages of senescent T-cell populations in transferred, preactivated naive T cells isolated from different organs were very similar. We also determined the suppressive activity of the recovered CD8+ T cells on the proliferation of responding T cells using 3H-thymidine incorporation assays. As expected, we found that the purified CD8+ T cells from different organs previously cotransferred with CD4+CD25hiFoxP3+ Treg cells could suppress the proliferation of responding CD4+ T cells potently in vitro. In contrast, purified CD8+ T cells previously cotransferred with or without CD4+CD25− T cells did not have any suppressive activity (Figure 7C).
Reversal of Treg-induced senescence by TLR8 ligand and MAPK inhibitors in vivo. (A) Colocalization of adoptively transferred naive CD8+ T cells and CD4+CD25hiFoxP3+ Treg cells in Rag1−/− mice. Naive CD8+ T cells (5 × 106/mouse) and CD4+CD25hiFoxP3+ Treg cells (5 × 106/mouse) were injected intravenously into Rag1−/− mice. Blood, lymph nodes (LN), and spleens (SP) were harvested from the mice on days 1 and 2 after injection. Cells were purified and analyzed for CD4+ and CD8+ T-cell populations using FACS with anti–human CD4 and CD8 Abs. (B) Increased SA-β-Gal+ cell populations were induced markedly in naive CD8+ T cells after cotransfer with CD4+CD25hiFoxP3+ Treg cells, whereas cotransfer with CD4+CD25− T cells did not induce increased senescent CD8+ T cells. (C) The purified CD8+ T cells cotransferred with CD4+CD25hiFoxP3+ Treg cells had potent suppressive activity on the proliferation of responding CD4+ T cells. Naive CD8+ T cells (5 × 106/mouse), CD4+CD25hiFoxP3+ Treg cells (3 × 106/mouse), and CD4+CD25− T cells (3 × 106/mouse) were preactivated with anti-CD3 Ab and adoptively cotransferred into Rag1−/− mice. Blood, lymph nodes, and spleens were harvested at 12 days after injection. The transferred human CD8+ T cells were isolated for subsequent SA-β-Gal staining (B) and 3H-thymidine incorporation (C) assays. *P < .05 and **P < .01 compared with the groups cotransferred with CD4+CD25− T cells or alone. (D-E) Pretreatment of CD4+CD25hiFoxP3+ Treg cells with poly-G3 or naive T cells with ERK and p38 inhibitors before coinjection was able to block significantly the induction of senescence and reverse the suppressive activity in transferred naive CD8+ T cells. Naive CD8+ T cells were pretreated with ERK and p38 inhibitors and/or CD4+CD25hiFoxp3+ Treg were pretreated with poly-G3 or poly-T3 (control) for 2 days before cotransfer. The transferred human CD8+ T cells in different organs were isolated at 12 days after injection for subsequent SA-β-Gal staining (D) and 3H-thymidine incorporation (E) assays. *P < .05 and **P < .01 compared with the medium-only and poly-T3 groups.
Reversal of Treg-induced senescence by TLR8 ligand and MAPK inhibitors in vivo. (A) Colocalization of adoptively transferred naive CD8+ T cells and CD4+CD25hiFoxP3+ Treg cells in Rag1−/− mice. Naive CD8+ T cells (5 × 106/mouse) and CD4+CD25hiFoxP3+ Treg cells (5 × 106/mouse) were injected intravenously into Rag1−/− mice. Blood, lymph nodes (LN), and spleens (SP) were harvested from the mice on days 1 and 2 after injection. Cells were purified and analyzed for CD4+ and CD8+ T-cell populations using FACS with anti–human CD4 and CD8 Abs. (B) Increased SA-β-Gal+ cell populations were induced markedly in naive CD8+ T cells after cotransfer with CD4+CD25hiFoxP3+ Treg cells, whereas cotransfer with CD4+CD25− T cells did not induce increased senescent CD8+ T cells. (C) The purified CD8+ T cells cotransferred with CD4+CD25hiFoxP3+ Treg cells had potent suppressive activity on the proliferation of responding CD4+ T cells. Naive CD8+ T cells (5 × 106/mouse), CD4+CD25hiFoxP3+ Treg cells (3 × 106/mouse), and CD4+CD25− T cells (3 × 106/mouse) were preactivated with anti-CD3 Ab and adoptively cotransferred into Rag1−/− mice. Blood, lymph nodes, and spleens were harvested at 12 days after injection. The transferred human CD8+ T cells were isolated for subsequent SA-β-Gal staining (B) and 3H-thymidine incorporation (C) assays. *P < .05 and **P < .01 compared with the groups cotransferred with CD4+CD25− T cells or alone. (D-E) Pretreatment of CD4+CD25hiFoxP3+ Treg cells with poly-G3 or naive T cells with ERK and p38 inhibitors before coinjection was able to block significantly the induction of senescence and reverse the suppressive activity in transferred naive CD8+ T cells. Naive CD8+ T cells were pretreated with ERK and p38 inhibitors and/or CD4+CD25hiFoxp3+ Treg were pretreated with poly-G3 or poly-T3 (control) for 2 days before cotransfer. The transferred human CD8+ T cells in different organs were isolated at 12 days after injection for subsequent SA-β-Gal staining (D) and 3H-thymidine incorporation (E) assays. *P < .05 and **P < .01 compared with the medium-only and poly-T3 groups.
We next investigated whether we could prevent the induction of senescent T cells mediated by Treg cells in vivo by the manipulation of TLR8 signaling and/or specific MAPK signaling inhibition in this adoptive transfer model. Treg cells were pretreated with TLR8 ligand (poly-G3) or control poly-T3, and naive T cells were pretreated with the MAPK kinase inhibitors U0126 and SB203580 for 24 hours. The treated or untreated cells were cotransferred into Rag1−/− mice following the same procedures described in Figure 7B and C. The senescent cell populations and suppressive activity of the recovered CD8+ T cells were then investigated 12 days after injection. As shown in Figure 7D, we found that pretreatment of CD4+CD25hiFoxP3+ Treg cells with poly-G3 and/or naive T cells with ERK and p38 inhibitors blocked the induction of cell senescence in transferred, preactivated naive CD8+ T cells significantly. Furthermore, we observed that anti-CD3–preactivated naive T cells pretreated with ERK and p38 inhibitors did not develop suppressive activity after being cotransferred with Treg cells. In addition, pretreatment of Treg cells with poly-G3, but not poly-T3, prevented these Treg cells from inducing senescent T cells with suppressive activity in vivo (Figure 7E). These results indicate that human Treg cells can convert responder T cells into senescent T cells with suppressive function both in vitro and in vivo, and that manipulation of TLR8 signaling in Treg cells and/or specific MAPK signaling inhibition in responder T cells can prevent Treg-mediated induction of T-cell senescence and subsequent immune suppression.
Discussion
In the present study, we investigated the suppression mediated by naturally occurring human CD4+CD25hiFoxP3+ Treg cells and found that human Treg cells can strongly suppress naive and effector T-cell proliferation through the induction of responder T-cell senescence. These studies identify a novel suppressive mechanism mediated by human Treg cells that is different from the previous findings in murine and human systems that Treg cells can mediate responder T-cell apoptosis or death.12,42 A better understanding of the biologic and functional changes and molecular processes responsible for these changes induced in responder T cells by Treg cells will be critical for the manipulation of human Treg-mediated immune suppression and therapeutic interventions.
Accumulation of senescent CD8+CD28null T cells has been found in patients with chronic viral infections and with certain types of cancers, but the mechanisms responsible for the induction of these senescent cells have been unclear.24-27,43,44 Considering elevated levels of different types of Treg cells in patients with various cancers and chronic infections, our current findings that human Treg cells induce T-cell senescence can at least partially explain the accumulation of senescent T cells in certain patients. We further characterized the Treg-induced senescent T cells and showed that these cells have significant phenotypic and functional changes. Our studies demonstrated that Treg-induced senescent T cells down-regulated the expression of the costimulatory molecules CD27 and CD28 significantly, indicating their dysfunction. Furthermore, these Treg-induced senescent T cells possessed a potent suppressive activity, which is consistent with the findings from other groups that senescent CD8+ T cells have negative regulatory functions on immune responses induced by vaccination and transplantation.26,30 The results of the present study strongly suggest that senescent T cells could amplify the immunosuppressive effects mediated by Treg cells indirectly. In addition to inhibiting immune cells directly through an unknown mechanism, these Treg-induced senescent T cells could also affect bystander immune cells through additional actions. First, these Treg-induced senescent T cells secreted large amounts of proinflammatory cytokines such as IL-6, IL-8, and TNF-α, which have been shown to be critical inducers of premature senescence.22,23 These elevated proinflammatory cytokines secreted by Treg-induced senescent responder T cells might also result in the induction of more senescent T cells under pathologic conditions, including within tumor-suppressive microenvironments and in chronically infected patients. In addition, our data clearly indicate that these Treg-induced senescent T cells secreted large amounts of IL-10 and moderate amounts of TGF-β1. These 2 suppressive cytokines play important roles in the induction of adaptive Treg cells and the maintenance of immunosuppressive microenvironments. Our results suggest that Treg cells are important inducers of senescent T cells and, conversely, that these Treg-induced senescent T cells may also be important mediators and amplifiers of immunosuppressive microenvironments under both physiologic and pathologic conditions (supplemental Figure 11).
Our present findings also partially address the critical question of what molecular processes are involved in the Treg-induced conversion of responder T cells into senescent cells. We found that Treg-induced senescent T cells have a distinct molecular signature that is different from that of anergic T cells (data not shown). Recent studies have demonstrated that MAPK signaling pathways play a major role in regulating cell-cycle reentry and oncogenic ras-induced senescence.39-41 The results of the present study show clearly that human Treg cells modulated the specific MAPK p38 and ERK1/2 signaling pathways selectively in responder T cells. In addition, our in vitro and in vivo studies suggest that specific MAPK signaling pathway inhibition in responder T cells can prevent the induction of T-cell senescence mediated by Treg cells. These studies open new avenues for the development of novel strategies to block Treg-mediated immune suppression and restore responder T-cell functions.
It is now widely acknowledged that a significant challenge for successful antitumor immunotherapy and active vaccination is the development of strategies to eliminate or reverse the suppressive functions of Treg cells within the tumor microenvironment and during chronic infections.3,5 Recently, several strategies, including depletion or blockage of Treg-suppressive activities through targeting of CD25, CTLA-4, or GITR molecules, have been used in animal models and clinical trials and have yielded promising results. However, these strategies can concurrently eliminate activated effector T cells, prevent effector T-cell activities, and induce Treg replenishment.45,46 Recent alternative strategies that manipulate TLR signaling via dendritic cells or in Treg cells to reverse the Treg-suppressive function have been developed.47,48 We have also demonstrated previously that human TLR8 signaling can completely reverse the suppressive functions of naturally occurring CD4+CD25+ Treg cells and tumor-derived CD4+, CD8+, and γδ Treg cells, but does not affect naive/effector CD4+ T-cell proliferation or function.32,33 The results of the present study also show that manipulation of TLR8 signaling in Treg cells can prevent human Treg-induced T-cell senescence by abrogating Treg-suppressive activity in vitro and in vivo. These studies not only indicate that TLR8 signaling is critically important for the prevention of human Treg-suppressive function, but also demonstrate the feasibility of developing novel strategies capable of augmenting immune responses directed against infectious diseases and cancer.
In summary, the results of the present study provide the first evidence that human Treg cells suppress naive and effector T-cell proliferation and function directly through the induction of responder T-cell senescence (supplemental Figure 11). We have also shown that Treg-induced senescent T cells altered their phenotypes and functions. In our efforts to identify the molecular mechanisms controlling Treg-induced T-cell senescence, we demonstrated that ERK1/2 and p38 signaling are critical for this molecular process. Our studies also demonstrated that Treg-induced responder T-cell senescence can be blocked by TLR8 signaling and/or by specific MAPK signaling inhibition. These studies provide new insights relevant for the development of strategies for controlling Treg-induced immune suppression in various clinical settings, including autoimmune diseases, transplantation, infectious diseases, and cancer.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Richard Di Paolo for providing Rag1−/− mice, Joy Eslick and Sherri Koehm for FACS sorting and analyses, Dr Seth Crosby for performing microarray analyses, Dr Macro Colonna for critical reading and discussion, and Dr Ratna B. Ray for kindly providing the human foreskin fibroblast cell line.
This work was supported in part by grants from the American Cancer Society (RSG-10-160-01-LIB to G.P.), the Melanoma Research Alliance (to G.P.), and the National Institutes of Health (to G.P.).
National Institutes of Health
Authorship
Contribution: J.Y. and G.P. designed the research, analyzed the data, prepared the figures, and wrote the manuscript; X.H., E.C.H., M.A.V., and D.F.H. provided advice on research design and reviewed the manuscript; Q.Z. performed the transcriptome analyses of Treg-induced senescent T cells; and J.Y., C.M., and Y.Z. performed the experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Guangyong Peng, MD, PhD, Division of Infectious Diseases, Allergy & Immunology and Department of Internal Medicine, St Louis University School of Medicine, 1100 S Grand Blvd, Doisy Research Ctr, Rm 813, St Louis, MO 63104; e-mail: gpeng@slu.edu.

![Figure 1. The suppression of responder T cells mediated by CD4+CD25hiFoxP3+ Treg cells is due to the induction of G0/G1 cell-cycle arrest. (A) Isolation of CD4+CD25hiFoxP3+ Treg cells and CD4+CD25− effector T cells from PBMCs of healthy donors by FACS sorting. FoxP3 expression in the isolated T cells was further confirmed by FACS analyses. (B) Relative FoxP3 methylation levels of different T cells were determined by real-time quantitative PCR with methylation-specific primers, normalized to β-actin expression, and compared with the expression level of methylated FoxP3 in CD4+CD25− T cells. CD4+CD25− and anti-CD3–activated CD4+ T cells were included as controls. All experiments were performed in triplicate. (C-D) Suppression of naive T-cell proliferation by CD4+CD25hiFoxp3+ Treg cells. CD4+CD25− effector T cells served as a negative control displaying no suppressive activity. Naive CD4+ T cells were cocultured with Treg cells or control T cells at a ratio of 10:1. The proliferation of naive CD4+ T cells in the presence of anti-CD3 Ab was determined by [3H]-thymidine incorporation assays (C) or CFSE dilution assays (D). (E) Suppression of naive CD4+ T-cell proliferation mediated by CD4+CD25hiFoxp3+ Treg cells is not due to the induction of apoptosis. Naive CD4+ T cells were cocultured with CFSE-labeled Treg cells or CD4+CD25− cells in the presence of plate-bound anti-CD3 Ab. Apoptosis in naive CD4+ T cells was analyzed after staining with PE-labeled annexin V and 7-amino-actinomycin D gating on CFSE− cell populations. (F) CD4+CD25hiFoxP3+ Treg cells promoted the accumulation of naive CD4+ T cells in G0/G1 cell-cycle arrest. Cell treatment was the same as in panel E. Cell-cycle distribution in naive CD4+ T cells was analyzed after incubation with 1 μg/mL of propidium iodide and 100 μg/mL of RNase A. Naive CD4+ T cells cocultured with or without CD4+CD25− T cells served as controls. Data are representative of 3 independent experiments with similar results.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/120/10/10.1182_blood-2012-03-416040/4/m_zh89991294780001.jpeg?Expires=1765143100&Signature=bcSoMZjWCLxOUg0E5s7M02OP8gMUbUNHKX6sS6xsoiR3VlVYw3oK2JSENb1TnxZORBy-TTxCrlvTguVviH8M9tvpHg6MzCipYfqmC~JsrREmISPDwRSQOXFunRHYim5sUReHRRSb1fjr~S~SgXpyBhO5ar8mPLizM-NU83WXTavg2CbnyQ-sC3Y6abirbQJn7j8mpXyJoPZtS~SoELweXDQ0RHH4XMSRhau3KLWE5OIZUDKDTk5L44ZdfkgL01Iqu-O8nodJsnGhIwq~Bv83dk1WGdPleInSLOB1rVwSe~YgJWV3o1mZqPahLF17XtR-6-0nNAORvj---JKl1Y92Rw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Characterization of human Treg-induced senescent T cells. (A) CD27 and CD28, but not other markers in naive CD4+ T cells, were decreased significantly by treatment with CD4+CD25hiFoxP3+ Treg cells. The expression markers on Treg-treated T cells were determined by the FACS analysis. (B) Cytokine profile of Treg-induced senescent CD4+ T cells. CD4+ T cells treated with CD4+CD25− effector T cells served as a control. Treated CD4+ T cells were purified and cytokine secretion evaluated using ELISA. Cytokine concentrations (pg/mL) are shown as the means ± SD from 3 independent experiments. (C) Suppressive function of Treg-induced senescent T cells. Both senescent CD4+ and CD8+ T cells induced by CD4+CD25hiFoxp3+ Treg cells inhibited the proliferation of responding CD4+ T cells strongly. In contrast, naive T cells treated or untreated with control CD4+CD25− effector T cells did not affect the proliferation of responding CD4+ T cells. CFSE-labeled naive CD4+ and CD8+ T cells were cocultured with CD4+CD25hiFoxP3+ Treg cells or control CD4+CD25− effector T cells for 3 days. Treated CD4+ and CD8+ T cells were purified and the suppressive activities on CD4+ T-cell proliferation were evaluated using [3H]-thymidine incorporation assays. Data are representative of 3 independent experiments with similar results. (D) Neutralizing Abs against IL-10 and TGF-β failed to block the suppressive activity mediated by Treg-induced senescent T cells. The suppression mediated by senescent T cells on the proliferative activity of additional naive CD4+ T cells was determined using [3H]-thymidine incorporation assays in the presence of mouse anti–human neutralizing Abs against IL-10 and TGF-β. Results shown are means ± SD from 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/120/10/10.1182_blood-2012-03-416040/4/m_zh89991294780005.jpeg?Expires=1765143100&Signature=C8VOK3xR6I5o3Kvvksk2GVrGwULB2HMjGzL3a77Ic30gCca7gWBwG4QXY-FeHzQPR175DpIoVHHEfyemGkJBx8l4Tk-RmskYvlNkW2DthEFw5S78R51inyWNRo6ct83lwc2fMBB6yxcrk-RTsA6-MqXGajcajRaJQGwIkVLYjrXuDsrvELyzzLFpqL5K1g0MTd1Rw-JtbwbiAO1~yHpj~Ip7vFd9IXhEa1sUa-6N3iICdZgesNcRPsD1t23KGA5svM3rd1473xT2T8pb-uNwvCCYgrgB0oDoSIhdy5FxudJjqxRzR0ThkrV2ck3x3dUJ01SEYsbcigJ3o8~FRWQ7sg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)