Abstract
Sjögren syndrome (SS) is a systemic autoimmune disease characterized by dry mouth and eyes, and the cellular and molecular mechanisms for its pathogenesis are complex. Here we reveal, for the first time, that bone marrow mesenchymal stem cells in SS-like NOD/Ltj mice and human patients were defective in immunoregulatory functions. Importantly, treatment with mesenchymal stem cells (MSCs) suppressed autoimmunity and restored salivary gland secretory function in both mouse models and SS patients. MSC treatment directed T cells toward Treg and Th2, while suppressing Th17 and Tfh responses, and alleviated disease symptoms. Infused MSCs migrated toward the inflammatory regions in a stromal cell–derived factor-1–dependent manner, as neutralization of stromal cell–derived factor-1 ligand CXCR4 abolished the effectiveness of bone marrow mesenchymal stem cell treatment. Collectively, our study suggests that immunologic regulatory functions of MSCs play an important role in SS pathogenesis, and allogeneic MSC treatment may provide a novel, effective, and safe therapy for patients with SS. This study was registered at www.clinicaltrials.gov as NCT00953485.
Introduction
Human Sjögren syndrome (SS) is a chronic, systemic autoimmune disorder characterized by inflammation of exocrine glands and functional impairment of the salivary and lacrimal glands.1 B cells have been shown to play a significant role in SS pathogenesis, as their depletion significantly alleviated disease symptoms.2-4 Remarkable reduction of Treg numbers in salivary glands and reduction of CD4+CD25+high T cells in peripheral blood were observed,5 and analysis of inflammatory tissue in the salivary glands showed a predominance of T cells, in particular Th1 cell infiltration,6,7 albeit Th2 and Th17 responses have been also reported,8 demonstrating the complexity of the SS pathogenesis. Moreover, because methods for salivary gland morphologic (sialogram) observation and functional (saliva flow rate) evaluation are both noninvasive and easy to operate, SS serves as a valuable model for autoimmune disease studies.
Mesenchymal stem cells (MSCs), such as bone marrow mesenchymal stem cells (BMMSCs) and umbilical cord mesenchymal stem cells (UCMSCs), are multipotent stem cells with the capacity to differentiate into osteoblasts, chondrocytes, adipocytes, and neural cells.9 MSCs express low levels of MHC class I but lack expression of MHC class II surface molecules, and thereby cannot serve as effective antigen-presenting cells to promote immune responses.10 Although the precise molecular mechanism remains unclear, MSCs have been reported to exert immunomodulatory effects on various activated lymphoid cells, including T cells, B cells, natural killer cells, and dendritic cells.11-13 Their low immunogenicity and immunoregulatory potential offer a promising new treatment for severe refractory autoimmune diseases.14-19 Indeed, the therapeutic efficacy of MSC infusion has been demonstrated in experimental and clinical systemic lupus erythematosus,20,21 systemic sclerosis,22 and type 1 diabetes mellitus.23 In addition, migrating MSCs may also represent a source of multipotent cells that could repair damaged tissues and organs. The underlying mechanisms responsible for the homing of infused MSCs remain unclear.
Currently, treatment of SS is difficult and challenging.24 For example, in contrast to other inflammatory autoimmune diseases, including rheumatoid arthritis, blocking TNF-α showed very little effect in treatment of SS.7 Because MSCs offer a promising new treatment for autoimmune diseases, we examined functions of MSCs in SS disease mouse models and human SS patients, determined whether allogeneic MSCs have therapeutic effects, and investigated the underlying mechanisms of MSC treatment in both experimental animal models and SS patients.
Methods
Mice
Female NOD/Ltj mice (Cdh23ahl) served as SS animal model, with the H-2 haplotype H-2g7; outbred strain ICR mice (from a subline of which the NOD strain was derived) served as control. Male BALB/c mice (H-2d) and C57BL/6-gfp (H-2b) transgenic mice served as allogeneic MSC donors. NOD/Ltj mice and ICR mice were purchased from Beijing HFK Bioscience Co. BALB/c mice and C57BL/6-gfp transgenic mice were obtained from the Institute of Laboratory Animal Science, Chinese Academy of Medical Sciences. Mice were maintained in a specific pathogen-free animal facility and kept under conventional conditions with free access to water and food. The Animal Care and Use Committee of Capital Medical University approved all experiments in this study.
Isolation, culture, differentiation, and CXCR4 blockade of BMMSCs in vitro
Bone marrow cells were flushed out from bone cavity of femurs and tibias with heat-inactivated medium. After 3 or 4 passages, BMMSCs were cultured under osteogenic or adipo-induction differentiation condition for 2 weeks, and the osteogenic and adipogenic differentiation potentials were determined. Neutralizing anti-CXCR4 antibody (5 μg/mL/5 × 105 cells, R&D Systems) was added and incubated in 37°C for 20 minutes. Then cells were harvested for infusion. Details are described in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
T-cell proliferation
After isolation, splenocytes and PBMCs were labeled by CFSE (Invitrogen) stimulated by anti-CD3 antibody, with or without BMMSCs from SS disease animals, normal animals, SS patients, or normal humans, respectively. After 4 days, lymphocytes were harvested and determined by flow cytometric analysis. Proliferation index was calculated, as the average number of cell divisions versus the original population by Modfit LT Version 3.0 software. Details are described in supplemental Methods.
Allogeneic BMMSC treatment and saliva flow rate measurement
For BMMSC treatment, NOD/Ltj mice were injected with BMMSCs (from BALB/c or C57BL/6-gfp; 1 × 105 cells/mouse) in 0.15 mL PBS via tail vein. Saliva flow rate was determined as described in supplemental Methods.
Histologic analysis of salivary glands
Samples were fixed with 4% PFA for 24 hours at 4°C; paraffin sections were used for hematoxylin and eosin staining, and frozen sections were used for green fluorescent protein (GFP)–BMMSC tracking analysis. After hematoxylin and eosin staining, the sections were photographed in room temperature by microscope (Olympus BX51) with CCD (Olympus DP72) and the area of inflammatory focus (containing > 50 lymphocytes per 4 mm2 tissue) was calculated per field at ×200 magnification (×20 objective lenses) by Image-Pro Plus Version 6.0 software (Media Cybernetics). Five entire salivary gland sections for each animal were counted with an average of 10 fields/gland by an experienced expert of histopathology under blinded fashion.
Real-time RT-PCR
Total RNA was isolated by RNA isolation kit (Sunbio) following the manufacturer's instructions, and cDNA was synthesized from 100 ng of total RNA in 3 μL using reverse transcription kit (Sunbio). Real-time PCR was analyzed using the δδ-Ct method. A detailed description of procedures and primers specific for gene regions is provided in supplemental Methods.
ELISA
Mice peripheral blood was collected from the retro-orbital plexus of treated mice and controls and centrifuged to obtain serum. Tissue lysates were extracted from mouse spleen, liver, lung, kidney, salivary gland, and lymph node. Bone marrow cells were flushed out from bone cavity of femurs and tibias with PBS. For human, serum samples were collected before UCMSC treatment and at each follow-up visit. Cytokines were assessed by mouse or human ELISA kit (R&D Systems) according to the manufacturer's instructions.
Flow cytometric analysis
Flow cytometric analysis was performed as previously described.25 Mouse splenocytes were used for cytometric analysis in the animal studies. Detail methods for Treg, Tfh, cytokine-producing cell (Th1, Th2, Th17 cells) staining are provided in supplemental Methods.
Patient enrollment
Twenty-four primary SS patients, ranging in age from 27 to 68 years, were enrolled in an MSC treatment trial. Inclusion and exclusion criteria are reported in supplemental Methods. All patients enrolled in the present study were poor responsive to glucocorticoid/glucocorticoid combined with immunosuppressant treatment before mesenchymal stem cell treatment (MSCT). Eleven patients had severe xerostomia and/or xerophthalmia at baseline, whereas the other 13 patients had refractory systemic comorbidities, including thrombocytopenia in 4 patients, anemia in 3 patients, interstitial lung disease in 2 patients, renal tubule acidosis in 4 patients, autoimmune hepatitis in 7 patients, nervous system involvements in 3 patients, and autoimmune enteritis in 1 patient. The trial was conducted in compliance with current Good Clinical Practice standards and in accordance with the principles set forth under the Declaration of Helsinki, 1989. This protocol was approved by the Ethics Committee at the Drum Tower Hospital of Nanjing University Medical School and registered at www.clinicaltrials.gov (phase 1 or 2; NCT00953485). All patients provided informed consent.
Umbilical cord MSC infusion
UCMSCs were prepared by the Stem Cell Center of Jiangsu Province, and details of UCMSCs purification and identification were described previously21 and in supplemental Methods. For treatment, UCMSCs (1 × 106/kg of body weight) were administered by intravenous infusion without premedication, such as steroids or antihistamines.
Follow-up procedures
After MSCT, all 24 patients were followed and data collected at baseline, 2 weeks, 1, 3, 6, and 12 months after MSCT. The SS Disease Activity Index (SSDAI) score26 and visual analog scale (VAS) for assessment of disease impact were assessed, and blood samples were taken for laboratory examinations, including hemoglobin, alanine aminotransferase, and aspartate aminotransferase, for each patient at each follow-up visit. For those with severe xerostomia and/or xerophthalmia at baseline, assessments performed at these time points included salivary flow rate test, determination of modified Treatment Emergent Symptom Scale for xerostomia score,27 and sialography. Systemic improvements were graded by VAS score and SSDAI score at each visit time for those without obvious xerostomia and/or xerophthalmia at baseline. Adverse events and their severity were assessed and recorded throughout the study. Sialography of the parotid gland,28,29 saliva flow rate measurements, VAS score, and SSDAI determination for SS patients are given in the supplemental Methods. Medication intake was tapered according to disease amelioration.
Statistical analysis
All data have a normal distribution and are presented as mean ± SEM (in data of infiltrating area statistics) or SD (in other data) of 3 independent experiments, and we used an α level of .05 for all statistical tests. The mice salivary flow rate and all clinical trial data were statistically analyzed with repeated measurement; other data were analyzed with 1-way ANOVA using SPSS Version 17.0. Each value was compared with the control values. P < .05 was regarded as statistically significant and was adjusted by the Bonferroni method to allow for multiple comparisons. The chart was made by Microsoft Office Excel 2007 and GraphPad Prism Version 5.0 software.
Results
Immunoregulatory activities of BMMSCs are impaired in disease mice and SS patients
Previous studies suggest that T lymphocyte overactivation is associated with functional impairment of BMMSCs.30 We first examined the immunoregulatory activity of BMMSCs in a nonobese diabetic mouse model (NOD/Ltj). Splenic cells from ICR mice were cultured with ICR or NOD/Ltj BMMSCs, plus anti-CD3 and anti-CD28 mAbs. As shown in Figure 1A and B, culturing T cells with NOD/Ltj BMMSCs resulted in much higher proliferative responses compared with culturing with ICR BMMSCs, indicating that immunoregulatory activities of BMMSCs were reduced in disease animals. Interestingly, we observed that the frequency of CD4+Foxp3+ T regulatory cell (Treg) was significantly lower (P = .0001, n = 12; Figure 1C,E) in T cells cultured with BMMSCs from disease animals, and the percentage of CD4+ cells in splenocytes cocultured with NOD/Ltj BMMSCs was significantly higher than that with ICR BMMSCs (P = .0005, n = 12; Figure 1C-D), suggesting a possible mechanism that reduced Treg development driven by BMMSC from disease animals contributed to their loss of immunosuppression activities. BMMSCs from NOD/Ltj mice have significantly lower proliferative capacity (P = 1.691 × 10−11, n = 6; supplemental Figure 1A-B), and less osteogenic (supplemental Figure 1C-E) and adipogenic (supplemental Figure 1F-H) differentiation potentials than those from ICR mice, indicating that other biologic functions of BMMSCs are also impaired. To determine whether the impairment of immunoregulatory activities we observed in disease animals also exist in SS patients, normal human PBMCs were cultured with BMMSCs obtained from normal persons or SS patients. As shown in Figure 1F and G, BMMSCs from SS patients showed significant defectiveness of immunoregulatory functions, as much higher proliferative responses were observed in cells cocultured with BMMSCs obtained from SS persons than from normal patients (P = .01; n = 4). Together, these results demonstrated that the loss of immunoregulatory functions by BMMSCs may play an important role in SS pathogenesis.
Impairment of immunoregulatory activity of BMMSCs during SS pathogenesis. (A-B) BMMSCs from NOD/Ltj mice failed to effectively suppress the proliferation of stimulated T cells. Stimulated normal T cells cocultured with NOD BMMSCs showed higher proliferative response compared with those cocultured with control ICR BMMSCs (P = .0002, n = 12). (C-E) CD4+Foxp3+Treg cells from splenocytes cocultured with ICR BMMSCs or NOD BMMSCs. *CD4+ cells in splenocytes cocultured with NOD/Ltj BMMSCs were significantly higher than that with ICR BMMSCs (P = .0005); n = 12. *ICR BMMSCs had better regulatory potential for Treg cells compared with BMMSCs from NOD mice (P = .0001); n = 12. (F-G) Impairment of immunoregulatory capacity was observed in the BMMSCs of SS patients. Normal PBMCs cultured with BMMSCs obtained from SS patients showed higher proliferative response compared with those cocultured with normal BMMSCs (P = .01, n = 5).
Impairment of immunoregulatory activity of BMMSCs during SS pathogenesis. (A-B) BMMSCs from NOD/Ltj mice failed to effectively suppress the proliferation of stimulated T cells. Stimulated normal T cells cocultured with NOD BMMSCs showed higher proliferative response compared with those cocultured with control ICR BMMSCs (P = .0002, n = 12). (C-E) CD4+Foxp3+Treg cells from splenocytes cocultured with ICR BMMSCs or NOD BMMSCs. *CD4+ cells in splenocytes cocultured with NOD/Ltj BMMSCs were significantly higher than that with ICR BMMSCs (P = .0005); n = 12. *ICR BMMSCs had better regulatory potential for Treg cells compared with BMMSCs from NOD mice (P = .0001); n = 12. (F-G) Impairment of immunoregulatory capacity was observed in the BMMSCs of SS patients. Normal PBMCs cultured with BMMSCs obtained from SS patients showed higher proliferative response compared with those cocultured with normal BMMSCs (P = .01, n = 5).
Treatment of normal allogeneic BMMSCs alleviates SS-like disease in NOD/Ltj mice
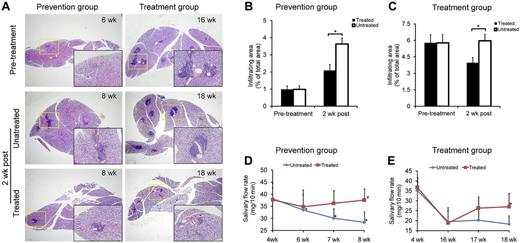
SS-like autoimmune disorders usually appear at age 7-8 weeks in NOD/Ltj mice. To investigate whether BMMSCs have preventive and therapeutic effects on SS-like inflammation in mice, we infused allogeneic BMMSCs from BALB/c mice into NOD/Ltj mice either at an early stage of SS (6 weeks of age, prevention group) or at the developed stage (16 weeks of age, treatment group). In NOD/Ltj mice, the inflammatory responses in the submandibular glands increased rapidly from 6 to 8 weeks of age (P = .00002, n = 6; Figure 2A-B). When NOD/Ltj mice were injected with allogeneic BMMSCs at 6 weeks of age, the area of inflammation in the submandibular glands was significantly smaller than untreated NOD/Ltj mice (P = .017, n = 6), and this protective effect was detectable as early as 2 weeks after BMMSC infusion (Figure 2A-B). A similar reduction of inflammatory responses was observed in the treatment group (BMMSCs were infused at 16 weeks of age) compared with untreated NOD/Ltj mice (P = .009, n = 6; Figure 2A,C). The saliva flow rate of untreated NOD/Ltj mice began to decrease at the age of 6 weeks and declined rapidly thereafter (P = .008, 8 weeks vs 4 weeks of age, n = 6; Figure 2D). In the prevention group, mice that received BMMSC infusion at 6 weeks of age showed sustained saliva flow rate 2 weeks after BMMSC infusion (P = .977, 8 weeks of age vs 4 weeks of age, n = 6; Figure 2D), and the saliva flow rate also improved significantly in the treatment group 2 weeks after BMMSC infusion (P = .045, 18 weeks vs 16 weeks of age, n = 6; Figure 2E). These findings indicated that allogeneic BMMSCs were effective in suppressing inflammation and restoring secretory function of the salivary glands in SS-like disease mice.
MSC treatment reduced inflamed tissue damage and improved salivary gland function in NOD/Ltj mice. (A-C) Histology of the submandibular glands of NOD/Ltj mice in untreated, prevention, and treatment groups. Pretreated indicates 6 weeks for prevention and 16 weeks for treatment groups; and 2 weeks post, 2 weeks later with or without MSC infusion (8 weeks for prevention and 18 weeks for treatment groups). Yellow box area is magnified in the black box. In the prevention group (B), the infiltrating area in the submandibular glands was significantly smaller than control (P = .017, n = 6). In the treatment group (C), the infiltrating area was significantly reduced 2 weeks after MSC infusion (P = .009, n = 6). (D-E) The saliva flow rate of NOD/Ltj mice before and after MSC infusion. The saliva flow rate began to decrease at ∼ 6 weeks of age and declined rapidly at 7 and 8 weeks of age. *Six, 7, and 8 weeks versus 4 weeks in the untreated group (all P < .05); n = 6. In the prevention group (D), the saliva flow rate returned to baseline (4 weeks level) 2 weeks after BMMSC infusion. #P = .977 (n = 6). (E) The saliva flow rate in the treatment group increased 2 weeks after BMMSC infusion (P = .045, n = 6), whereas the untreated group remained at a lower level.
MSC treatment reduced inflamed tissue damage and improved salivary gland function in NOD/Ltj mice. (A-C) Histology of the submandibular glands of NOD/Ltj mice in untreated, prevention, and treatment groups. Pretreated indicates 6 weeks for prevention and 16 weeks for treatment groups; and 2 weeks post, 2 weeks later with or without MSC infusion (8 weeks for prevention and 18 weeks for treatment groups). Yellow box area is magnified in the black box. In the prevention group (B), the infiltrating area in the submandibular glands was significantly smaller than control (P = .017, n = 6). In the treatment group (C), the infiltrating area was significantly reduced 2 weeks after MSC infusion (P = .009, n = 6). (D-E) The saliva flow rate of NOD/Ltj mice before and after MSC infusion. The saliva flow rate began to decrease at ∼ 6 weeks of age and declined rapidly at 7 and 8 weeks of age. *Six, 7, and 8 weeks versus 4 weeks in the untreated group (all P < .05); n = 6. In the prevention group (D), the saliva flow rate returned to baseline (4 weeks level) 2 weeks after BMMSC infusion. #P = .977 (n = 6). (E) The saliva flow rate in the treatment group increased 2 weeks after BMMSC infusion (P = .045, n = 6), whereas the untreated group remained at a lower level.
SDF-1/CXCR4 axis is critical for MSCs to migrate and improve salivary gland function
We next investigated the underlying mechanisms by which the injected allogeneic BMMSCs improved salivary gland function. We first examined whether the injected BMMSCs could migrate to the salivary glands. BMMSCs isolated from C57BL/6-gfp transgene mice were injected into NOD/Ltj mice and examined recipients' salivary glands. One week after MSC infusion, we observed GFP-positive cells in the submandibular glands of the MSC-treated NOD/Ltj mice (Figure 3A top right panel), and inflammation in affected salivary glands was also suppressed (not shown). We did not observe GFP-positive cells in submandibular glands of normal control mice injected with GFP-BMMSCs (Figure 3A), indicating that the presence of GFP-BMMSCs in the submandibular glands was not the result of a simple passive distribution, and the different microenvironment in NOD/Ltj mice versus normal ICR mice actively attracted BMMSCs to salivary glands.
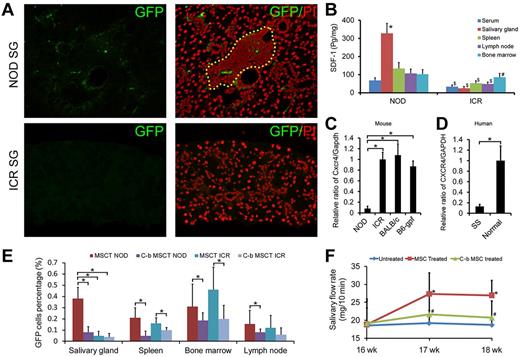
A critical role of SDF-1/CXCR4 for MSC trafficking and anti-inflammatory functions. (A) GFP-positive cells were detected in the submandibular glands of NOD/Ltj mice but not in control mice 1 week after allogeneic GFP-labeled BMMSC infusion. (B) ELISA (n = 6) showed that SDF-1 was significantly higher in serum ($P = .001), salivary gland ($P = 1.287 × 10−7), spleen ($P = .0002), and lymph nodes homogenates ($P = .0004) of NOD/Ltj mice than in control mice. In NOD/Ltj, the concentration of SDF-1 in salivary gland was the highest (*all P < .05), and in ICR mice bone marrow contained the highest SDF-1 (#all P < .05). (C) Real-time PCR for Cxcr4 expression in mice BMMSCs. The level of BMMSCs Cxcr4 gene from NOD/Ltj mice of 0.082 ± 0.043 was ∼ 5-fold lower than that from ICR, BALB/c, or C57BL/6-gfp mice (*all P < .05, n = 9). (D) Real-time PCR for CXCR4 expression in human BMMSCs. The level of BMMSCs CXCR4 gene from SS patients was ∼ 8-fold lower than that from healthy people (P = .009, n = 7). (E) In NOD/Ltj mice, higher numbers of GFP+ BMMSCs were detected in salivary glands after C57BL/6-gfp MSC infusion (MSCT NOD) compared with the CXCR4-blocked C57BL/6-gfp BMMSCs group (C-b MSCT NOD) 1 week after transplantation (P = 4.5 × 10−9, n = 12). A similar trend was observed for GFP+ BMMSCs in spleen, bone marrow, and lymph node in both NOD/Ltj and ICR mice (*all P < .05, n = 12). (F) Salivary flow rate (n = 6) of CXCR4-blocked BALB/c BMMSC-treated NOD/Ltj mice was significantly lower than mice in the normal BALB/c BMMSC infusion group (*P = .044 at 17 weeks and P = .036 at 18 weeks) and were similar to the untreated control group (#P = .475 at 17 weeks and P = .522 at 18 weeks).
A critical role of SDF-1/CXCR4 for MSC trafficking and anti-inflammatory functions. (A) GFP-positive cells were detected in the submandibular glands of NOD/Ltj mice but not in control mice 1 week after allogeneic GFP-labeled BMMSC infusion. (B) ELISA (n = 6) showed that SDF-1 was significantly higher in serum ($P = .001), salivary gland ($P = 1.287 × 10−7), spleen ($P = .0002), and lymph nodes homogenates ($P = .0004) of NOD/Ltj mice than in control mice. In NOD/Ltj, the concentration of SDF-1 in salivary gland was the highest (*all P < .05), and in ICR mice bone marrow contained the highest SDF-1 (#all P < .05). (C) Real-time PCR for Cxcr4 expression in mice BMMSCs. The level of BMMSCs Cxcr4 gene from NOD/Ltj mice of 0.082 ± 0.043 was ∼ 5-fold lower than that from ICR, BALB/c, or C57BL/6-gfp mice (*all P < .05, n = 9). (D) Real-time PCR for CXCR4 expression in human BMMSCs. The level of BMMSCs CXCR4 gene from SS patients was ∼ 8-fold lower than that from healthy people (P = .009, n = 7). (E) In NOD/Ltj mice, higher numbers of GFP+ BMMSCs were detected in salivary glands after C57BL/6-gfp MSC infusion (MSCT NOD) compared with the CXCR4-blocked C57BL/6-gfp BMMSCs group (C-b MSCT NOD) 1 week after transplantation (P = 4.5 × 10−9, n = 12). A similar trend was observed for GFP+ BMMSCs in spleen, bone marrow, and lymph node in both NOD/Ltj and ICR mice (*all P < .05, n = 12). (F) Salivary flow rate (n = 6) of CXCR4-blocked BALB/c BMMSC-treated NOD/Ltj mice was significantly lower than mice in the normal BALB/c BMMSC infusion group (*P = .044 at 17 weeks and P = .036 at 18 weeks) and were similar to the untreated control group (#P = .475 at 17 weeks and P = .522 at 18 weeks).
Stromal cell-derived factor-1 (SDF-1)/C-X-C chemokine receptor 4 (CXCR4) axis has been recognized as an inflammatory chemokine that regulates MSC trafficking.31 We measured the levels of SDF-1 and found that the salivary gland had the highest concentration of SDF-1 in NOD/Ltj (supplemental Figure 2A) and NOD/Ltj mice had significantly higher levels of SDF-1 in serum (P = .001), salivary gland (P = 1.287 × 10−7), spleen homogenates (P = .0002), and lymph node (P = .0004) than ICR mice (n = 6; Figure 3B), suggesting that the differential SDF-1 production may play a role in determining whether BMMSCs trafficked into the inflammation site. The reduction of Cxcr4 (receptor for SDF-1) transcription in BMMSCs of NOD/Ltj mice was further confirmed by real-time PCR, and it was almost 5-fold lower than in BMMSCs from ICR, BALB/c, or C57BL/6-gfp mice (Figure 3C), and CXCR4 in BMMSCs of SS patients was also ∼ 8-fold lower than healthy human (P = .009, n = 7; Figure 3D), suggesting that lower CXCR4 expression by BMMSCs from disease animals and patients, consequent failure to migrate into inflammation site, may play a role in their loss of immunoregulatory functions and consequent SS disease development.
To track migration of MSCs, we infused BMMSCs from C57BL/6-gfp mice to NOD/Ltj and found that the distributions of GFP MSCs corresponded with their SDF-1 expression 1 week after infusion (Figure 3E; supplemental Figure 2C). Similar trends were also observed 1 day after infusion (supplemental Figure 2B).When treated BMMSCs from C57BL/6-gfp with CXCR4 neutralizing antibody before infusion, 0.38% ± 0.1% GFP-positive cells were detected in salivary gland single-cell suspensions in the nonblocked group at 1 week after treatment, but only 0.08% ± 0.05% GFP-positive cells were detected in the CXCR4-blocked group (P = 4.5 × 10−9, n = 12; Figure 3E).
Furthermore, CXCR4 blocking completely abolished effectiveness of BALB/c BMMSCs to restore secretory function of salivary glands in NOD/Ltj mice (Figure 3F). These data indicated that the SDF-1/CXCR4 axis is critical in directing BMMSCs to migrate toward inflammatory sites to control autoimmunity.
MSC treatment favored Treg and Th2 while suppressing Th17 and Tfh responses
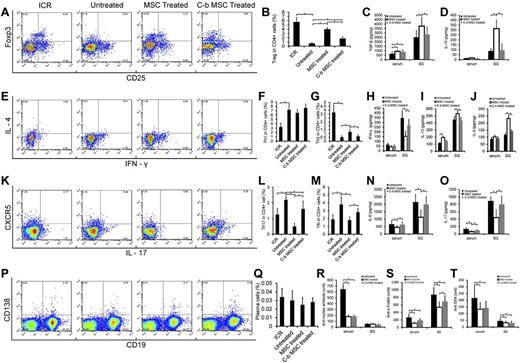
We next investigated how MSCs directed CD4 T-cell responses. We observed that the frequency of Treg cells in the spleen of NOD/Ltj mice were far less than that of ICR control mice (P = 2.597 × 10−7, n = 6; Figure 4A-B), and allogeneic BMMSCs from BALB/c mice significantly restored the number of Treg cells in NOD/Ltj mice (P = 4.790 × 10−9, n = 6) in the spleen as early as 1 week. Similar results were also observed in submandibular lymph nodes (supplemental Figure 3A-B). In addition, when we performed experiments on splenectomized NOD/Ltj mice, we observed that allogeneic MSCs were similarly effective in inhibiting disease development (supplemental Figure 3C), and there were similar higher levels of Treg cells in submandibular lymph nodes (supplemental Figure 3A-B), indicating that Tregs in spleen are not required for immune regulation and the therapeutic effects of MSCs probably occurred locally. When BMMSCs were treated with anti-CXCR4 Abs, the effect of Treg cell restoration was abrogated (P = 2.397 × 10−6, n = 6). TGF-β and IL-10 are important for Treg cell proliferation and function.32-34 We found that the levels of TGF-β increased both in blood (P = .035, n = 6) and salivary gland homogenates (P = .034, n = 6; Figure 4C) in NOD/Ltj mice 1 week after BMMSC infusion, and blocking CXCR4 resulted in some reduction. Interestingly, IL-10 only increased in salivary gland homogenates (P = .001, n = 6; Figure 4D), and CXCR4 blocking completely abolished these effects, suggesting that IL-10 may play a more direct and specific role for MSCs to control inflammatory responses in salivary gland than TGF-β (Figure 4C-D).
MSC treatment mediated immune regulation. (A-D) Tregs and related cytokines. (A) CD25 and FoxP3 in CD4 T cells of splenocytes in ICR control mice (ICR), NOD/Ltj untreated mice (Untreated), BALB/c BMMSC-infused NOD/Ltj mice (MSC Treated), and CXCR4-blocked BALB/c BMMSC-infused NOD/Ltj mice (C-b MSC Treated). (B) Tregs in spleen of NOD/Ltj untreated mice were far less than control ICR mice (P = 2.597 × 10−7, n = 6), and allogeneic BMMSC partly restored Treg cells (P = 4.790 × 10−9, n = 6), but CXCR4 blocking reduced MSC-mediated Treg generation compared with normal BMMSCs (P = 2.397 × 10−6, n = 6). (E-J) Th1, Th2, and related cytokines, intracellular staining for IFN-γ and IL-4 in CD4+ splenocytes. (E) Higher numbers of Th1 cells were observed in NOD/Ltj untreated mice than control ICR mice (F; P = 7.518 × 10−5, n = 6), and there was no change of Th1 response 1 week after allogeneic BMMSC or CXCR4-block BMMSC infusion. Th2 responses in the spleen of NOD/Ltj untreated mice were less than that of control ICR mice (G; P = 6.917 × 10−6, n = 6). Allogeneic BMMSC could partly restore Th2 (P = .0008, n = 6), and blocking of CXCR4 abolished this effect (P = .323, n = 6). (K-O) Th17, Tfh, and related cytokines. Flow cytometry for CXCR5 (Tfh) and IL-17 (Th17) expression in CD4 splenocytes. (K) Th17 were significantly higher in NOD/Ltj untreated mice than in control ICR mice (L; P = .0004, n = 6). Allogeneic BMMSCs mitigated the percentage of Th17 (P = 1.386 × 10−7, n = 6), and CXCR4 blocking resulted in less suppression (P = .025, n = 6). (M) MSC treatment suppressed Tfh response. Allogeneic BMMSC regulated T-cell cytokines IFN-γ (H) and IL-17 (O), regulatory cytokines IL-10 (D), IL-13 (I), and IL-4 (J), and other cytokines TGF-β (C) and IL-6 (N) production in serum and/or salivary gland homogenates. Blocking of CXCR4 of BMMSCs resulted in impairment of immunoregulatory activities. (P-T) Plasma cells and autoantibodies, CD19 and CD138 gated splenocytes in the 4 groups. (P) There was no significant difference of plasma cells in these 4 groups (Q). Both allogeneic BMMSC and CXCR4 blocked BMMSC transplantation decreased the SS-related autoantibodies: antinucleic antibody (R), anti–α-fodrin (S), and anti–SSA/Ro (T) in serum.
MSC treatment mediated immune regulation. (A-D) Tregs and related cytokines. (A) CD25 and FoxP3 in CD4 T cells of splenocytes in ICR control mice (ICR), NOD/Ltj untreated mice (Untreated), BALB/c BMMSC-infused NOD/Ltj mice (MSC Treated), and CXCR4-blocked BALB/c BMMSC-infused NOD/Ltj mice (C-b MSC Treated). (B) Tregs in spleen of NOD/Ltj untreated mice were far less than control ICR mice (P = 2.597 × 10−7, n = 6), and allogeneic BMMSC partly restored Treg cells (P = 4.790 × 10−9, n = 6), but CXCR4 blocking reduced MSC-mediated Treg generation compared with normal BMMSCs (P = 2.397 × 10−6, n = 6). (E-J) Th1, Th2, and related cytokines, intracellular staining for IFN-γ and IL-4 in CD4+ splenocytes. (E) Higher numbers of Th1 cells were observed in NOD/Ltj untreated mice than control ICR mice (F; P = 7.518 × 10−5, n = 6), and there was no change of Th1 response 1 week after allogeneic BMMSC or CXCR4-block BMMSC infusion. Th2 responses in the spleen of NOD/Ltj untreated mice were less than that of control ICR mice (G; P = 6.917 × 10−6, n = 6). Allogeneic BMMSC could partly restore Th2 (P = .0008, n = 6), and blocking of CXCR4 abolished this effect (P = .323, n = 6). (K-O) Th17, Tfh, and related cytokines. Flow cytometry for CXCR5 (Tfh) and IL-17 (Th17) expression in CD4 splenocytes. (K) Th17 were significantly higher in NOD/Ltj untreated mice than in control ICR mice (L; P = .0004, n = 6). Allogeneic BMMSCs mitigated the percentage of Th17 (P = 1.386 × 10−7, n = 6), and CXCR4 blocking resulted in less suppression (P = .025, n = 6). (M) MSC treatment suppressed Tfh response. Allogeneic BMMSC regulated T-cell cytokines IFN-γ (H) and IL-17 (O), regulatory cytokines IL-10 (D), IL-13 (I), and IL-4 (J), and other cytokines TGF-β (C) and IL-6 (N) production in serum and/or salivary gland homogenates. Blocking of CXCR4 of BMMSCs resulted in impairment of immunoregulatory activities. (P-T) Plasma cells and autoantibodies, CD19 and CD138 gated splenocytes in the 4 groups. (P) There was no significant difference of plasma cells in these 4 groups (Q). Both allogeneic BMMSC and CXCR4 blocked BMMSC transplantation decreased the SS-related autoantibodies: antinucleic antibody (R), anti–α-fodrin (S), and anti–SSA/Ro (T) in serum.
A predominance of Th1 rather than Th2 cell responses in patients with primary SS has been documented.35,36 We showed here that, although allogeneic BMMSC treatment did not suppress Th1 cells (IFN-γ; P = .262, n = 6; Figure 4E-F), it increased the percentage of Th2 cells (IL-4, IL-13; all P < .01, n = 6; Figure 4E,G; supplemental Figure 4A-B), and CXCR4-blocked BMMSCs failed to do so. CD4+ Th17 cells are involved during autoimmune diseases pathogenesis, and IL-6 participates in driving Th17 differentiation with or without TGF-β.25,37 We found that allogeneic BMMSCs significantly reduced the frequency of Th17 cells (P = 1.386 × 10−7, n = 6), and blocking CXCR4 abolished this suppression in NOD/Ltj mice (Figure 4K-L). In addition, IL-6 was reduced in normal BMMSC-treated but not CXCR4-blocked BMMSC-treated NOD/Ltj mice compared with untreated NON/Ltj mice, which correlated with the decrease in IL-17 levels in the same mice (Figure 4N-O), demonstrating that BMMSCs suppressed Th17 responses. T follicular helper T (Tfh) cells are a distinct subset of CD4+ helper T cells that regulate the development of antigen-specific B-cell immunity. On exposure to a foreign antigen, Tfh cells help B cells generate antibody-producing plasma cells and long-lived memory B cells.38 We next examined whether MSCs regulated Tfh cell development. One week after allogeneic BMMSC treatment, the proportion of Tfh cells (CD4+CXCR5+, Figure 4K) decreased significantly (P = .001, n = 6; Figure 4M), and CXCR4 blocking reduced this inhibitory effect (P = .053, n = 6; Figure 4M). There was no difference in plasma cell concentration between groups (Figure 4P-Q), but SS-related autoantibodies (antinucleic antibody, anti–α-fodrin, and anti-SSA/Ro, Figure 4R-T) were significantly decreased in the serum of NOD/Ltj mice treated with normal BMMSCs, whereas CXCR4 blocking partially abated autoantibody suppression effects (Figure 4R-T). Together, these data collectively demonstrated that BMMSCs suppressed inflammatory responses by favoring Treg and Th2 differentiation while inhibiting Th17 and Tfh responses, further supporting that the SDF-1/CXCR4 axis is critical for MSCs to exert immune-regulatory activities.
Allogeneic MSC treatment suppresses diseases in patients with primary SS
Given the therapeutic effects of allogeneic MSCs on experimental SS in NOD/Ltj mice, we next performed a clinical evaluation of allogeneic MSC treatment on patients with primary SS. Twenty-four patients with primary SS (23 females and 1 male; age, 45 ± 12 years; range, 27-68 years), were admitted to the hospital (Drum Tower Hospital of Nanjing University Medical School) and underwent umbilical cord MSCT (NCT00953485). Eleven patients reported symptoms of xerostomia and/or xerophthalmia, and 13 presented with severe systemic comorbidities. Mean disease duration was 76.7 ± 82.5 months (range, 3-384 months). All patients completed a 12-month follow-up, and clinical and laboratory data were collected before MSCT and at 2 weeks, 1, 3, 6, and 12 months after MSCT. The demographic and clinical manifestations are shown in supplemental Table 1.
All patients tolerated allogeneic MSCT well, and no adverse events occurred during or after MSC infusion. All patients showed improvements in symptoms after MSCT, although with a different response time (from 2 weeks to 6 months). Mean SSDAI scores of all the 24 patients decreased from 5.63 ± 1.44 (baseline) to 4.58 ± 1.67 at 2 weeks, 4.33 ± 1.79 at 1 month, 4.08 ± 1.44 at 3 months, 3.46 ± 1.18 at 6 months, and 3.08 ± 1.21 at 12 months (all P < .05; Figure 5A). SSDAI scores of ∼ 45.8% patients decreased > 30% at 3 months from baseline, ∼ 70.8% patients decreased > 30% at 6 months from baseline, and at 12s month, ∼ 83.3% of patients have their SSDAI score decreased > 30% from baseline. Global assessment by VAS also improved (Figure 5B) at 2 weeks (P = 8.98 × 10−6, n = 24) and showed further amelioration at 1, 3, 6, and 12 months after MSCT. At 3 months, 37.5% of patients have their VAS score decreased > 30% from baseline, at 6 and 12 months, ∼ 58.3% and 75% of patients have their VAS score decreased > 30% from baseline, respectively.
Allogeneic UCMSC treatment improves salivary gland function and suppresses disease activity and autoimmunity in SS patients. (A) SSDAI scores for all 24 patients decreased 2 weeks and 1, 3, 6, and 12 months after UCMSCT (all P < .05). (B) The VAS of all 24 patients decreased significantly after UCMSCT (all P < .05). Unstimulated (C) and stimulated (D) salivary flow rate of the 11 patients with xerostomia increased significantly at 2 weeks after UCMSCT (all P < .05) and maintained this level at 1, 3, 6, and 12 months of follow up. (E) The Treatment Emergent Symptom Scale score of the 11 patients with xerostomia decreased 2 weeks after UCMSCT (P = .05) and maintained at this low level on subsequent visits. (F-G) Allogeneic UCMSCT regulated SS-related autoantibodies in serum. (F) Anti-SSA/Ro decreased from 84.76 ± 62.19 U/mL at baseline to 0.51 ± 0.22 U/mL 1 month after treatment (n = 7), and (G) anti-SSB/La decreased from 146.62 ± 83.08 U/mL to 52.61 ± 38.67 U/mL (n = 6) 1 month after treatment.
Allogeneic UCMSC treatment improves salivary gland function and suppresses disease activity and autoimmunity in SS patients. (A) SSDAI scores for all 24 patients decreased 2 weeks and 1, 3, 6, and 12 months after UCMSCT (all P < .05). (B) The VAS of all 24 patients decreased significantly after UCMSCT (all P < .05). Unstimulated (C) and stimulated (D) salivary flow rate of the 11 patients with xerostomia increased significantly at 2 weeks after UCMSCT (all P < .05) and maintained this level at 1, 3, 6, and 12 months of follow up. (E) The Treatment Emergent Symptom Scale score of the 11 patients with xerostomia decreased 2 weeks after UCMSCT (P = .05) and maintained at this low level on subsequent visits. (F-G) Allogeneic UCMSCT regulated SS-related autoantibodies in serum. (F) Anti-SSA/Ro decreased from 84.76 ± 62.19 U/mL at baseline to 0.51 ± 0.22 U/mL 1 month after treatment (n = 7), and (G) anti-SSB/La decreased from 146.62 ± 83.08 U/mL to 52.61 ± 38.67 U/mL (n = 6) 1 month after treatment.
Unstimulated salivary flow rate (Figure 5C) of all 11 patients with symptoms of xerostomia increased significantly 2 weeks after MSCT (vs baseline, P = .0005, n = 11) and showed a 2-fold increase at 1 month (P = .0098, n = 11). This index continued to increase on subsequent follow-up visits. Stimulated salivary flow rate of these 11 patients also significantly increased at follow-up visits (P = .008, at 2 weeks, P = .043 at 1 month, P = .016 at 3 months, P = .017 at 6 months, and P = .016 at 12 months vs baseline, n = 11; Figure 5D). The determination of modified Treatment Emergent Symptom Scale score (Figure 5E) decreased 2 weeks after MSCT (P = .05, n = 11) and was maintained at this low level on subsequent visits.
For those with organ involvement at baseline, platelet counts increased significantly 2 weeks after MSCT for all 4 patients: [(85.75 ± 37.92) × 103/μL vs (37.50 ± 22.19) × 103/μL at baseline, P = .0001], and continued to increase on subsequent visits [(106.5 ± 36.7) × 103/μL at 1 month, (84.8 ± 8.4) × 103/μL at 3 months, (74.3 ± 17.2) × 103/μL at 6 months, and (106.0 ± 34.6) × 103/μL at 12 months]. Refractory hemolytic anemia also improved in all 3 patients after MSCT, hemoglobin level increased from 5.77 ± 1.26 g/dL at baseline, to 7.13 ± 0.61 g/dL at 2 weeks (P = .166), 7.93 ± 1.27 g/dL at 1 month (P = .103), (8.23 ± 1.3.82) g/dL at 3 months (P = .079), (8.53 ± 0.55) g/dL at 6 months (P = .025), and (8.87 ± 1.33) g/dL at 12 months (P = .043). SS-related autoimmune hepatitis improved in all 7 patients, as shown by an improved liver function index. The level of alanine aminotransferase was 105.3 ± 59.9 U/L before MSCT, 70.7 ± 31.9 U/L at 2 weeks, 60.7 ± 38.7 U/L at 1 month, significantly decreased at 3 months (49.9 ± 20.0 U/L, P = .039), 6 months (42.3 ± 24.8 U/L, P = .025), and 12 months (50.43 ± 19.78 U/L, P = .04). The concentration of aspartate aminotransferase was 130.8 ± 45.9 U/L at baseline, 99.3 ± 34.9 U/L at 2 weeks, 79.0 ± 33.1 U/L at 1 month, significantly decreased at 3 months (61.5 ± 20.0 U/L, P = .007), 6 months (45.2 ± 12.2 U/L, P = .01), and 12 months (38.5 ± 11.17 U/L, P = .01). For 1 patient with refractory enteritis, diarrhea improved after MSCT, in parallel with increased body weight. Nervous system involvement in 3 patients (myelitis, leukodystrophy, and peripheral nervous system involvement, respectively) showed no satisfactory improvements after MSCT. Medications used for each patient were tapered according to disease amelioration. Two patients discontinued immunosuppressive drugs, and 1 patient had prednisone withdrawn 6 months after MSCT, with sustained disease remission. The treatment protocol for each patient before and after MSCT is shown in supplemental Table 2.
We investigated whether MSCT regulated the immune response in SS patients. We observed that MSCT completely abolished production of anti-SSA/Ro (Figure 5F) in serum, decreased from 84.76 ± 62.19 U/mL at baseline to 0.51 ± 0.22 U/mL 1 month after treatment (n = 7); and also resulted in more than 50% of inhibition of anti-SSB/La (Figure 5G) in serum, decreased from 146.62 ± 83.08 U/mL to 52.61 ± 38.67 U/mL (n = 6), further suggesting the important role of MSCs in suppressing Tfh differentiation and function. Together, these data demonstrated that UCMSC treatment substantially increased salivary flow rate, ameliorated disease symptoms, and inhibited the inflammatory responses, and allogeneic MSC treatment is a novel, effective, and safe therapy for the patients with SS.
Discussion
In the present study, we revealed, for the first time, that immunoregulatory functions and biologic properties of MSCs in SS patients are significantly impaired. We found that treatment of allogeneic MSCs prevented and suppressed experimental SS-like diseases in NOD/Ltj mice. More importantly, we showed a novel therapeutic approach to alleviate diseases in patients with primary SS by infusing allogeneic UCMSCs. We demonstrated that the therapeutic effects of MSC treatment were attributed to their immunoregulatory activities in driving CD4 T cells favoring Treg and Th2 development while inhibiting Th17 and Tfh inflammatory responses. Notably, we also discovered a critical role of the SDF-1/CXCR4 axis in directing MSC trafficking toward inflammation sites, to exert suppressive activities and improve salivary gland function.
MSCs have been successfully used to treat a variety of human diseases for their wide ranging differentiation potential, possibility of engraftment, and immunoregulatory effects. MSCs are reported to modulate immune responses through multiple mechanisms, including prostaglandin E2, nitric oxide, indoleamine 2,3-dioxygenase, and Toll-like receptor signaling pathways.14 Exposure to IFN-γ did not ablate MSC inhibited T-cell proliferation but rather antagonized TGF-β suppressed allo-responsiveness.39 MSCs induce Treg cells, thereby interfering with the immune response to alloantigens.40 For local immunity, inflammation after tissue damage can recruit MSCs into the inflamed sites where they participate in tissue regeneration and modulation of immune response. SDF-1/CXCR4 interaction played a critical role in directing hematopoietic cells and MSC trafficking in vivo. In our study, we observed that GFP MSCs distributed most in the kidney and lung, followed by spleen and liver at day 1 after infusion both in NOD/Ltj and ICR mice, suggesting that GFP MSCs at 1 day after infusion was not only affected by SDF-1 concentration but also by blood supply and histologic situation. Then distributions in kidney, spleen, and lung both in NOD/Ltj and ICR at 1 week after infusion were decreased, but GFP cells in salivary gland of NOD/Ltj remained at a high level and corresponded well with the SDF-1 expression level, further demonstrating the critical role of SDF-1 in directing MSCs migrating toward inflammatory salivary glands to exert their immunoregulatory functions. This notion was supported by our data that blockade of CXCR4 in BMMSCs with specific antibody abrogated the immunoregulatory activity and consequently the therapeutic effects of normal BMMSCs. Moreover, we found the similar higher level of Treg cells in submandibular lymph nodes after MSC treatment with or without spleen, and allogeneic MSCs were similarly effective in inhibiting disease development in splenectomized NOD/Ltj mice, indicating that Treg cells in spleen were not required for immune regulation and the therapeutic effects MSCs probably occurred locally.
Infusion of MSCs from bone marrow is considered safe and has been widely tested in clinical trials of cardiovascular, neurologic, and immunologic disease with encouraging results.20,41,42 Compared with BMMSCs, UCMSCs have significant advantages as a cellular source for MSC-mediated therapy: (1) collection from the umbilical cord is easy and noninvasive for the donor43 ; (2) umbilical cords can be stored in advance, whereas bone marrows specimens have to be collected from the donor immediately before infusion; (3) MSCs from the umbilical cord are more primary than MSCs isolated from some other tissue sources44 ; and (4) UCMSCs have greater proliferative potential and possess therapeutic effects in experimental and human autoimmune diseases.21,45 Clinically, treatment for hypofunction of salivary gland in primary SS remained a challenge, long-term use of sialagogue can only alleviate xerostomia and usually be with adverse effects. Here we showed that treatment of UCMSCs in 24 patients with primary SS resulted in considerable improvements in disease activity and organ function, demonstrating UCMSCs as a reliable cellular source of MSCs and their infusion as a novel therapeutic approach for SS.
In conclusion, our study showed, for the first time, that allogeneic MSC infusion is an effective treatment for SS and has important implication for further exploration of MSCs as a novel therapy for patients with SS and other autoimmune diseases. Our data demonstrate that SDF-1-mediated MSC migration plays a key role in their immunoregulatory functions and controlling SS and other autoimmune diseases pathogenesis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Songtao Shi, Center for Craniofacial, Molecular Biology, Ostrow School of Dentistry, University of Southern California for his insights and thoughtful input.
This work was supported by the National Nature Science Foundation of China (Key project grant 30430690, S.W.), the National Basic Research Program of China (2007CB947304 and 2010CB944801, S.W.), the Funding Project for Academic Human Resources Development in Institutions of Higher Learning Under the Jurisdiction of Beijing Municipality (PHR20090510, S.W.), the Funding Project to Science Facility in Institutions of Higher Learning Under the jurisdiction of Beijing Municipality (PXM 2009-014226-074691, S.W.; PXM2011-014226-07-000066, Z.F.), Major International (Regional) Joint Research Project (81120108021, L.S.), the National Nature Science Foundation of China (30972736, L.S.), Jiangsu Province Natural Science Foundation (BK2009034, L.S.), Jiangsu Province Kejiao Xingwei Program (L.S.), and the Intramural Research Program of National Institutes of Health, National Institute of Dental and Craniofacial Research (W.C.).
National Institutes of Health
Authorship
Contribution: S.W. conceived and designed the study and obtained funding; J.X. and D.W. acquired the data; J.X. performed the statistical analysis; S.W., L.S., Y.D., J.S.B., and W.C. supervised the study; Z.F., H.Z., and C.Z. provided administrative, technical, and material support; S.W., L.S., J.X., and Y.D. drafted the manuscript; and all authors analyzed and interpreted the data, critically revised the report for intellectual content, and provided final approval of the submitted version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Songlin Wang, Capital Medical University School of Stomatology, Tian Tan Xi Li No. 4, Beijing 100050, China; e-mail: slwang@ccmu.edu.cn.
References
Author notes
J.X. and D.W. contributed equally to this study.