Abstract
Immune thrombocytopenia (ITP) is a bleeding disorder in which antibodies and/or T cells lead to enhanced peripheral platelet destruction and reduced bone marrow platelet production. Several reports have observed that ITP is associated with a peripheral deficiency of tolerance-inducing CD4+CD25+FoxP3+ T regulatory cells (Tregs). Using a murine model of ITP, we analyzed Tregs in the spleen and thymus. CD61 knockout mice were immunized against wild-type (CD61+) platelets, and their splenocytes were transferred into severe combined immunodeficient (SCID) mice. Compared with SCID mice receiving naive splenocytes, within 2 weeks after transfer, the ITP SCID mice became thrombocytopenic (< 200 × 109 platelets/L) and had increased serum anti-CD61 antibodies. The quantity of thymic Tregs by 2 weeks after transfer was significantly elevated, whereas Tregs in the spleens were significantly reduced. Treatment of the ITP mice with 2 g/kg intravenous immunoglobulin raised the platelet counts, reduced antibody production, and normalized the thymic and splenic Treg populations. Compared with thymocytes from ITP mice treated with intravenous immunoglobulin, thymocytes from untreated ITP mice delayed the onset of ITP when administered before engraftment with immune splenocytes. These results suggest that ITP in mice is associated with a peripheral Treg deficiency because of thymic retention and therapy normalizes the Tregs.
Introduction
Immune thrombocytopenia (ITP) is an autoimmune bleeding disorder characterized by the presence of plasma antibodies that recognize platelet autoantigens, which leads to premature platelet clearance by Fcγ receptor (R)–mediated phagocytosis in the reticuloendothelial system.1-5 It has also been suggested that the immune response against platelets can affect the bone marrow, leading to megakaryocyte destruction and/or inhibition and impaired platelet production.6,7 Of interest, the early self-administration experiments of Harrington et al who infused healthy volunteers with the plasma from ITP patients only caused thrombocytopenia in 17 of the 26 infusions (65%), suggesting that a mechanism of thrombocytopenia other than antibodies may be present.8 In 2003, Olsson et al9 confirmed this finding by showing that, in patients with no detectible antiplatelet antibodies, thrombocytopenia was indeed the result of platelet destruction by CD8+ cytotoxic T cells, and this was confirmed by other laboratories.10,11 We subsequently developed a mouse model of ITP that demonstrated both antibody- and cell-mediated thrombocytopenia in which the antibody-mediated form was sensitive to intravenous gammaglobulin (IVIg) treatment, whereas the cell-mediated thrombocytopenia was not.11 Taken together, these results suggest that the immunopathogenesis of ITP is more complex than was originally thought, in that some patients with ITP, particularly chronic ITP, have a cell-mediated form of thrombocytopenia in addition to autoantibody-mediated platelet destruction.
Within the last 6 years, the immunopathologic complexity of ITP has been further supported by many laboratories demonstrating that patients with active ITP have a peripheral deficiency of the CD4+CD25+FoxP+ T helper subset known as T regulatory cells (Tregs).12-29 Tregs are thymic-derived T cells that are critical in maintaining self-tolerance, and genetic deficiencies of this T-cell population lead to overwhelming autoimmunity, such as the immunodysregulation polyendocrinopathy enteropathy X-linked syndrome in humans and the Scurfy phenotype in mice.30,31 Many autoimmune diseases, such as rheumatoid arthritis, type I diabetes, and multiple sclerosis, now are associated with peripheral Treg deficiencies.32,33 The reasons for the Treg deficiency in active ITP is unknown, but several therapies that raise platelet counts do so in association with normalizing the observed Treg deficiency.15,16,18,20,26 Because our murine model of ITP was dependent on the presence of CD4+ T cells, we determined whether the low platelet counts were associated with Treg abnormalities. We demonstrate here that active murine ITP is associated with a peripheral (splenic) Treg deficiency, but concomitantly, the thymus of ITP mice had significant elevations in Treg percentages. IVIg treatment raised platelet counts and normalized the thymic and splenic Treg proportions. The results suggest that the peripheral Treg deficiency in active ITP may be the result of a thymic sequestration of Tregs, and these retained T cells are released when platelet counts are increased by therapy.
Methods
Mice
For platelet donors or splenocyte transfer recipients, 8- to 10-week-old female BALB/c (H-2d) and CB.17 (H-2d) severe combined immunodeficient (SCID) mice, respectively, were obtained from The Jackson Laboratory. BALB/c CD61 KO mice were obtained from H.N. All mice were housed in the Li Ka Shing Knowledge Institute's Research Vivarium, and all animal studies were approved by the St Michael's Hospital Animal Care Committee.
Platelet preparation and immunization of CD61 KO mice
Leukoreduced platelets were prepared as previously described.34 Briefly, blood was drawn by cardiac puncture from anesthetized donor mice into PBS containing citrate/phosphate, dextrose, acetate (CPDA buffer). The blood was pooled, diluted with PBS-CPDA, centrifuged at 150g for 15 minutes, and the platelet-rich plasma was collected. The platelet-rich plasma was washed 3 times by centrifuging at 450g for 18 minutes. The washed platelets were resuspended in PBS-CPDA, counted, and their concentration adjusted to 1 × 109 cells/mL. For immunization, BALB/c CD61 KO mice were bled 24 hours before the first transfusion and then injected with 100 μL (108 platelets) CD61+ platelets via the tail vein weekly for 3 weeks. Their sera was tested weekly for the presence of anti-CD61 antibodies by flow cytometry using wild-type (WT) CD61 platelets; and when the titers were found to be > 1:1600, the immune mice were killed by cervical dislocation and their spleens removed, homogenized in RPMI 1640, and washed by centrifugation at 400g for 15 minutes. Spleen homogenates were further treated with ACK lysing solution and washed to remove RBCs. The splenocytes were then adjusted in PBS to a concentration of 1.5 × 105 cells/mL. Nontransfused (naive) BALB/c mice were used as source of control splenocytes.
Generation of active ITP
ITP was induced as previously described but with modifications.11 Briefly, CB.17 SCID mice were first γ-irradiated (2 cGy) and NK cell–depleted by an intraperitoneal infusion of 50 μL of a rabbit anti-asialo GM1 antibody (Wako Pure Chemical Industries) on day −1; and on day 0, 100 μL (1.5 × 104) of the indicated splenocytes was transferred intraperitoneally. At this low dose of transferred splenocytes, the IVIg-resistant cell-mediated thrombocytopenia is eliminated so that only the antibody-mediated form of ITP is present. Platelet counts were measured weekly using a Beckman Coulter Counter-LH750 hematology analyzer.
Serum anti-CD61 antibody production
To detect recipient IgG anti-CD61 production in the transferred SCID mice, platelets from BALB/c mice were prepared as described in “Platelet preparation and immunization of CD61 KO mice” and 1 × 106 platelets were incubated with 3 μL of undiluted serum for 30 minutes at room temperature. The platelet-serum mixtures were washed once with PBS and then labeled with an FITC-conjugated goat anti–mouse IgG (FITC-GAM, Fc specific; Caltag Laboratories) in the dark at room temperature for 30 minutes. The mixture was washed before being analyzed by flow cytometry.
IVIg treatment
The indicated groups of engrafted SCID mice were injected intraperitoneally with 2 g/kg IVIg (Gamunex 10%; Talecris Biotherapeutics) 1 day before splenocyte transfer and twice weekly thereafter as previously described.11 All mice were bled weekly for platelet counts and serum antibody levels.
Thymus and spleen preparations for Treg enumeration
Mice from the indicated groups were killed each week, and their spleens and thymuses were removed, cleaned of fat and any apparent lymph nodes, weighed, and prepared into single-cell suspensions by homogenization in RPMI. The splenocyte suspension was further treated with ACK lysing buffer to remove RBCs. Cells were washed 2 times by centrifugation at 400g for 15 minutes. Cells were adjusted in cell medium to a concentration of 1 × 108 cells/mL and were used to enumerate the percentage of Tregs using a commercially available Treg kit (eBioscience) according to the manufacturer's instructions. Briefly, thymocytes and splenocytes were surface stained with FITC-conjugated anti–mouse CD4 and PE-conjugated anti–mouse CD25 for 45 minutes at 4°C, washed, fixed, and permeabilized using the Foxp3 staining buffer, and subsequently stained with anti–mouse PE-Cy5 Foxp3. Cells were analyzed using a BD FACSort flow cytometer (BD Biosciences) by gating 10 000 CD4+CD25hi+ cells and then enumerating the percentage of gated FoxP3+ cells. The proportional data were adjusted for differences in splenic and thymic mass to reflect the total lymphocyte content of these organs as previously described35,36 and to indicate whether there were absolute changes in lymphocyte numbers in addition to proportional changes in Treg distribution. It was calculated by the formula: organ weight × percent Tregs.
Thymus histology
Mice were killed and thymuses were removed, cleaned of fat and any bronchial lymph nodes, and placed in buffered 10% formalin, pH 6.8-7.2 at room temperature for 2-3 days, followed by auto-processing of the tissue using a Leica TP1020 auto tissue processor (Leica Microsystem), and the fixed tissue was embedded in wax (Leica EG1160) and sectioned with a manual microtome (Leica RM 2145). The sections were placed on a glass microscope slide, fixed, and stained with hematoxylin and eosin (Leica autostainer XL). Histologic sections were visualized at 40× magnification (numerical aperture, objective lens, 0.75) with an Olympus BX50 upright microscope.
Thymic function
To determine the ability of thymocytes to affect ITP development, 105 of the indicated thymocytes were injected intraperionteally on day −5 before the intraperitoneal administration of splenocytes from CD61 immune mice. Platelet counts were performed at weekly intervals.
Statistical analysis
Data were analyzed using Student t test; P less than .05 was considered significant.
Results
Platelet counts and anti-CD61 antibody titers
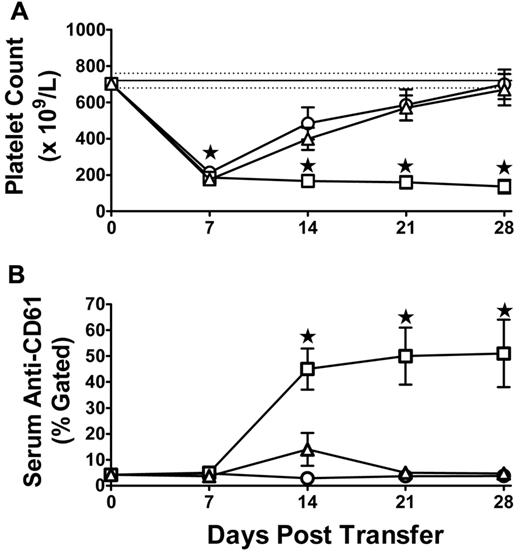
To ensure that ITP was induced, platelet counts and serum anti-CD61 antibody levels were analyzed. The initial fall in the platelet count at 7 days (Figure 1A) results from the irradiation treatment as previously described.11 Compared with SCID mice engrafted with splenocytes from naive mice, splenocytes transferred from CD61 immune mice caused significant thrombocytopenia throughout the 28-day protocol (Figure 1A). This correlated with an increase in anti-CD61 antibody titer by day 14 after splenocyte administration (Figure 1B). Treatment of the ITP mice with 2 g/kg IVIg rescued the platelet counts (Figure 1A) and significantly reduced the anti-CD61 antibody titers (Figure 1B).
Platelet counts and anti-CD61 antibody levels in transferred mice. Induction of (A) thrombocytopenia and (B) anti-CD61 antibody production in irradiated SCID mice transferred with 1.5 × 104 splenocytes from naive BALB/c mice (N = 10, ○), CD61 KO mice immunized against BALB/c platelets (N = 15, □), or SCID mice transferred with 1.5 × 104 splenocytes from CD61 KO mice immunized against WT BALB/c platelets and treated biweekly with 2 g/kg IVIg (N = 15, ▵). (A) Thrombocytopenia at day 7 occurred in all irradiated mice as previously described.11 Data are platelet counts (×109/L; ± SEM) over time (days). The solid horizontal line represents the normal mean platelet count (± SEM; hatched lines) from 250 healthy SCID mice (mean platelet count = 720 ± 41 × 109/L, SD). (B) Anti-CD61 specific antibody production was performed by flow cytometry, and the data are expressed as percent gated BALB/c platelets. *P < .01, ITP mice (□) versus IVIg-treated mice (▵).
Platelet counts and anti-CD61 antibody levels in transferred mice. Induction of (A) thrombocytopenia and (B) anti-CD61 antibody production in irradiated SCID mice transferred with 1.5 × 104 splenocytes from naive BALB/c mice (N = 10, ○), CD61 KO mice immunized against BALB/c platelets (N = 15, □), or SCID mice transferred with 1.5 × 104 splenocytes from CD61 KO mice immunized against WT BALB/c platelets and treated biweekly with 2 g/kg IVIg (N = 15, ▵). (A) Thrombocytopenia at day 7 occurred in all irradiated mice as previously described.11 Data are platelet counts (×109/L; ± SEM) over time (days). The solid horizontal line represents the normal mean platelet count (± SEM; hatched lines) from 250 healthy SCID mice (mean platelet count = 720 ± 41 × 109/L, SD). (B) Anti-CD61 specific antibody production was performed by flow cytometry, and the data are expressed as percent gated BALB/c platelets. *P < .01, ITP mice (□) versus IVIg-treated mice (▵).
CD4+CD25hi+FoxP3+ Treg distribution is significantly skewed in the lymphoid organs of ITP mice
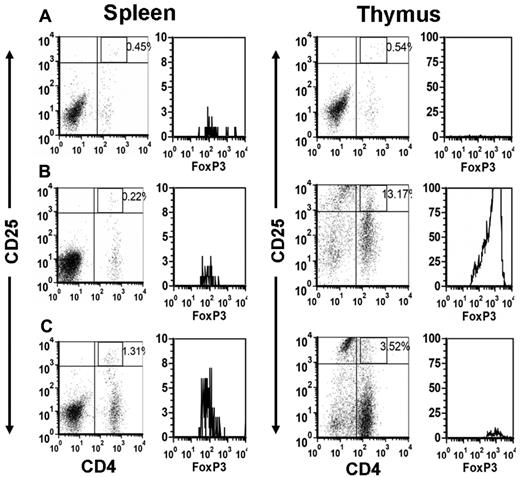
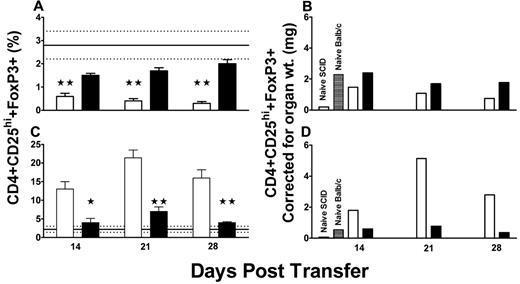
The percentage of Tregs was quantified within the mouse thymi and spleens by flow cytometry (Figure 2). Compared with control BALB/c mice or with SCID mice transferred with splenocytes from naive mice, the ITP mice developed a significant splenic Treg deficiency by 14 days after splenocyte transfer (Figure 3A). In contrast, there was a concomitant significant increase in the percentage of Tregs within the thymi of the ITP mice (Figure 3B). Treatment of the ITP mice with 2 g/kg IVIg normalized the peripheral splenic deficiency by day 28 after transfer (Figure 3A) and significantly reduced the thymic Treg proportions to near control levels (Figure 3B). The proportional data were adjusted for differences in splenic and thymic mass to reflect the total lymphocyte content of these organs and therefore to indicate whether there were absolute changes in lymphocyte numbers in addition to proportional changes in Treg distribution (Figure 3C-D). The normalization of the Tregs in the IVIg-treated mice also closely correlated with changes in thymic architecture (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Compared with untreated mice, IVIg therapy transformed the thymic architecture to that resembling healthy BALB/c mice (supplemental Figure 1).
A representative flow cytometric dot plot analysis of CD4+CD25+ T cells and the corresponding gated histograms of FoxP3+ T cells in the spleen (left panels) and thymus (right panels) of the indicated mice at 21 days after transfer. (A) Irradiated SCID mice transferred with 1.5 × 104 splenocytes from naive BALB/c mice. (B) Irradiated SCID mice transferred with 1.5 × 104 splenocytes from CD61 KO mice immunized against BALB/c platelets and (C) irradiated SCID mice transferred with 1.5 × 104 splenocytes from CD61 KO mice immunized against WT BALB/c platelets and treated biweekly with 2 g/kg IVIg. Freshly isolated splenocytes and thymocytes were stained and analyzed by flow cytometry using a lymphocyte gate based on anti-CD4 green and CD25 red fluorescence in the dot plots. The histograms of FoxP3+ cells in the CD4+CD25hi+ fluorescent gate are shown.
A representative flow cytometric dot plot analysis of CD4+CD25+ T cells and the corresponding gated histograms of FoxP3+ T cells in the spleen (left panels) and thymus (right panels) of the indicated mice at 21 days after transfer. (A) Irradiated SCID mice transferred with 1.5 × 104 splenocytes from naive BALB/c mice. (B) Irradiated SCID mice transferred with 1.5 × 104 splenocytes from CD61 KO mice immunized against BALB/c platelets and (C) irradiated SCID mice transferred with 1.5 × 104 splenocytes from CD61 KO mice immunized against WT BALB/c platelets and treated biweekly with 2 g/kg IVIg. Freshly isolated splenocytes and thymocytes were stained and analyzed by flow cytometry using a lymphocyte gate based on anti-CD4 green and CD25 red fluorescence in the dot plots. The histograms of FoxP3+ cells in the CD4+CD25hi+ fluorescent gate are shown.
T regulatory cell levels in transferred mice. The percentages of CD4+CD25hi+FoxP3+ Tregs in the (A) spleens and (C) thymuses of irradiated SCID mice engrafted with 1.5 × 104 splenocytes from CD61 KO mice immunized against BALB/c platelets (N = 10, open columns). The percentages of Tregs in organs from irradiated SCID mice engrafted with 1.5 × 104 splenocytes from CD61 KO mice immunized against BALB/c platelets and treated biweekly with 2 g/kg IVIg (N = 10, black columns) are shown for comparison. Data are expressed as the percent CD4+CD25hi+ T cells (± SEM) in the fluorescence gates, as shown in Figure 2. The solid horizontal line in the panels represents the normal mean percentage (± SEM hatched lines) of CD4+CD25hi+FoxP3+ Tregs in aged-matched BALB/c mice (N = 10). (A,C) *P < .05, **P < .01, statistical significance between the ITP mice (open columns) and IVIg-treated mice (black columns). (B,D) The proportional data were adjusted for differences in splenic and thymic mass, respectively, to reflect the total lymphocyte content of these organs.
T regulatory cell levels in transferred mice. The percentages of CD4+CD25hi+FoxP3+ Tregs in the (A) spleens and (C) thymuses of irradiated SCID mice engrafted with 1.5 × 104 splenocytes from CD61 KO mice immunized against BALB/c platelets (N = 10, open columns). The percentages of Tregs in organs from irradiated SCID mice engrafted with 1.5 × 104 splenocytes from CD61 KO mice immunized against BALB/c platelets and treated biweekly with 2 g/kg IVIg (N = 10, black columns) are shown for comparison. Data are expressed as the percent CD4+CD25hi+ T cells (± SEM) in the fluorescence gates, as shown in Figure 2. The solid horizontal line in the panels represents the normal mean percentage (± SEM hatched lines) of CD4+CD25hi+FoxP3+ Tregs in aged-matched BALB/c mice (N = 10). (A,C) *P < .05, **P < .01, statistical significance between the ITP mice (open columns) and IVIg-treated mice (black columns). (B,D) The proportional data were adjusted for differences in splenic and thymic mass, respectively, to reflect the total lymphocyte content of these organs.
Thymocyte function in modulating the development of ITP
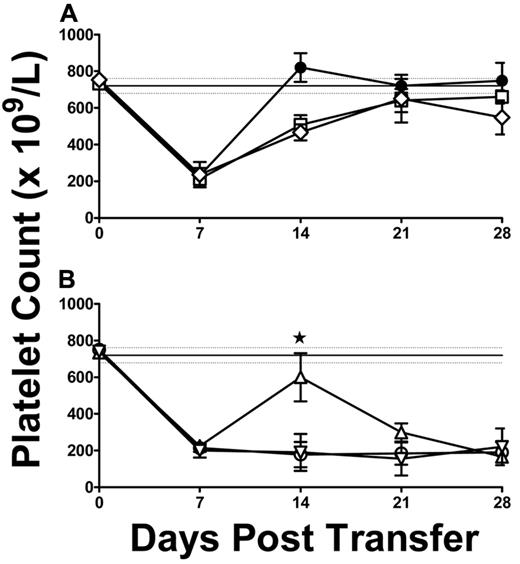
Irradiated and NK cell–depleted SCID mice were injected intraperitoneally with 105 thymocytes harvested from thrombocytopenic SCID mice; and 5 days later, the mice were engrafted with splenocytes from CD61 immune KO mice to assess the effect of the thymocytes on the development of ITP. Thymocytes from either ITP SCID mice or ITP SCID mice treated with IVIg could not, on their own, induce thrombocytopenia when transferred into SCID mice (Figure 4A). In contrast, however, thymocytes from SCID ITP mice were able to delay the onset of thrombocytopenia induced by the subsequent administration of CD61 immune KO splenocytes (Figure 4B). The platelet counts of mice receiving thymocytes were significantly better sustained at day 14, and ITP development was delayed (Figure 4B). ITP development was not affected by thymocytes from ITP SCID mice treated with IVIG (Figure 4B).
Platelet counts in thymocyte-treated ITP mice. (A) Platelet counts in control SCID mice transferred with 1.5 × 104 splenocytes from naive BALB/c mice (N = 5, ●) or SCID mice transferred with 105 thymocytes from either SCID ITP mice (N = 5, □) or SCID ITP mice treated biweekly with 2 g/kg IVIg (N = 15, ♢). (B) Platelet counts in SCID mice transferred with 1.5 × 104 splenocytes from CD61 immune KO mice (SCID ITP, N = 5, ○) or SCID mice transferred with 105 thymocytes from SCID ITP mice 5 days before receiving splenocytes from CD61 immune KO mice (N = 5, ▵) or SCID mice transferred with 105 thymocytes from SCID ITP mice treated with IVIg 5 days before receiving splenocytes from CD61 immune KO mice (N = 5, ▿). Thrombocytopenia at day 7 occurred in all irradiated mice as previously described.11 The data are expressed as platelet counts (× 109/L; mean ± SEM) over time (days). The solid horizontal lines represents the normal mean platelet count (± SEM; hatched lines) from 250 healthy SCID mice (mean ± SD platelet count = 720 ± 41). (B) *P < .01, the SCID mice administered thymocytes (▵) and control ITP SCID mice (○).
Platelet counts in thymocyte-treated ITP mice. (A) Platelet counts in control SCID mice transferred with 1.5 × 104 splenocytes from naive BALB/c mice (N = 5, ●) or SCID mice transferred with 105 thymocytes from either SCID ITP mice (N = 5, □) or SCID ITP mice treated biweekly with 2 g/kg IVIg (N = 15, ♢). (B) Platelet counts in SCID mice transferred with 1.5 × 104 splenocytes from CD61 immune KO mice (SCID ITP, N = 5, ○) or SCID mice transferred with 105 thymocytes from SCID ITP mice 5 days before receiving splenocytes from CD61 immune KO mice (N = 5, ▵) or SCID mice transferred with 105 thymocytes from SCID ITP mice treated with IVIg 5 days before receiving splenocytes from CD61 immune KO mice (N = 5, ▿). Thrombocytopenia at day 7 occurred in all irradiated mice as previously described.11 The data are expressed as platelet counts (× 109/L; mean ± SEM) over time (days). The solid horizontal lines represents the normal mean platelet count (± SEM; hatched lines) from 250 healthy SCID mice (mean ± SD platelet count = 720 ± 41). (B) *P < .01, the SCID mice administered thymocytes (▵) and control ITP SCID mice (○).
Discussion
The immunopathogenesis of ITP has been extensively investigated in the last decade, and it appears that one of the major contributing factors in the development of this disorder is a peripheral deficiency of Tregs.12-29 Tregs are a T-cell subset marked by CD4+CD25hi Foxp3+ and constitute approximately 5%-10% of peripheral CD4+ T cells and play an important role in self-tolerance.32,33 The reasons for the Treg deficiency in ITP are unclear, and we attempted to address this question by studying our murine model of active ITP. We report here that one of the reasons for the peripheral Treg deficiency during active disease may be the result of sequestration of functional Tregs within the thymus. Treatment of the ITP mice with IVIg increased platelet counts and significantly reduced the thymic accumulation of functional Tregs, thereby correcting the peripheral deficiency.
Natural Tregs originate from the thymus as CD4+ cells expressing high levels of CD25 together with the transcription factor (and lineage marker) FoxP3.32,33 It is clear that Tregs are essential for maintaining self-tolerance because genetic deficiencies of the transcriptional factor FoxP3 leads to overwhelming autoimmunity.32,33 Indeed, many autoimmune diseases in both humans and mice are associated with a peripheral blood and splenic deficiency of Tregs, respectively.30,31 This observation now appears to be true for patients with chronic ITP.12-29 Of interest in patients with ITP, therapies that raise platelet counts, including dexamethasone, rituxan, thrombopoietin receptor agonists, and IVIg, do so in association with rescuing the peripheral Treg deficiency.15,16,18,20,26 Our data also support this contention where active ITP had low numbers of peripheral splenic Tregs (Figures 2 and 3) and IVIg therapy, for example, normalized the peripheral deficiency (Figure 3). What these results additionally show is that the peripheral Treg deficiency is associated with a large thymic accumulation of Tregs, and IVIg therapy reduced the thymic Treg numbers by allowing them to be released into the periphery. The mechanism of how IVIg may accomplish this is unknown, but there is evidence that IVIg can modulate Treg numbers and movements.37
IVIg has been shown to induce Treg development, and it has been hypothesized that the anti-inflammatory nature of IVIg may be the result of Treg induction. For example, Kessel et al showed that IVIg could stimulate the expansion of CD4+CD25+Foxp3+ Tregs in vitro,38 and Chi et al demonstrated that the beneficial effect of IVIg treatment of Guillian-Barré syndrome was the result of its ability to rescue their low Treg numbers.39 Subsequently, Ephrem et al demonstrated that IVIg therapy could also protect mice from experimental autoimmune encephalopathy through induction of Tregs.40 More recently, 2 reports now have shown that IVIg treatment of patients with acute Kawasaki disease alleviates the disorder by increasing the number and function of Tregs.41,42 Similarly, IVIg was shown to enhance allograft survival by the induction of Tregs43 and the beneficial effect of IVIg on murine diabetes is associated with Treg induction.44 Of interest, IVIg can alter Tregs as early as 24-48 hours after therapy, the time, for example, when platelet counts begin to rise in IVIg-treated patients with ITP.43,44 Collectively, these reports suggest that IVIg's protective anti-inflammatory effects are mediated by expanding the suppressive effects of Tregs. This is supported by the data in Figure 4, which shows that development of ITP is delayed by the Treg-laden thymocytes. Perhaps the thymocyte infusions delay antibody production via direct inhibition of immunoglobulin production or inhibition of T helper-B lymphocyte interactions. We are currently studying this.
The mechanism of how Tregs accumulate within the thymus during active ITP is unknown; however, thymic epithelial cells, which are the major antigen-presenting cells involved in thymic selection of T cells, can express the CD61 antigen associated with CD51 to form the vitronectin receptor.45-47 It is possible that the transferred anti-CD61 immune splenocytes may damage thymic epithelial cells and cause faulty thymic adhesion or selection processes that lead to Treg retention. Related to this, compared with healthy age-matched BALB/c mice, histologic staining of the thymus in SCID mice transferred with anti-CD61 immune splenocytes revealed a thymic architecture that had an increased density of epithelial and stromal cells with a relatively moderate but homogeneous scattering of lymphocytes (supplemental Figure 1). In contrast, however, treatment of the transferred mice with IVIg completely transformed the thymic architecture more resembling the BALB/c mouse controls (supplemental Figure 1). It is possible that the changes in thymic architecture are related to the release of Tregs into the periphery.
Thymocytes from either the untreated or IVIg-treated ITP SCID mice could not themselves induce ITP when transferred into naive SCID mice (Figure 4); however, if thymocytes from the ITP SCID mice were administered before engraftment with CD61 immune splenocytes, there was a delay in the development of ITP at 2 weeks after engraftment (Figure 4). Of interest, the thymocytes from ITP mice treated with IVIg did not affect the development of ITP, suggesting that IVIg may allow for the release of the sequestered functional Tregs to mediate suppression of the immune mechanisms producing the ITP. Nonetheless, the sequestered thymic Tregs in mice with active ITP appear to be functional in that they can at least delay the onset of ITP.
In conclusion, we have demonstrated that active murine ITP is associated with a peripheral splenic Treg deficiency and corresponding thymic accumulation of Tregs. Treatment with IVIg normalizes the platelet counts and significantly reduces the thymic Treg numbers, and this correlates with a rescuing of the peripheral Treg pool in the spleen. The data suggest that a novel potential mechanism of peripheral Treg deficiency in ITP, central thymic retention of Tregs, starves the peripheral tolerance mechanism and allows the antiplatelet immune response to proceed.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Alan Lazarus (Canadian Blood Services and St Michael's Hospital, Toronto, ON) for his helpful discussions.
Authorship
Contribution: R.A. designed research, performed and supervised all experiments, collected, analyzed, and interpreted data, performed statistical analysis, and wrote the manuscript first draft; Y.H., S.G., G.B.S., E.R.S., L.G., and M.K. performed experiments and collected, analyzed, and interpreted data; H.N. and J.F. designed research, edited the manuscript, and contributed animals; and J.W.S. provided financial resources, designed research, analyzed and interpreted data, and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John W. Semple, St Michael's Hospital, 30 Bond Street, Toronto, ON, Canada, M5B 1W8; e-mail: semplej@smh.ca.