Abstract
Chronic graft-versus-host disease (cGVHD) is a prognosis limiting complication of allogeneic stem cell transplantation. The molecular mechanisms underlying cGVHD are incompletely understood, and targeted therapies are not yet established for clinical use. Here we examined the role of the hedgehog pathway in sclerodermatous cGVHD. Hedgehog signaling was activated in human and murine cGVHD with increased expression of sonic hedgehog and accumulation of the transcription factors Gli-1 and Gli-2. Treatment with LDE223, a highly selective small-molecule antagonist of the hedgehog coreceptor Smoothened (Smo), abrogated the activation of hedgehog signaling and protected against experimental cGVHD. Preventive therapy with LDE223 almost completely impeded the development of clinical and histologic features of sclerodermatous cGVHD. Treatment with LDE223 was also effective, when initiated after the onset of clinical manifestations of cGVHD. Hedgehog signaling stimulated the release of collagen from cultured fibroblasts but did not affect leukocyte influx in murine cGVHD, suggesting direct, leukocyte-independent stimulatory effects on fibroblasts as the pathomechanism of hedgehog signaling in cGVHD. Considering the high morbidity of cGVHD, the current lack of efficient molecular therapies for clinical use, and the availability of well-tolerated inhibitors of Smo, targeting hedgehog signaling might be a novel strategy for clinical trials in cGVHD.
Introduction
Allogeneic bone marrow transplantation (alloBMT) represents the standard care of a wide variety of genetic, immunologic, and oncologic disorders.1 Immune reactions of the transplanted graft against tumor cells contribute as graft-versus-leukemia (GVL) or graft-versus-tumor reactions to the therapeutic effects of alloBMT. However, the recognition of polymorphic major or minor histocompatibility antigens by donor T cells initiates an immune response against host tissues, which manifests as graft-versus-host disease (GVHD).2 GVHD is associated with a high morbidity and mortality emerging as an important risk of alloBMT.3 GVHD can further be subclassified into an acute form (aGVHD) and a chronic form (cGVHD). Although several therapeutic options have improved the outcome of aGVHD, therapeutic options for cGVHD are limited and cGVHD still represents a major challenge after alloBMT.4-6 Approximately 40%-60% of long-term survivors after alloBMT develop cGVHD,7 whereas frequencies up to 80% have been reported for non HLA-identical donor-recipient constellations.8 cGVHD can manifest on virtually every organ system. However, the skin is most commonly affected, with marked xerosis at early-stage progressing to dermal subcutaneous fibrosis associated with neuropathy and painful ulcers at end stage.
Three different hedgehog proteins have been identified: Sonic hedgehog (Shh), Indian hedgehog (Ihh), and Desert hedgehog (Dhh). Although all hedgehog proteins share significant sequence homology, their distribution patterns differ, with Shh being the most abundant hedgehog protein in the skin.9,10 The hedgehog pathway is activated by binding of hedgehog proteins to the membrane receptor Patched homolog 1 (Ptch-1). In the absence of hedgehog proteins, Ptch-1 inhibits the co-receptor Smoothened (Smo). Binding of hedgehog proteins induces conformational changes of Ptch-1 that prevent the inhibitory effects on Smo. Smo then translocates into the primary cilium and initiates a series of intracellular events that result in the stabilization of Gli family zinc finger transcription factors and the transcription of target genes.11,12 The hedgehog pathway plays a critical role for cellular differentiation during embryonic development.13-16 In the adult, the activation of the hedgehog pathway is tightly controlled and hedgehog signaling is inhibited by an array of different regulatory mechanisms. This strict control is essential because uncontrolled activation alters cellular fate and differentiation and has been identified as a crucial step in the pathogenesis of several malignancies.17-22 The prominent role of hedgehog signaling in those diseases fostered the development of pharmacologic inhibitors of the hedgehog pathway. Several small-molecule inhibitors of Smo are currently evaluated for the treatment of basal cell carcinoma and medulloblastoma. First results from those trials indicate that hedgehog signaling can be targeted efficiently in humans and that this treatment is not limited by toxicity.23
We investigated in the present study the role of hedgehog signaling in sclerodermatous cGVHD. We demonstrate that hedgehog signaling is activated in human and murine cGVHD and that pharmacologic inhibition of Smo is effective for prevention and treatment of cGVHD. Thus, Smo might be a promising molecular target for the treatment of sclerodermatous cGVHD.
Methods
Mice
Female BALB/c (H-2d) mice were purchased from Janvier. B10.D2 (H-2d) mice were purchased from The Jackson Laboratory. BMT was performed at the age of 6-8 weeks under specific pathogen-free conditions. Male mice were used as bone marrow donors and females as recipients. All mice received sterile pellet food and water ad libitum. All animal experiments were approved by the local ethic committee.
Mice were bred and maintained at the central animal facility of the University of Erlangen-Nuremberg (Franz-Penzoldt-Zentrum) under specific pathogen-free conditions. This study was carried out in strict accordance with international guidelines for animal care and use and in accordance with the guidelines of the Animal Care and Use Committee of the Government of Bavaria and the institutional guidelines of the University of Erlangen-Nuremberg. The protocol was approved by the Government of Bavaria (Regierung von Mittelfranken).
Human samples
Human sample collection was approved by the local ethical boards and authorities in Erlangen, Kiel, and Manchester. Human skin samples were obtained for diagnostics with the informed consent of the patients and used in compliance with the regulations of the local ethics committees. This study was conducted in accordance with the Declaration of Helsinki. A clinical characterization of cGVHD patients is given in Table 1. Nonfibrotic skin samples were obtained from 10 age- and sex-matched healthy persons and 2 patients that underwent with allogeneic BMT but did not develop cGVHD.
BMT
The B10.D2→Balb/c [H-2(d)] minor histocompatibility antigen-mismatched model, which reflects clinical and pathologic symptoms of human sclerodermatous cGVHD, was used in this study.24,25 PBS was used to flush bone marrow cells from bone marrow cavities of tibias and femurs followed by erythrocyte hemolysis. No further purification or in vitro expansion of a particular subset of cells was performed. Recipient mice [BALB/c (H-2d)] underwent total body irradiation with 2 doses of 6 Gy separated by 3 hours. In a modified protocol, low-dose total body irradiation was performed with 700 cGy. Sixteen hours after the second irradiation, each recipient mouse received 2 × 106 splenocytes and 1 × 106 bone marrow cells, dissolved in 100 μL PBS, from either BALB/c (H-2d) in a syngeneic or B10.D2 (H-2d) in an allogeneic, multiple minor mismatched transplantation via tail vein injection.24,25 The treatment was either started after stable engraftment 10 days after BMT or at the onset of first clinical signs of disease 21 days after BMT.26 Engraftment was confirmed by quantification of leukocyte and thrombocyte count and measurement of the Hb content.
Treatment with Smo inhibitor LDE223
LDE223, a novel and selective inhibitor of Smo, was kindly provided by Novartis. Structurally closely related inhibitors, such as LDE225, are currently evaluated in clinical trials in patients with basal cell carcinoma. LDE223 was dissolved in 10% DMSO and administered in polyethylenglycol-300 (PEG 300), as suspension, once daily in a total volume of 100 μL via oral gavage. Mice were treated with LDE223 in a pharmacologically relevant dose of 40 mg/kg per day. Control mice received sham treatment with the solvent PEG 300. Animals were killed 6 weeks after BMT by cervical dislocation after anesthesia with CO2.
Induction of GVL
The P815 leukemia model was used for the graft-versus-leukemia (GVL) experiments as described.27 P815 is a mastocytoma (H2d) cell line derived from DBA/2 mouse. Briefly, 500, 1500, or 10 000 P815 cells (Leibniz-Institute DSMZ) were injected to each recipient along with syngeneic or allogeneic bone marrow and splenocytes. A subgroup of allogenically transplanted mice was treated with LDE223. Animals were monitored daily for survival and the cause of death determined by postmortem gross pathology examination.
Scoring of the clinical manifestations of cGVHD
Mice were monitored daily to determine the incidence and severity of cutaneous cGVHD as well as other signs of disease, such as reduced mobility, diarrhea, and weight loss. The following established scoring system for cutaneous cGVHD was used: healthy appearance = 0; skin lesions with alopecia ≤ 1 cm2 in area = 1; skin lesions with alopecia 1-2 cm2 in area, 2; and skin lesions with alopecia > 2 cm2 in area = 3.25,28
Histologic analysis
Skin samples of 1 cm2 were obtained from the upper back, fixed in 4% formalin and embedded in paraffin. Sections were stained with hematoxylin and eosin. Dermal thickness was analyzed with a Nikon Eclipse 80i microscope (Nikon) at 100-fold magnification by measuring the distance between the epidermal-dermal junction and the dermal-subcutaneous fat junction at sites of induration at 3 consecutive skin sections of each animal.29 If not indicated otherwise, human and mouse skin samples were treated alike.
Immunohistochemistry for Vimentin, Shh, and Gli transcription factors
Sections of human and murine cGVHD and controls were incubated with polyclonal rabbit anti–Gli-1 (Thermo Scientific), polyclonal rabbit anti–Gli-2 (Abnova), polyclonal goat anti–Gli-3 (Santa Cruz Biotechnology) or polyclonal rabbit anti–human Shh antibodies (Abcam), respectively, overnight at 4°C. Irrelevant rabbit immunogloblulins were used as controls. The expression of Gli-1, Gli-2, Gli-3, and Shh was visualized with secondary antibodies labeled with horseradish peroxidase (Dako North America) and DAB peroxidase substrate solution (Sigma-Aldrich). Counterstaining has been performed with Vimentin (Abcam) where AlexaFluor-488 (Invitrogen) has been used as secondary antibody.
Quantification of Shh and Gli transcription factors
Shh and Gli transcription factors staining were quantified in a semiquantitative manner, where 0 indicates no staining; 1, weak staining; 2, moderate staining; and 3, strong staining of fibroblasts at 400-fold magnification at 3 randomly chosen high-power fields per patient.
Quantification of myofibroblasts
Myofibroblasts were identified by staining for α-SMA using monoclonal anti-actin α-smooth muscle antibodies (Sigma-Aldrich) as described previously.30 In each section, α-SMA–positive myofibroblasts were counted at 200-fold magnification at 3 randomly chosen high-power fields.
Quantification of the collagen content in lesional skin
The collagen content in the skin was analyzed with the hydroxyproline assay as described.31 Skin punch biopsies of 3 mm in diameter obtained from the upper back were digested in 6M HCl at 120°C for 3 hours, chloramine T (0.06M) was added and samples were mixed and incubated at room temperature for 20 minutes. Perchloric acid (3.15M) and p-dimethylaminobenzaldehyde (20%) were added, and samples were incubated at 60°C for an additional 20 minutes. The absorbance was determined at 557 nm with a Spectra MAX 190 microplate spectrophotometer (Molecular Devices).
Collagen measurements
Total soluble collagen in cell culture supernatants was quantified using the SirCol collagen assay (Biocolor) as described previously.31 Cell culture supernatants were incubated for 30 minutes with Sirius Red dye, an anionic dye that binds specifically the basic side chain groups of collagens under assay conditions. The formed Sirius Red-collagen complex was redissolved in 0.5M NaOH. The collagen content was determined by the absorbance of Sirius Red at 540 nm with a Spectra MAX 190 microplate spectrophotometer (Molecular Devices).
FISH with murine Y chromosome paint
Tissue section were deparaffinized in xylene, rehydrated in an ethanol series, followed by an antigen retrieval in 10mM sodium citrate for 15 minutes at 96°C. Further pretreatment of the slides and hybridization with red labeled Y chromosome probes were performed according to guidelines of the Mouse IDetect Chromosome Paint Probes (ID Labs). Nuclei were visualized with 4,6-diamidino-2-phenylindole.
Quantification of infiltrating leukocytes, B cells, and regulatory T cells
Infiltrating leukocytes were quantified in a blinded manner in skin sections stained with hematoxylin and eosin. All images were captured with a Nikon Eclipse 80i microscope (Nikon) at 400-fold magnification at 8 randomly chosen high-power fields per mouse.32
Leukocyte counts in the skin were also determined by FACS for CD45-positive cells in skin single-cell suspensions as described.33 The number of B cells was measured as cells positive for CD45 and CD19 (both antibodies from eBioscience) by FACS analysis.
Regulatory T cells were stained in skin sections with monoclonal antibodies against mouse anti-FoxP3 (eBioscience) and visualized with AlexaFluor-488–labeled secondary antibodies (Invitrogen).
Determination of BAFF levels
B-cell activating factor (BAFF) levels in murine serum were quantified by ELISA using the Mouse BAFF/BLyS/TNFSF13B Immunoassay (R&D Systems) according to the guidelines of the manufacturer.
Stress fiber staining
To analyze the potential of Shh to induce stress fiber development, healthy human dermal fibroblasts were stimulated with 1 μg/mL Shh for 24 hours. After fixation with 4% paraformaldehyde, permeabilization with 0.25% Triton X-100 and blocking with 1% BSA, stress fibers were visualized with phalloidin as describe previously.29 Quantification of stress fibers was performed via ImageJ (Version 1.42q, National Institutes of Health).
Quantitative real time PCR
NucleoSpin RNA II (Machery-Nagel) was used for total RNA extraction. Reverse transcription into cDNA was performed using random hexamers.34,35 Quantification of Col1a2 gene expression was done by SYBR Green real-time PCR using the ABI Prism 7300 Sequence Detection System (Applied Biosystems). The following primer pairs were used: human col1a2 forward primer: 5′-GGTCAGCACCACCGATGTC-3′, human col1a2 reverse primer: 5′-CACGCCTGCCCTTCCTTT-3′. A predeveloped β-actin assay (Applied Biosystems) was used to normalize for the amounts of loaded cDNA. Samples without enzyme in the reverse transcription reaction served as negative controls. Unspecific signals caused by primer dimers were excluded by no template controls and by dissociation curve analysis. Differences were calculated with the threshold cycle (Ct) and the comparative Ct method for relative quantification. The expression at baseline was set to 100% for control experiments and all other results were normalized to that and are shown as percent expression of baseline.
Statistics
Data are expressed as mean ± SEM. The Wilcoxon signed rank tests for related samples and the Mann-Whitney U test for nonrelated samples were used for statistical analyses. P < .05 was considered statistically significant.
Results
Hedgehog signaling is activated in cGVHD
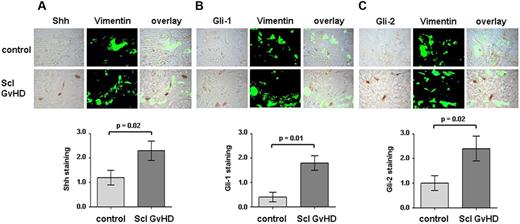
To analyze whether hedgehog signaling is activated in human cGVHD, we performed immunohistochemistry for Shh in skin sections obtained from patients with sclerodermatous cGVHD and controls. Shh was overexpressed in skin sections of cGVHD patients. Double staining of Shh with the fibroblast marker vimentin demonstrated a prominent expression of Shh in dermal fibroblasts (Figure 1A). To investigate whether the overexpression of Shh results in activation of the hedgehog pathway, we stained for Gli-1, Gli-2, which accumulates on activated hedgehog signaling. The expression of Gli-1 and Gli-2 was increased in cGVHD patients compared with controls. Gli-1 and Gli-2 accumulated in particular in fibroblasts in section of cGVHD patients, whereas only few vimentin-positive cells stained positive for Gli-1 or Gli-2 in nontransplanted healthy controls (Figure 1B-C). We also did not observe an activation of hedgehog signaling in 2 patients with allogeneic BMT and without cGVHD. Semiquantitative analyses confirmed a significant induction of Shh, Gli-1, and Gli-2 in sclerodermatous cGVHD compared with controls (Figure 1A-C). Gli-3, which has been reported to counteract the stimulatory effects of Gli-1 and Gli-2, was significantly lower than that of Gli-1 and Gli-2 without detectable differences between cGVHD and controls (data not shown).
Hedgehog signaling is activated in human sclerodermatous cGVHD. Shh, Gli-1, and Gli-2 were detected by immunohistochemistry in skin biopsies of cGVHD patients and healthy volunteers. Fibroblasts were identified by staining with vimentin (n = 8 each). Representative examples of cGVHD patients and controls are shown at 1000-fold magnification. (A) Shh expression was strongly increased in cGVHD patients (Scl GVHD) compared with controls. (B-C) Gli-1 and Gli-2, both downstream transcription factor of the hedgehog pathway, accumulated in cGVHD patients with increased staining for Gli-1 (B) and Gli-2 (C), respectively, compared with controls.
Hedgehog signaling is activated in human sclerodermatous cGVHD. Shh, Gli-1, and Gli-2 were detected by immunohistochemistry in skin biopsies of cGVHD patients and healthy volunteers. Fibroblasts were identified by staining with vimentin (n = 8 each). Representative examples of cGVHD patients and controls are shown at 1000-fold magnification. (A) Shh expression was strongly increased in cGVHD patients (Scl GVHD) compared with controls. (B-C) Gli-1 and Gli-2, both downstream transcription factor of the hedgehog pathway, accumulated in cGVHD patients with increased staining for Gli-1 (B) and Gli-2 (C), respectively, compared with controls.
Hedgehog signaling is activated in experimental cGVHD and blocked by LDE223
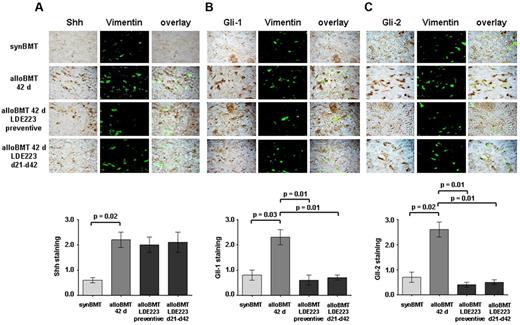
We next determined whether the hedgehog signaling is also activated in murine cGVHD. Indeed, the expression of Shh, Gil-1 and Gli-2 was increased in the skin of recipients of alloBMT compared with syngeneic controls. Double staining of skin sections of murine cGVHD with vimentin demonstrated a prominent staining in fibroblasts as in human sclerodermatous cGVHD (Figure 2A-C). Gli-3 was hardly detectable in murine skin and was not induced by alloBMT. Consistent with the proposed mode of action as an inhibitor of the coreceptor Smo, LDE223 did not alter the expression of Shh (Figure 2A). However, application of LDE223 either in a preventive manner or as treatment initiated after onset of clinical disease effectively inhibited hedgehog signaling and reduced the accumulation of Gli-1 and Gli-2 in fibroblasts on alloBMT (Figure 2B-C).
Increased Hedgehog signaling in murine sclerodermatous cGVHD. Shh, Gli-1, and Gli-2 were detected by immunohistochemistry in skin biopsies of syngeneic controls (synBMT), sham-treated mice receiving alloBMT (alloBMT), and recipients treated with LDE223, either in preventive or therapeutic regimens (n = 6 each). Fibroblasts were identified by double staining for vimentin. Representative images of the treatment groups and the control groups are shown at 1000-fold magnification. (A) Shh expression was strongly increased 42 days after alloBMT compared with syngeneic controls. Consistent with the proposed mechanism of action, LDE223 did not affect the expression of Shh in mice receiving alloBMT. (B-C) Gli-1 and Gli-2 signaling was activated in skin of mice receiving alloBMT with increased staining for Gli-1 (B) and Gli-2 (C) compared with syngeneic controls. Preventive treatment as well as treatment of clinically manifest sclerodermatous cGVHD with LDE223 diminished the expression of both Gli-1 and Gli-2. synBMT indicates syngeneic controls; and alloBMT, mice receiving alloBMT.
Increased Hedgehog signaling in murine sclerodermatous cGVHD. Shh, Gli-1, and Gli-2 were detected by immunohistochemistry in skin biopsies of syngeneic controls (synBMT), sham-treated mice receiving alloBMT (alloBMT), and recipients treated with LDE223, either in preventive or therapeutic regimens (n = 6 each). Fibroblasts were identified by double staining for vimentin. Representative images of the treatment groups and the control groups are shown at 1000-fold magnification. (A) Shh expression was strongly increased 42 days after alloBMT compared with syngeneic controls. Consistent with the proposed mechanism of action, LDE223 did not affect the expression of Shh in mice receiving alloBMT. (B-C) Gli-1 and Gli-2 signaling was activated in skin of mice receiving alloBMT with increased staining for Gli-1 (B) and Gli-2 (C) compared with syngeneic controls. Preventive treatment as well as treatment of clinically manifest sclerodermatous cGVHD with LDE223 diminished the expression of both Gli-1 and Gli-2. synBMT indicates syngeneic controls; and alloBMT, mice receiving alloBMT.
LDE223 prevents experimental GVHD
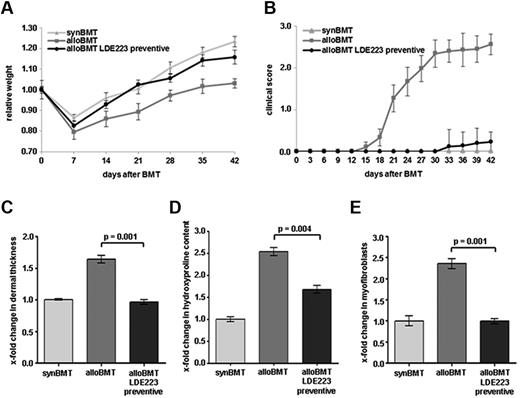
Sclerodermatous GVHD occurred in all Balb/c recipients receiving B10.D2 bone marrow, whereas syngeneic controls showed no signs of cGVHD. Clinical signs of systemic cGVHD in mice receiving alloBMT included reduced mobility, diarrhea, and weight loss. Weight loss was observed in all mice after BMT. The minimum body weight was measured 7 days after BMT with a weight loss of 14% ± 1% of the total body weight in syngeneic controls and of 20% ± 4% in mice receiving alloBMT. The differences in body weight between syngeneic controls and sham-treated mice receiving alloBMT increased further from 6% ± 4% at day 7 to 12% ± 4% at day 21 and 20% ± 3% at day 42 (Figure 3A). Alopecia and skin ulcers as clinical signs of cGVHD became evident 18 days after allogeneic BMT. The common clinical composite score for cutaneous cGVHD progressively increased from 1.27 at day 21 to 2.6 at day 42 (Figure 3B). In contrast, syngeneic control mice did not show alopecia or skin ulcers (Figure 3B). Histologically, a scleroderma-like picture with dermal fibrosis, infiltration of leukocytes, and atrophy of the subcutis was observed in recipients of alloBMT. The dermal thickness and the hydroxyproline content increased by 1.6 ± 0.1–fold and 2.5 ± 0.1–fold, respectively, after 42 days compared with control mice (P = .003 and P = .004, respectively; Figure 3C-D). The numbers of myofibroblasts, which represent activated fibroblasts and are considered as key players in the pathogenesis of fibrotic disease, were 2.4 ± 0.1–fold higher in mice receiving alloBMT (P < .0001; Figure 3E). No differences in clinical or histologic signs of sclerodermatous cGVHD were observed between mice with low- and high-dose total body irradiation.
Inhibition of hedgehog signaling by LDE223 is well tolerated and prevents the clinical features of sclerodermatous cGVHD. (A) Preventive treatment with LDE223 impeded weight loss after alloBMT and resulted in a significantly higher body weight. (B) The clinical composite score for cutaneous cGVHD was significantly lower in mice treated with LDE223 in a preventive manner compared with sham-treated mice. (C) LDE223 prevented the histologic changes of experimental sclerodermatous cGVHD. Preventive regimen of LDE223 reduced dermal thickening, decreased the hydroxyproline content of the skin (D) and diminished the differentiation of resting fibroblasts into myofibroblasts (E) compared with sham-treated mice. Histologic analyses were performed at day 42 after transplantation. Six mice per group were analyzed. synBMT indicates syngeneic controls; and alloBMT, mice receiving alloBMT.
Inhibition of hedgehog signaling by LDE223 is well tolerated and prevents the clinical features of sclerodermatous cGVHD. (A) Preventive treatment with LDE223 impeded weight loss after alloBMT and resulted in a significantly higher body weight. (B) The clinical composite score for cutaneous cGVHD was significantly lower in mice treated with LDE223 in a preventive manner compared with sham-treated mice. (C) LDE223 prevented the histologic changes of experimental sclerodermatous cGVHD. Preventive regimen of LDE223 reduced dermal thickening, decreased the hydroxyproline content of the skin (D) and diminished the differentiation of resting fibroblasts into myofibroblasts (E) compared with sham-treated mice. Histologic analyses were performed at day 42 after transplantation. Six mice per group were analyzed. synBMT indicates syngeneic controls; and alloBMT, mice receiving alloBMT.
Treatment with LDE223 effectively prevented the clinical manifestations of cGVHD. LDE223 reduced weight loss observed in mice with cGVHD. The body weight of LDE223-treated mice did not differ from that of syngeneic controls until day 23. Thereafter, a modest difference in body weight was observed. However, the body weight of LDE223-treated mice remained significantly higher compared with sham-treated recipients of alloBMT. The relative decrease in body weight was ameliorated by 65% ± 3% in LDE223-treated mice compared with sham-treated mice at day 42 (Figure 3A). Treatment with LDE223 also delayed the onset and reduced the severity of cutaneous cGVHD. In contrast to sham-treated mice, in which first signs of cGVHD were observed 18 days after allogeneic BMT, no clinical signs of cutaneous cGVHD were observed in LDE223-treated mice until day 30. At the end of the follow-up period (42 days after BMT), a mild clinical cGVHD with a maximal composite score of 0.2 occurred in LDE223-treated mice compared with a score of 2.6 in the sham-treated mice (Figure 3B). Of note, the formation of cutaneous ulcers was completely prevented by LDE223.
Inhibition of hedgehog signaling also ameliorated the histologic changes of experimental cGVHD and reduced dermal fibrosis and atrophy of the subcutis. Preventive treatment with LDE223 after allogeneic BMT abrogated dermal thickening and maintained the dermal thickness at the levels of syngeneic controls (P = .001 vs sham-treated mice; Figure 3C). The hydroxyproline content was also significantly reduced by 56% ± 9% (P = .004; Figure 3D). Moreover, the differentiation of resting fibroblasts into myofibroblasts in mice receiving alloBMT was completely prevented by LDE223 (P = .001; Figure 3E).
LDE223 is effective for the treatment of clinically manifest experimental cGVHD
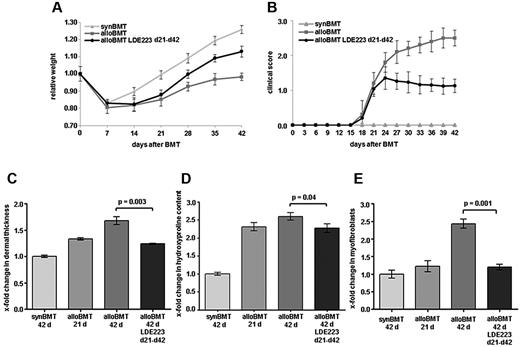
After demonstrating that LDE223 can prevent clinical and histologic manifestations of experimental cGVHD, we next evaluated LDE223 for the treatment of established cGVHD. In these experiments, treatment with LDE223 was initiated at day 21 after the onset of first clinical manifestations of experimental cGVHD. Mice treated with LDE223 gained significantly more weight than sham-treated controls (Figure 4A). The body weight increased by 27% ± 4% after initiation of LDE223 at day 21 until the end of the observation period at day 42 compared with an increase of 13% ± 3% during the same observation period in sham-treated mice (Figure 4A).
Treatment with LDE223 initiated after onset of clinical disease effectively ameliorates clinical and histologic changes of sclerodermatous cGVHD. Treatment with LDE223 on first clinical signs improved clinical features of cGVHD as evidenced by a increased recovery of the body weight after alloBMT (A) and by decreased composite score (B). Treatment with LDE223 (C) reduced dermal thickening, (D) prevented further increase of the hydroxyproline content, and (E) reversed the differentiation of resting fibroblasts into myofibroblasts. Six mice per group were analyzed. synBMT indicates syngeneic controls; and alloBMT, mice receiving alloBMT.
Treatment with LDE223 initiated after onset of clinical disease effectively ameliorates clinical and histologic changes of sclerodermatous cGVHD. Treatment with LDE223 on first clinical signs improved clinical features of cGVHD as evidenced by a increased recovery of the body weight after alloBMT (A) and by decreased composite score (B). Treatment with LDE223 (C) reduced dermal thickening, (D) prevented further increase of the hydroxyproline content, and (E) reversed the differentiation of resting fibroblasts into myofibroblasts. Six mice per group were analyzed. synBMT indicates syngeneic controls; and alloBMT, mice receiving alloBMT.
LDE 223 treatment after onset of clinical disease of cGVHD completely inhibited progression of cutaneous cGVHD. The clinical composite score in LDE223 remained at 1.1 ± 2 after 42 days in LDE223-treated mice, whereas it further increased to 2.5 ± 2 in sham-treated mice (Figure 4B). In contrast to sham-treated mice, no cutaneous ulcers were observed in mice treated with LDE223 after the onset of cGVHD.
Consistent with the stabilization of clinical disease, inhibition of hedgehog signaling in clinically manifest cGVHD abrogated histologic progression. In mice receiving alloBMT, dermal thickening at day 42 was significantly reduced by 65% on treatment with LDE223 compared with sham-treated mice (P = .003; Figure 4C). The hydroxyproline content and the myofibroblast counts were also significantly lower in LDE223-treated compared with sham-treated recipients (54% for the hydroxyproline content, P = .04) and 86% for the numbers of myofibroblasts (P = .001; Figure 4D-E). Furthermore, the dermal thickness and the numbers of myofibroblasts did not differ between alloBMT mice treated therapeutically with LDE223 and recipients killed before initiation of LDE223 treatment at day 21. This suggests that inhibition of hedgehog signaling completely inhibited histological progression of experimental cGVHD.
LDE223 treatment does not reduce leukocyte infiltration
Inflammatory infiltrates are characteristic features of human and murine sclerodermatous cGVHD. The infiltrating leukocytes, in particular T cells, stimulate the synthesis of collagen in resident fibroblasts by the release of profibrotic cytokines. To analyze whether the therapeutic effects of LDE223 result from inhibition of leukocyte influx, we quantified the number of leukocytes. Only a minor, nonsignificant reduction in the number of leukocytes was observed between mice receiving alloBMT treated with LDE223 and sham-treated mice (395% ± 4% vs 456% ± 10% increase compared with syngeneic controls) by histologic analyses. FISH analyses further confirmed that LDE223 did not affect the number of infiltrating donor leukocytes. Consistently, no differences were observed by FACS analyses for CD45-positive cells, suggesting that the hedgehog pathway does not regulate leukocyte infiltration in cGVHD. Further analyses also demonstrated no changes in B cell and Treg counts as well as in the levels of BAFF (data not shown).
Hedgehog signaling directly activates fibroblasts
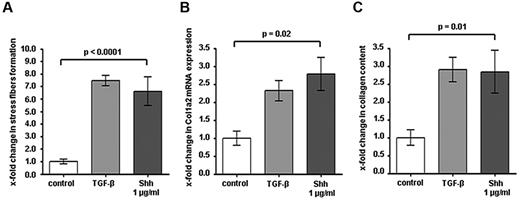
Based on the prominent activation of hedgehog signaling in fibroblasts in cGVHD and the comparable degree of inflammatory cell infiltration in LDE223- and sham-treated mice, we assumed that hedgehog signaling may directly stimulate the release of collagen in fibroblasts. To address this hypothesis, cultured fibroblasts were stimulated with recombinant Shh. Shh induced the formation of stress fibers in resting fibroblasts, which serve as markers of fibroblast activation and differentiation into metabolically active myofibroblasts (P < .0001; Figure 5A). Incubation with Shh significantly increased the mRNA levels of col 1a2 by 2.8 ± 0.5–fold (P = .04; Figure 5B). Consistently, Shh stimulated also the release of collagen protein into the cell culture supernatant by 2.9 ± 0.4–fold (P = .03; Figure 5C). Of note, the stimulatory effects of Shh were comparable with those of TGF-β, which is considered as one of the most potent profibrotic mediators.
The Hedgehog pathway regulates fibroblast activation. (A) Stimulation of human cultured fibroblasts with Shh induced stress fiber formation in resting fibroblasts to the levels that can also be achieved by TGF-β stimulation. Stress fibers were visualized with phalloidin and quantified with ImageJ Version 1.44p software. (B) Incubation of human dermal fibroblasts with Shh increased the mRNA levels of col 1a2 as well as the release of collagen protein (C) similar to that obtained on stimulation with TGF-β (n = 5 each).
The Hedgehog pathway regulates fibroblast activation. (A) Stimulation of human cultured fibroblasts with Shh induced stress fiber formation in resting fibroblasts to the levels that can also be achieved by TGF-β stimulation. Stress fibers were visualized with phalloidin and quantified with ImageJ Version 1.44p software. (B) Incubation of human dermal fibroblasts with Shh increased the mRNA levels of col 1a2 as well as the release of collagen protein (C) similar to that obtained on stimulation with TGF-β (n = 5 each).
Effects of the pharmacologic inhibition of Smo on GVL
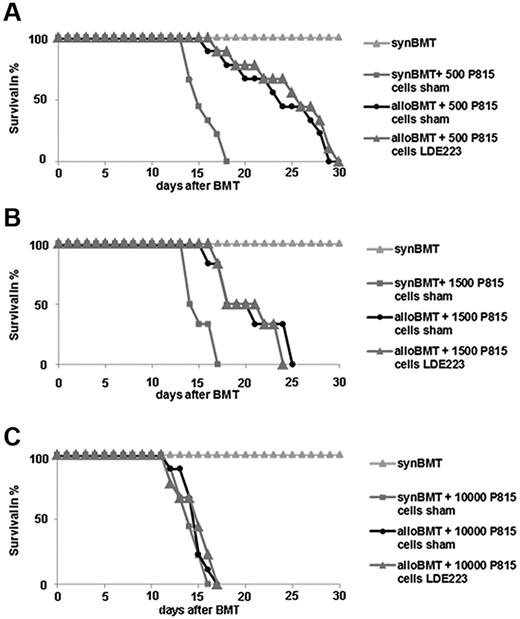
To investigate potential effects of targeting Smo on GVL, a P815 leukemia model with injection of different numbers of P815 cells was analyzed. All of the syngeneic recipients that did not receive P815 leukemia cells survived throughout the observation period, whereas all of the syngeneic recipients that were injected with 500 P815 cells after irradiation died by day 18 (Figure 6A). Sham-treated, allogeneically transplanted mice that received the allogeneic bone marrow and splenocytes died by day 30. The survival period of LDE223-treated, allogeneically transplanted mice did not differ from that of sham-treated, allogeneically transplanted mice. No differences in survival between LDE223-treated and sham-treated mice were also noted on injection of 1500 P815 cells (Figure 6B). However, injection of 10 000 cells P815 led to rapid tumor progression and death of all mice within 17 days (Figure 6C). A survival advantage because of GVL in allogeneically transplanted mice was no longer detectable with 10 000 tumor cells. Survival did not differ between LDE223-treated mice and sham-treated mice.
Effects of LDE223 on GVL and survival in the P815 leukemia model. (A-B) In mice injected with 500 (A) or 1500 (B) P815 cells, comparable survival times were noted in LDE223-treated, allogeneically transplanted mice and sham-treated, allogeneically transplanted mice. (C) Injection of 10 000 cells P815 led to death of all mice within 16 days. No survival advantage of allogeneically transplanted mice was detectable in mice injected with 10 000 tumor cells, and survival did not differ between LDE223-treated mice and sham-treated mice. Survival over time is shown as Kaplan-Meier curves. Nine mice per group were analyzed.
Effects of LDE223 on GVL and survival in the P815 leukemia model. (A-B) In mice injected with 500 (A) or 1500 (B) P815 cells, comparable survival times were noted in LDE223-treated, allogeneically transplanted mice and sham-treated, allogeneically transplanted mice. (C) Injection of 10 000 cells P815 led to death of all mice within 16 days. No survival advantage of allogeneically transplanted mice was detectable in mice injected with 10 000 tumor cells, and survival did not differ between LDE223-treated mice and sham-treated mice. Survival over time is shown as Kaplan-Meier curves. Nine mice per group were analyzed.
Discussion
In the present study, we demonstrate that hedgehog signaling plays an important role in the pathogenesis of sclerodermatous cGVHD. We observed an overexpression of Shh in human and murine cGVHD that resulted in activation of hedgehog signaling with accumulation of the downstream transcription factors Gli-1 and Gli-2. Consistent with findings in other fibrotic conditions, we did not observe changes in the expression of Gli-3 in human or murine cGVHD. We did not find any trends between activation of hedgehog signaling and clinical characteristics of patients with sclerodermatous cGVHD. However, further studies with larger patient numbers are required to finally assess potential correlations. The overexpression of Gli-1 and Gli-2 was particularly pronounced in fibroblasts, suggesting that fibroblasts may be the major effector cells for the pathogenetic effects of hedgehog signaling in cGVHD. Consistent with this hypothesis, we demonstrate that Shh potently activates resting fibroblasts and stimulates the release of collagen in vitro. Of note, the stimulatory effects of Shh were comparable to that of TGF-β, which is considered as the key cytokine for the activation of fibroblasts. In contrast, inhibition of hedgehog signaling did not reduce the number of infiltrating leukocytes. Although we cannot exclude fine-tuning of leukocyte activation by hedgehog signaling, these findings suggest direct stimulatory effects on fibroblasts as the major mechanism of hedgehog signaling in cGVHD. The stimulatory effects of Shh on fibroblasts are likely not restricted to cGVHD but may also occur in other diseases with aberrant activation of fibroblasts. Indeed, recent studies in mouse models of experimental bile duct ligation and nonalcoholic fatty liver disease implicate hedgehog signaling in different types of liver fibrosis.36 Moreover, tumors with activated hedgehog signaling, such as pancreatic tumors, are typically surrounded by an excessive extracellular matrix capsule.37,38
Our study demonstrates that targeting the hedgehog pathway is effective for the treatment of experimental cGVHD. Pharmacologic inhibition of Smo abolished hedgehog activation in cGVHD and ameliorated clinical and histologic features of cGVHD. Inhibition of Smo not only reduced weight loss, alopecia, and cutaneous ulcers but also prevented dermal thickening, accumulation of collagen, and differentiation of resting fibroblasts into metabolically active myofibroblasts. Targeting Smo was effective not only as preventive but also as a therapeutic regimen administered after onset of clinical disease. Although further studies are essential to better characterize the role of hedgehog signaling in sclerodermatous cGVHD, including additional models of cGVHD with mortality as endpoint, our findings may have direct translational implications. The prominent role of the hedgehog pathway in multiple human cancers, such as basal cell carcinoma, pancreatic cancer, and medulloblastoma, fostered the development of small-molecule inhibitors against Smo, several of which are currently evaluated in clinical trials (www.clinicaltrials.gov). In addition to Smo, other components of hedgehog signaling, such as Shh, may also represent interesting candidates for targeted therapies. Despite the deleterious consequences of perturbed hedgehog signaling during embryonic development in mice, results from first clinical trials indicate that pharmacologic targeting of the hedgehog pathway is not limited by toxicity in adults.23,39 No dose-limiting toxicity was reported with the oral Smo inhibitor GDC449 in a phase 1/2 trial at doses that efficiently inhibited hedgehog signaling.23 Moreover, only one grade 4 adverse event and no grade 5 adverse events had occurred and only 1 of 68 treated patients withdrew because of adverse events.23 Based on these results, GDC449 has recently been approved by the FDA. Thus, pharmacologic inhibition of hedgehog signaling is well tolerated, and several inhibitors of Smo would be available for clinical trials in cGVHD. The development of novel therapeutic approaches is warranted because of the high morbidity and mortality of cGVHD and the current lack of efficient molecular therapies for clinical use.2,4
In conclusion, the pathophysiology of cGVHD Hedgehog signaling does not affect leukocyte infiltration but appears to directly regulate fibroblast activation. Inhibition of hedgehog with small-molecule inhibitors of Smo potently ameliorates clinical and histologic changes of murine cGVHD in both preventive and therapeutic regimens. Because inhibitors of Smo are available and well tolerated, targeting hedgehog signaling might be a novel strategy for clinical trials in cGVHD.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Maria Halter and Anna-Maria Herrmann for excellent technical assistance.
This work was supported by the Interdisciplinary Center of Clinical Research in Erlangen (grants A20 and A40, J.H.W.D.), Deutsche Forschungsgesellschaft (grants DI 1537/1-1, DI 1537/2-1, DI 1537/4-1, DI-1537/5-1, AK 144/1-1, and SCHE 1583/7-1), CMH Research Projects (no. 00000023728, M.T.), the Wilhelm Sander Foundation (B.M.S. and J.H.W.D.), and the Ernst Jung Foundation (Career Support Award of Medicine, J.H.W.D.). LDE223 was kindly provided by Novartis. Novartis had no influence on the design of the study or the interpretation of the results.
Authorship
Contribution: P.Z., K.P.-Z., A.D., C.D., M.T., L.E.M., F.E., S.V., C.B., I.T., and F.D.G. performed experiments and/or analyzed data; and P.Z., K.P.-Z., A.D., O.D., G.S., B.M.S., and J.H.W.D. designed the research plan and wrote the manuscript.
Conflict-of-interest disclosure: O.D. has consultancy relationships and/or has received research funding from Actelion, Pfizer, Ergonex, BMS, Sanofi-Aventis, United BioSource Corporation, medac, Biovitrium, Boehringer Ingelheim, Novartis, 4D Science and Active Biotec in the area of potential treatments of scleroderma; J.H.W.D. has consultancy relationships and/or has received research funding from Actelion, Pfizer, Ergonex, BMS, Celgene, Bayer Pharma, Boehringer Ingelheim, JB Therapeutics, Sanofi-Aventis, Novartis, Array Biopharma and Active Biotec in the area of potential treatments of scleroderma and is stock owner of 4D Science GmbH.
Correspondence: Jörg H. W. Distler, Department of Internal Medicine 3 and Institute for Clinical Immunology, University of Erlangen-Nuremberg, Ulmenweg 18, 91054 Erlangen, Germany; e-mail: joerg.distler@uk-erlangen.de.