In this issue of Blood, Ye et al1 and Al Hawas et al2 clarify the roles of 2 key fusion proteins that regulate the agonist-stimulated release of bioactive factors from platelets, and thereby explain the defective hemostasis in patients with 2 rare genetic diseases.
On stimulation at sites of blood vessel damage, platelets release an array of soluble factors that facilitate platelet adhesion and other physiologic responses required for hemostasis and thrombus formation and remodeling. These factors are released from 3 types of storage compartments (α granules, dense granules, and lysosomes) after their fusion with the platelet plasma membrane or open canalicular system. Fusion requires cognate interactions between the cytoplasmic domains of Soluble NSF Attachment Protein Receptor (SNARE) proteins. Three coiled-coil domains from a plasma membrane target SNARE (tSNARE) engage a single coiled-coil domain on a granule membrane vesicle SNARE (vSNARE) to form a 4-helix bundle that drives membrane deformation and fusion. In addition, members of the Sec1/Munc18 (SM) family enhance the efficiency and fidelity of cognate SNARE interactions and subsequent fusion. Deficiencies in either SNAREs or SM proteins impair secretion and consequent physiologic function, as exemplified by the neurologic impairments induced by bacterial toxins that cleave neuronal SNAREs3 or by a lethal disorder caused by mutations in the endosomal SM protein VPS33B.4 Similarly, genetic deficiencies in the vSNARE, VAMP8, cause impaired mediator release from all 3 platelet granule types and consequent bleeding defects in mice,5,6 suggesting that VAMP8 is the major vSNARE for granule fusion in platelets. However, the identity of the tSNARE and SM components has remained controversial (see figure).
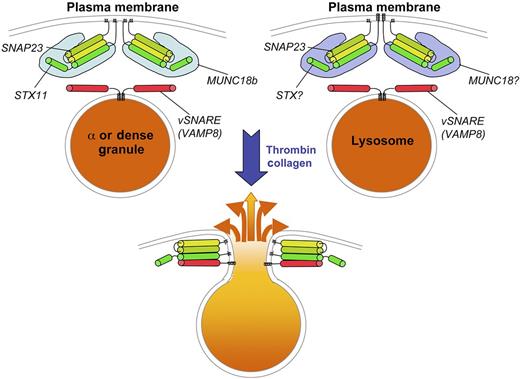
SNARE-dependent fusion of platelet granules with the plasma membrane. Top panels depict α granules or dense granules (left) or lysosomes (right) in resting platelets. The vSNARE VAMP8 is present on the cytosolic surface of each of the granules. At the plasma membrane, a tSNARE complex consisting of SNAP23 bound to either syntaxin 11 (STX11; left) or another STX (right) is held in a conformationally active state by the SM protein MUNC18b (left) or another MUNC variant (right). On platelet stimulation by agonists such as thrombin or collagen, the granules fuse with the plasma membrane by engagement of the vSNARE and tSNARE to form a 4-helix bundle. The contents of the granules are then released to the extracellular space.
SNARE-dependent fusion of platelet granules with the plasma membrane. Top panels depict α granules or dense granules (left) or lysosomes (right) in resting platelets. The vSNARE VAMP8 is present on the cytosolic surface of each of the granules. At the plasma membrane, a tSNARE complex consisting of SNAP23 bound to either syntaxin 11 (STX11; left) or another STX (right) is held in a conformationally active state by the SM protein MUNC18b (left) or another MUNC variant (right). On platelet stimulation by agonists such as thrombin or collagen, the granules fuse with the plasma membrane by engagement of the vSNARE and tSNARE to form a 4-helix bundle. The contents of the granules are then released to the extracellular space.
Previous work using a permeabilized cell granule release assay and inhibitory antibodies or recombinant soluble SNARE domains implicated syntaxin (STX) 2 and STX4, 2 ubiquitously expressed plasma membrane tSNARE subunits, as being essential for granule release.7,8 However, several inconsistencies in the data raised questions about the conclusions. In Ye et al, the group comes to a different conclusion by using clean genetic systems.1 Specifically, by exploiting a STX2 knockout mouse and a second mouse strain engineered to lack STX4 specifically in platelets, they demonstrate that neither STX2 nor STX4 is required for efficient secretion from platelet α granules, dense granules, or lysosomes. By contrast, platelets from human patients lacking STX11, a tissue-restricted tSNARE component that they show to be the most highly expressed syntaxin isoform in platelets, have severely defective α and dense granule release and partially impaired lysosome release. Moreover, STX11 physically interacts with the relevant partners, the vSNARE VAMP8 and the additional tSNARE component, SNAP23 (see figure).
Similarly, although earlier data using the reconstituted secretion system had implicated MUNC18C, an SM protein that complexes with STX4 and regulates insulin secretion in other cell types, as critical for granule secretion in platelets,9 Al Hawas et al now show that platelets from patients lacking the more abundant MUNC18B have granule defects similar to those observed in STX11-deficient platelets.2 Moreover, a heterozygous Munc18b mutant showed partial inhibition of granule secretion, whereas platelets from Munc18c heterozygotes had no defect at all. Finally, MUNC18B-deficient platelets are depleted for STX11, and STX11-deficient platelets are depleted for MUNC18B, suggesting that the syntaxin and SM protein stabilize each other. Together, these data definitively identify STX11 and MUNC18B as the syntaxin tSNARE and SM components involved in platelet α and dense granule secretion.
Given the high homology among syntaxin isoforms and among SM proteins, why should we care which ones specifically function in platelets? The reason is that the genes encoding STX11 and MUNC18B are mutated in variants of the lethal heritable disease, familial hemophagocytic lymphohistiocytosis (FHL). FHL is characterized by severe immunodeficiency because of a loss of lytic function by natural killer and cytolytic T lymphocytes. Whereas FHL2 (and perhaps FHL1) patients lack specific lytic granule contents, FHL types 3 (lacking UNC13D/Munc13-4, a protein previously implicated in platelet secretion10,11 ), 4 (lacking STX11), and 5 (lacking MUNC18B) exhibit impaired lytic granule secretion. Thus, the new findings indicate that (1) secretion of lytic granules and platelet granules are under generally similar modes of regulation; (2) patients with FHL types 3, 4, and 5 have bleeding deficiencies in addition to immunodeficiency; and (3) simple tests of agonist-induced platelet secretion from patient blood samples can provide an excellent first line diagnosis for FHL.
While the findings of these 2 papers appear to be definitive, they also raise several new questions. First, STX11 has been reported on intracellular organelles in other cell types, and is thought to regulate late endosome: lysosome fusion in macrophages.12,13 Granule morphology and content are normal in STX11- or MUNC18B-deficient platelets, nominally ruling out a role for these effectors in platelet granule maturation. How, then, would the same syntaxin/SM pair be delivered to the plasma membrane to function as a tSNARE in platelets? Second, STX11 is unusual among syntaxin family members in that it lacks a traditional transmembrane domain,12 and its partner, SNAP23, is also linked to the plasma membrane by a lipid anchor (see figure). How, then, does this tSNARE function in destabilizing the plasma membrane to facilitate fusion? Third, the effects of STX11- or MUNC18B-deficiency on lysosome secretion are much more modest than those on α or dense granule release,1,2 implying distinct controls for secretion from lysosomes relative to dense and α granules. Either of 2 models might explain this phenomenon: either the effects on lysosomal secretion are indirect (eg, a consequence of reduced signaling because of the lack of ADP release from dense granules) and a distinct syntaxin isoform, such as the less abundant STX7, participates in lysosome secretion, or lysosomal secretion is mediated by a set of redundant syntaxins. Finally, if release of dense and α granules (and to at least some extent, lysosomes) is under similar molecular control, then how does one account for the different kinetics of their release after platelet stimulation? Surely the answers to these questions will be unlocked in the years ahead.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
REFERENCES
National Institutes of Health