Abstract
Dendritic cells (DCs) support only low levels of HIV-1 replication, but have been shown to transfer infectious viral particles highly efficiently to neighboring permissive CD4 T lymphocytes. This mode of cell-to-cell HIV-1 spread may be a predominant mode of infection and dissemination. In the present study, we analyzed the kinetics of fusion, replication, and the ability of HIV-1–specific Abs to inhibit HIV-1 transfer from immature DCs to autologous CD4 T lymphocytes. We found that neutralizing mAbs prevented HIV-1 transfer to CD4 T lymphocytes in trans and in cis, whereas nonneutralizing Abs did not. Neutralizing Abs also significantly decreased HIV-1 replication in DCs, even when added 2 hours after HIV-1 infection. Interestingly, a similar inhibition of HIV-1 replication in DCs was detected with some nonneutralizing Abs and was correlated with DC maturation. We suggest that the binding of HIV-1-specific Abs to FcγRs leads to HIV-1 inhibition in DCs by triggering DC maturation. This efficient inhibition of HIV-1 transfer by Abs highlights the importance of inducing HIV-specific Abs by vaccination directly at the mucosal portal of HIV-1 entry to prevent early dissemination after sexual transmission.
Introduction
Transmission via the sexual mucosa is the main route of HIV-1 infection worldwide.1 HIV-1 infection generally begins in the genital mucosal tissues, but the cellular mechanisms leading to HIV-1 infection remain unclear. The mucosal tissues contain various types of immune cells, including dendritic cells (DCs), which are among the cells initially targeted by HIV-1.2,3 The DCs lining the genital mucosa are APCs initiating a potent immune response.2,4 HIV-1 subverts Ag processing in DCs, resulting in viral uptake, infection, and transfer.2
Several studies have shown that different types of DCs are infected by HIV-1 in vivo, in the SIV-infected macaque model,5,6 or ex vivo in vaginal epithelial explants.3 In vitro, immature DCs replicate CCR5 (R5)–tropic viruses, albeit less efficiently than primary CD4 T lymphocytes.7-9 Furthermore, interactions between DCs and HIV-1 have highlighted their important role in HIV-1 transmission. The efficient transfer of HIV-1 from DCs to CD4 T lymphocytes, first described in 1992,10 may largely contribute to HIV-1 propagation and dissemination through the body,10-16 but the precise mechanisms leading to HIV-1 transfer remain unclear.17,18 DCs bind native free viral particles, but viruses are rarely colocalized with endolysosomal markers, suggesting that viral infection may alter endolysosomal trafficking.19
Two modes of HIV-1 transfer have been well described: trans-infection and cis-infection.19,20 Infectious viral particles in the intracellular compartments of DCs can be rapidly redirected to virological synapses formed at the site of contact between infected DCs and CD4 T lymphocytes.11,19-22 This trans-infection may also be mediated by DC-derived exosomes released from immature monocyte-derived dendritic cells (MoDCs).23 In addition, HIV-1 bound to C-type lectins, such as DC-SIGN, on the surface of immature DCs can be efficiently captured and transferred directly to CD4 T lymphocytes during cell-to-cell contact.12,17,24,25 This HIV-1 transfer, also referred to as trans-infection, occurs in the absence of viral replication in DCs.12 This trans-infection of CD4 T lymphocytes is transient, because HIV-1 transfer decays within 24 hours19 after the addition of CD4 T lymphocytes to infected DCs.26 A second transfer phase is then detected in immature DCs after 48 hours as an azidothymidine (AZT)–sensitive increase in proviral DNA levels.19 This second type of transfer, in cis, occurs over a longer period, after conventional HIV-1 fusion and de novo virus production in immature DCs.19 The mechanism of viral transfer from DCs to CD4 T lymphocytes can therefore be addressed as a function of time: the addition of CD4 T lymphocytes to DCs just after their exposure to HIV-1 may allow the analysis of HIV-1 transfer in trans, whereas the addition of CD4 T lymphocytes 1 day after DC infection is likely to promote transfer in cis.
It has been suggested that the transfer of viral particles from MoDCs to CD4 T lymphocytes is resistant to neutralizing Abs (NAbs).8,25 The investigators suggested that the virological synapse between infected DCs and CD4 T lymphocytes impeded the binding of NAbs to the transferred virus.8 In contrast, other studies have reported HIV-1 transfer to be sensitive to NAbs.18,27,28 We previously showed that HIV-1–exposed immature MoDCs produce large numbers of new viral particles when cocultured with primary CD4 T lymphocytes.26 The HIV-1 particles produced by DCs and released into the supernatant may interfere with the Ab-mediated inhibition of HIV-1 transfer to T cells, because Abs can inhibit HIV-1 transfer to T cells but not HIV-1 production in DCs. This would result in the release of HIV-1 into the supernatant despite the efficient inhibition of HIV-1 transfer to CD4 T lymphocytes. We therefore analyzed the intracellular p24 of HIV-1 produced in DCs and in CD4 T lymphocytes directly, rather than assessing HIV-1 release into the supernatant, to make it possible to distinguish between the virus produced by DCs and that transferred to CD4 T lymphocytes. We found that the addition of NAbs to HIV-1–loaded DCs at the same time as CD4 T lymphocytes strongly inhibited the trans- and cis-infection of primary CD4 T lymphocytes with HIV-1, whereas nonneutralizing Abs (NNAbs) had no inhibitory activity. Surprisingly, both NAbs and some NNAbs decreased the percentage of infected MoDCs in the coculture. This decrease was correlated with the level of DC maturation.
Methods
Abs
Mouse anti–human CD3-VioBlue (BW264/56) and CD83-APC (HB15) mAbs were purchased from Miltenyi Biotec. PerCP-Cy5.5–conjugated mouse mAb against human CD209 (DC-SIGN, DCN46) was purchased from BD Pharmingen. HIV-1 Ag p24 KC57-FITC (FH190-1-1) and goat F(ab′)2 fragment anti–human IgG (Fcγ)-PE Abs were purchased from Beckman-Coulter. The IgG1 2G12 (directed against gp120), 2F5, and 4E10 (against gp41) NAbs were obtained from Polymun Scientific. For monoclonal NAbs directed against the CD4-binding site, the IgG1 b12 was kindly provided by D. Burton (The Scripps Research Institute, La Jolla, CA) and the VRC01 and VRC03 by J. R. Mascola (National Institutes of Health [NIH], Bethesda, MD). Inhibitory mAbs IgG1 246-D, 4B3, and 5F3 (against gp41) were kindly provided by S. Zolla-Pazner (New York University School of Medicine, NY) and Polymun, respectively. The NNAbs IgG1 1570-D (NIH 1172 against the CD4 binding site) was obtained from the NIH (Bethesda, MD). Monoclonal anti-dengue Ab (DEN-3) was obtained from BEI Resources (National Institute of Allergy and Infectious Diseases [NIAID], NIH). Endotoxin levels in the anti–HIV-1–specific Abs preparations were determined using a commercial limulus amebocyte lysate chromogenic endotoxin quantitation kit (Pierce/Thermo Scientific).
Cell preparation
All human blood samples were collected from donors seronegative for HIV-1 and HCV (EFS). Immature MoDCs were obtained by the differentiation of purified human blood CD14+ monocytes with immunomagnetic bead isolation after Ficoll-Hypaque sedimentation (AutoMACS; Miltenyi Biotec). As described previously,26,29,30 autologous CD4 T lymphocytes were purified by positive selection after CD14 purification. Primary purified CD4 T lymphocytes were activated by incubation with phytohemagglutinin-A (PHA; 1 μg per million cells, 1 mg/mL) for 3 days. Cells were frozen and thawed 1 day before use.26
Virus preparation
Primary HIV-1 isolates were amplified on human blood leukocytes, as described previously.29 Virus stocks collected at peak virus production were concentrated 70-fold with a 100-kDa cutoff polyethersulfone filter (Centricon Plus-70 Biomax Filter; Millipore). Primary R5 HIV-1BaL (subtype B) was provided by S. Gartner, M. Popovic, and R. Gallo (NIH). HIV-1TV1 (subtype C, R5 strain) was obtained from S. Engelbrecht (NHLS, University of Stellenbosch, Tygerberg, South Africa). HIV-1QH0 (subtype B, R5 strain) was obtained from the National Institute for Biologic Standards and Control (NIBSC).
HIV-1–transfer experiments
The conditions used for MoDC infection and coculture with PHA-activated autologous CD4 T lymphocytes were similar to those described previously.26 Briefly, immature MoDCs were infected with primary R5 HIV-1 at a concentration of 500 ng/mL of viral p24 Ag. After 2 hours of incubation, the cells were washed thoroughly to remove unbound free viral particles. Next, 25 μL of HIV-1–loaded MoDCs (at 6 × 106 cells/mL) were incubated with 25 μL of PHA-activated CD4 T lymphocytes at 24 × 106 cells/mL and 25 μL of human mAbs (in serial 2-fold dilutions beginning from a concentration of 100 μg/mL, except for VRC01 and VRC03, for which the starting concentration was 50 μg/mL) in 96-well plates. Where indicated, 5μM AZT (Sigma-Aldrich), a reverse transcriptase inhibitor, was added at the same time as the CD4 T lymphocytes, to prevent HIV-1 replication. The detection of viral p24 Ag in AZT-treated wells was considered to represent residual p24 detected in the absence of de novo virus synthesis. Unless otherwise stated, productive infection was quantified by flow cytometry based on the detection of intracellular viral p24 Ag in both cell populations after 48 hours of culture.
Kinetics of HIV-1 fusion, replication, and detection of a single cycle of HIV-1 replication in cocultured MoDCs and CD4 T lymphocytes
For studies of the kinetics of HIV-1 fusion and replication in MoDCs and autologous PHA-activated CD4 T lymphocytes, we added 5 μg/mL of Fuzeon (T20, an HIV-1 fusion inhibitor; AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH) or 5μM HIV-1 AZT at various times after infection. We added the HIV-1 protease inhibitor indinavir (IDV; AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH) at a concentration of 1μM31 to MoDCs at the same time as CD4 T lymphocytes for analysis of a single cycle of HIV-1 replication.
Ab neutralizing activity on purified CD4 T lymphocytes
The neutralizing activity of Abs was assessed as described previously.32 Briefly, we incubated 25 μL of primary HIV-1BaL isolate (concentration of 500 ng/mL of viral p24 Ag to give > 2% infected cells) for 1 hour with 25 μL of human mAbs. We then added 25 μL of PHA-stimulated CD4 T lymphocytes at 15 × 106 cells/mL. After 48 hours, the percentage of infected CD4 T lymphocytes was determined by flow cytometric analysis of intracellular p24 viral Ag.
Cell staining and flow cytometry assay
Briefly, cells were labeled with Live/Dead Fixable Dead Cell Stain fluorescence kits (Invitrogen/Applied Biosystems) for 10 minutes at room temperature. After washing, a mixture of Abs directed against the human CD209, CD83, and CD3 cell-surface molecules was incubated for 10 minutes at 4°C. Cells were fixed and permeabilized with the Cytofix/Cytoperm wash kit solutions (BD Biosciences) and subjected to intracellular p24 Ag staining. The percentages of infected DC-SIGN+ (CD209+) CD3− MoDCs and infected CD3+ DC-SIGN− CD4 T lymphocytes were determined by flow cytometry. Multicolor samples were acquired with an LSRII SORP cytometer (BD Biosciences). We used cytometer setup and tracking calibration particles to ensure that the fluorescence intensity measurement was consistent in all experiments. We used a flow cytometry CompBeads kit (BD Biosciences) for compensation. Forward-angle and side-scatter light gating were used to exclude cell debris from the analysis. Forward width and forward area were used to exclude doublet cells, and dead cells were excluded with the Live/Dead Kit solution. The final analysis was performed with FACSDiva Version 6.1.2 software (BD Biosciences), which generated a graphical output.
Ab-binding detection assay
Cells were incubated with various anti–HIV-1–specific Abs (at a concentration of 100 μg/mL, except for VRC01 and VRC03, used at a concentration of 50 μg/mL) for 10 minutes at 4°C, washed with PBS, and incubated for 10 minutes at 4°C with goat F(ab′)2 fragment anti–human IgG (Fcγ)–PE (Beckman-Coulter). Cells were washed and fixed in Cytofix solution. We determined the percentage of cells positive for Ab binding by flow cytometry (LSRII SORP). In parallel, we blocked FcγRs on the surface of MoDCs by incubating MoDCs with a mixture of purified anti-FcγRI (CD64, clone 10.1), anti-FcγRII (CD32, clone 3D3), and anti-FcγRIII (CD16, clone 3G8) Abs (BD Pharmingen) at 10 μg/mL for 30 minutes at 4°C before adding Abs specific for HIV-1. The percentage of Ab binding via FcγRs on MoDCs was defined as the percentage of Ab binding to MoDCs minus the percentage of Ab binding to MoDCs treated with anti-FcγR Abs.
ELISA
We determined the amount of HIV-1 p24 Ag released into the supernatant of cocultured cell populations by ELISA (Innogenetics/Ingen).
Statistical analysis
Groups were compared by 1-way ANOVA (Kruskal-Wallis test), with t tests used for pairwise comparisons (Mann-Whitney test). P < .05 was considered statistically significant. Correlations were analyzed by calculating the Pearson correlation coefficient, with P < .05 considered statistically significant. All statistical calculations were performed with Prism Version 5.04 software (GraphPad).
Results
Productive HIV-1 infection of MoDCs and CD4 T lymphocytes
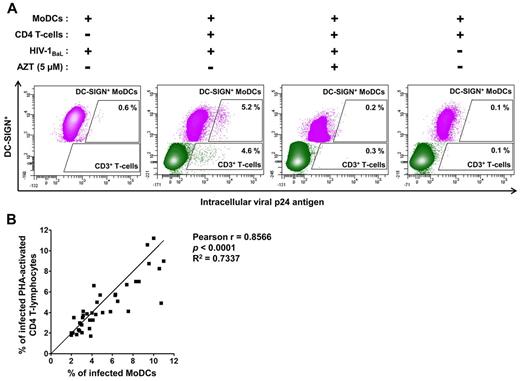
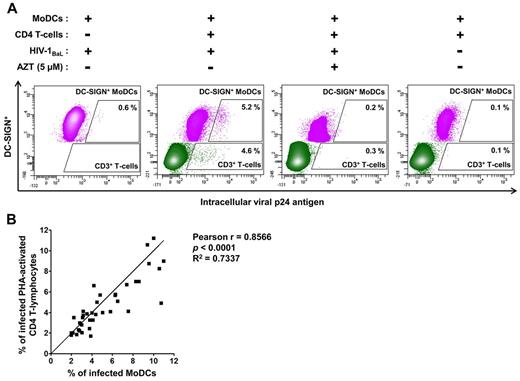
We selected HIV-1–transfer conditions suitable for assessment of the early stage of HIV-1 transfer in trans from MoDCs to CD4 T lymphocytes (Figure 1): PHA-activated CD4 T lymphocytes and anti–HIV-1–specific Abs were added to immature MoDCs incubated for 2 hours with HIV-1. We determined HIV-1 replication after 48 hours of infection to minimize multiple cycles of reverse transcription leading to transfer in cis (Figure 1). At this time point, HIV-1 transfer from MoDCs to PHA-activated CD4 T lymphocytes was efficient (4.6% of CD3+ T lymphocytes were p24+; Figure 2A). In addition, infected immature MoDCs were detected, because 5.2% of these cells were p24+ (Figure 2A). This infection corresponded to newly synthesized virions, because the low percentages of p24+ cells were detected in the presence of the reverse transcriptase inhibitor AZT (Figure 2A) and were similar to uninfected MoDCs cocultured with CD4 T lymphocytes (Figure 2A). In the absence of CD4 T lymphocytes, only a few (0.6%) immature DC-SIGN+ MoDCs displayed HIV-1 replication after 48 hours (Figure 2A).
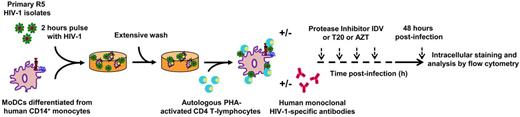
Schematic diagram of the HIV-1–transfer assay. Immature MoDCs were pulsed with primary R5 HIV-1 isolates for 2 hours, thoroughly washed, and added to uninfected primary autologous PHA-activated CD4 T lymphocytes. The percentage of HIV-1–infected cells in each cell population was determined on the basis of intracellular viral p24 Ag detection by flow cytometry.
Schematic diagram of the HIV-1–transfer assay. Immature MoDCs were pulsed with primary R5 HIV-1 isolates for 2 hours, thoroughly washed, and added to uninfected primary autologous PHA-activated CD4 T lymphocytes. The percentage of HIV-1–infected cells in each cell population was determined on the basis of intracellular viral p24 Ag detection by flow cytometry.
Detection of intracellular HIV-1 p24 Ag production in immature MoDCs and primary autologous PHA-activated CD4 T lymphocytes. (A) Dot-plot representations of MoDCs (in pink), infected with primary HIV-1 or uninfected, and CD4 T lymphocytes (in green) in the coculture. We differentiated between MoDCs and CD4 T lymphocytes on flow cytometry by analyzing the expression of DC-SIGN+ (CD209+) and CD3+, respectively. The HIV-1 reverse transcriptase inhibitor AZT was added to the coculture at the same time as CD4 T lymphocytes as a negative control for HIV-1 replication. Experiments were performed in duplicate and the mean percentages of intracellular p24+ MoDCs or CD4 T lymphocytes are shown in the dot plot. (B) Curve for the correlation between the mean values of the percentage of infected MoDCs and infected primary PHA-activated CD4 T lymphocytes in coculture conditions. The Pearson correlation coefficient and its significance are shown. n = 38 experiments performed with cells from different healthy blood donors for panels A and B.
Detection of intracellular HIV-1 p24 Ag production in immature MoDCs and primary autologous PHA-activated CD4 T lymphocytes. (A) Dot-plot representations of MoDCs (in pink), infected with primary HIV-1 or uninfected, and CD4 T lymphocytes (in green) in the coculture. We differentiated between MoDCs and CD4 T lymphocytes on flow cytometry by analyzing the expression of DC-SIGN+ (CD209+) and CD3+, respectively. The HIV-1 reverse transcriptase inhibitor AZT was added to the coculture at the same time as CD4 T lymphocytes as a negative control for HIV-1 replication. Experiments were performed in duplicate and the mean percentages of intracellular p24+ MoDCs or CD4 T lymphocytes are shown in the dot plot. (B) Curve for the correlation between the mean values of the percentage of infected MoDCs and infected primary PHA-activated CD4 T lymphocytes in coculture conditions. The Pearson correlation coefficient and its significance are shown. n = 38 experiments performed with cells from different healthy blood donors for panels A and B.
Efficient HIV-1 transfer from MoDCs to CD4 T lymphocytes was observed repeatedly (n = 38). The percentage of HIV-1 replication in CD4 T lymphocytes was correlated with that in MoDCs (Figure 2B), suggesting a high degree of cooperation between MoDCs and CD4 T lymphocytes to promote HIV-1 replication in these 2 cell types.
Kinetics of HIV-1 fusion and replication
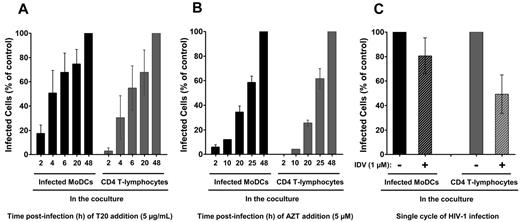
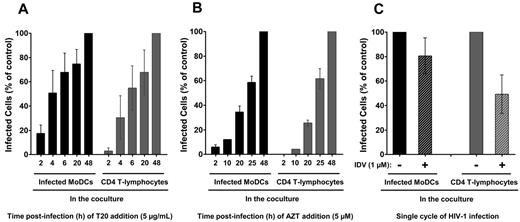
We analyzed the kinetics of HIV-1 membrane fusion in coculture conditions by adding a fusion inhibitor (T20) at various time points after infection (Figure 3A). When T20 was added to HIV-1–loaded MoDCs at the same time as CD4 T lymphocytes, approximately 20% of the MoDCs were found to be positive for intracellular p24 Ag 48 hours later compared with control MoDCs in the absence of T20. Therefore, approximately 80% of the HIV-1 particles had yet to fuse with immature MoDCs after 2 hours of incubation (Figure 3A) and were still accessible to HIV-1–specific Abs. Moreover, at this time point, T20 almost completely inhibited HIV-1 transfer to primary CD4 T lymphocytes, demonstrating the requirement for fusion in HIV-1 transfer to CD4 T lymphocytes, as described previously (Figure 3A).33,34
Kinetics of HIV-1 fusion and replication in cocultured MoDCs and primary autologous PHA-activated CD4 T lymphocytes. We added 5 μg/mL T20 (HIV-1 fusion inhibitor; A) or 5μM AZT (reverse transcriptase inhibitor; B) to HIV-1–loaded MoDCs/CD4 T lymphocytes cocultures at various times. Infection was assessed 48 hours after infection with HIV-1BaL and the percentage of infection compared with control (without T20 or AZT) was calculated. Data are expressed as means ± SD for n = 6 (A) or n = 4 (B) independent experiments performed with cells from different healthy blood donors. (C) Percentages of infection in each cocultured cell population in the presence of the HIV-1 protease inhibitor IDV compared with control cells (in the absence of IDV). Data are expressed as the means ± SD for n = 14 independent experiments performed with cells from different healthy blood donors.
Kinetics of HIV-1 fusion and replication in cocultured MoDCs and primary autologous PHA-activated CD4 T lymphocytes. We added 5 μg/mL T20 (HIV-1 fusion inhibitor; A) or 5μM AZT (reverse transcriptase inhibitor; B) to HIV-1–loaded MoDCs/CD4 T lymphocytes cocultures at various times. Infection was assessed 48 hours after infection with HIV-1BaL and the percentage of infection compared with control (without T20 or AZT) was calculated. Data are expressed as means ± SD for n = 6 (A) or n = 4 (B) independent experiments performed with cells from different healthy blood donors. (C) Percentages of infection in each cocultured cell population in the presence of the HIV-1 protease inhibitor IDV compared with control cells (in the absence of IDV). Data are expressed as the means ± SD for n = 14 independent experiments performed with cells from different healthy blood donors.
We also investigated the kinetics of HIV-1 reverse transcription by adding the reverse transcriptase inhibitor AZT at various time points after infection. The reverse transcription profiles of MoDCs and CD4 T lymphocytes were similar in coculture conditions (Figure 3B). Indeed, 40% of MoDCs and 30% of CD4 T lymphocytes were already infected when AZT was added 20 hours after infection compared with control cells in the absence of AZT. The reverse transcription in MoDCs alone (in the absence of CD4 T lymphocytes) began after 48 hours and the first round of viral replication was not detected until 72 hours (data not shown).26,29 Therefore, an increased rate of reverse transcription was observed in MoDCs when cocultured with CD4 T lymphocytes. This increase of reverse transcription rate may account for the higher percentage of HIV-1 p24+ MoDCs observed when they were cocultured with CD4 T lymphocytes.
Single cycle of HIV-1 infection in MoDCs and CD4 T lymphocytes in coculture
We investigated whether the HIV-1 replication in MoDCs and CD4 T lymphocytes corresponded to a single cycle of HIV-1 infection after 48 hours of incubation by adding a protease inhibitor, IDV. IDV prevent new cycles of infection by blocking final assembly and maturation of newly synthesized virions. In the presence of IDV, infected MoDCs correspond to 80% of control infected MoDCs (in the absence of IDV; Figure 3C). The HIV-1 replication detected in these cells after 48 hours was therefore due essentially to a single cycle of infection. Conversely, approximately half of the lymphocyte population was found to be positive for intracellular p24 Ag production in the presence of IDV (Figure 3C), indicating the occurrence of a new cycle of HIV-1 replication in CD4 T lymphocytes. Therefore, approximately half of the HIV-1 replication detected in these lymphocytes corresponds to HIV-1 transfer in trans, the other half corresponding to HIV-1 transfer in cis. However, further studies are required to determine whether these new infectious particles were produced by MoDCs or CD4 T lymphocytes. Coculture for 24 hours was not sufficient for the analysis of p24 in HIV-1–infected MoDCs and CD4 T lymphocytes by flow cytometry (data not shown). We therefore chose to use an incubation period of 48 hours, corresponding mainly to transfer in trans from DCs to CD4 T lymphocytes, for analysis of the inhibition of HIV-1 transfer by Abs.
Ab-mediated inhibition of HIV-1 transfer
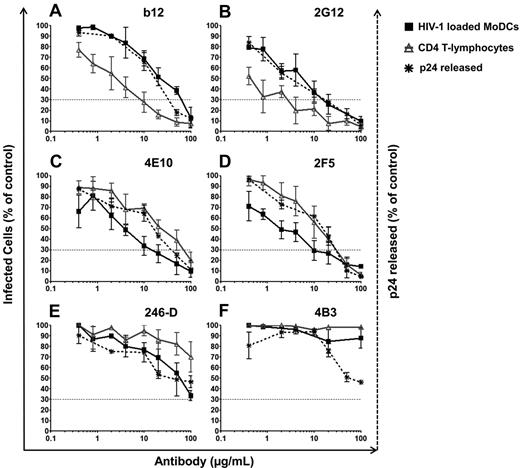
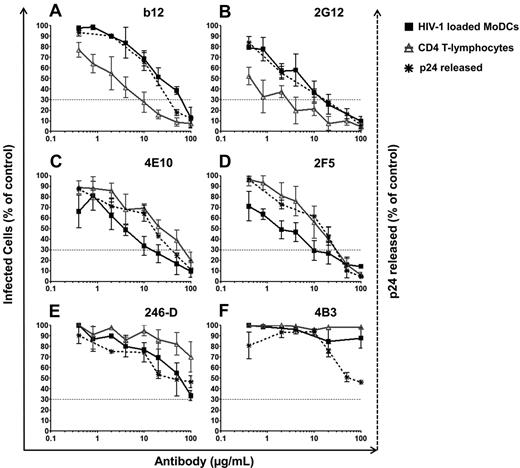
We analyzed the inhibition of HIV-1 transfer from immature DCs to CD4 T lymphocytes by Abs using a panel of specific anti–HIV-1 Abs at various concentrations, and the concentration of Ab resulting in a 70% decrease in the percentage of infected cells (inhibitory concentration 70, IC70) values were calculated (Figure 4 and Table 1). Indeed, because 20% of HIV-1 had already fused with MoDCs before the addition of Abs (Figure 3A), IC90 would have been too stringent. In addition, IC50 was not very reproducible because of the intrinsic variability among primary HIV-1 isolates infecting primary human cells. We found that the 6 NAbs (anti-gp120: 2G12, b12, VRC01, and VRC03 and anti-gp41: 2F5 and 4E10) effectively prevented HIV-1 transfer from MoDCs to PHA-activated CD4 T lymphocytes (Figure 4A-D and Table 1). These NAbs had IC70 values between 4 and 47 μg/mL for HIV-1 transfer to CD4 T lymphocytes (Table 1), whereas the NNAbs 5F3 and 1570-D and the NNAbs Fcγ-mediated inhibitory Abs 246-D and 4B3 did not prevent HIV-1 transfer to CD4 T lymphocytes (Figure 4E-F and Table 1). We further analyzed the inhibition by Abs of HIV-1 transfer in trans only by adding the protease inhibitor IDV to the coculture. Inhibition efficiency was similar in the presence and absence of IDV (supplemental Figure 1C-D, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), demonstrating the capacity of NAbs to inhibit trans-infection. Moreover, this inhibition was maintained over a period of 5 days, indicating a subsequent inhibition by Abs of transfer in cis (data not shown). This Ab-mediated inhibition of HIV-1 transfer was observed after the infection of MoDCs with subtype C HIV-1TV1 and subtype B HIV-1QH0 viruses in addition to HIV-1BaL (data not shown).
Inhibition of HIV-1 trans-infection by various HIV-1–specific Abs. HIV-1–specific Abs were added 2 hours after the infection of immature MoDCs at the same time as PHA-activated CD4 T lymphocytes. After 48 hours of culture, infection was determined by the detection of HIV-1 intracellular p24 Ag production by flow cytometry or by the detection of viral p24 Ag released into the supernatant by ELISA. Percentages of infected MoDCs (black squares) or CD4 T lymphocytes (gray triangles) after coculture in the presence of a panel of different anti–HIV-1–specific Abs: anti-gp120 NAbs b12 (A) and 2G12 (B); anti-gp41 NAbs 4E10 (C) and 2F5 (D); and NNAbs 246-D (E) and 4B3 (F). The percentage of viral p24 released into the supernatant of the coculture is indicated by black dots and asterisks. Dashed lines correspond to the IC70 concentrations of Abs. The curve corresponds to the means ± SD of at least 4 independent experiments performed with cells from 4 different healthy blood donors.
Inhibition of HIV-1 trans-infection by various HIV-1–specific Abs. HIV-1–specific Abs were added 2 hours after the infection of immature MoDCs at the same time as PHA-activated CD4 T lymphocytes. After 48 hours of culture, infection was determined by the detection of HIV-1 intracellular p24 Ag production by flow cytometry or by the detection of viral p24 Ag released into the supernatant by ELISA. Percentages of infected MoDCs (black squares) or CD4 T lymphocytes (gray triangles) after coculture in the presence of a panel of different anti–HIV-1–specific Abs: anti-gp120 NAbs b12 (A) and 2G12 (B); anti-gp41 NAbs 4E10 (C) and 2F5 (D); and NNAbs 246-D (E) and 4B3 (F). The percentage of viral p24 released into the supernatant of the coculture is indicated by black dots and asterisks. Dashed lines correspond to the IC70 concentrations of Abs. The curve corresponds to the means ± SD of at least 4 independent experiments performed with cells from 4 different healthy blood donors.
In addition to inhibiting HIV-1 transfer, NAbs also inhibited MoDC infection in these coculture conditions despite the loading of MoDCs with HIV-1 2 hours before the addition of Abs (Figure 4A-D, supplemental Figure 1A-B, and Table 1). Moreover, slightly lower levels of HIV-1 replication in MoDCs were detected in the presence of NNAb 246-D (Figure 4E and Table 1), although no inhibition of HIV-1 transfer to CD4 T lymphocytes was observed. For NAbs directed against gp120, the inhibition of HIV-1 transfer from MoDCs to CD4 T lymphocytes was more efficient than the inhibition of HIV-1 replication in MoDCs (Figure 4A-B, supplemental Figure 1A,C, and Table 1). In contrast, for the anti-gp41 NAbs and 2 NNAbs (246-D and 4B3), HIV-1 replication in MoDCs was inhibited more strongly than HIV-1 transfer to CD4 T lymphocytes (Figure 4C-F, supplemental Figure 1B,D, and Table 1). These differences were observed reproducibly in at least 4 independent experiments with cells from different healthy blood donors, suggesting that Ab function may depend on the glycoprotein subunit targeted. Indeed, the NAbs 4E10 and 2F5 have been shown to be active even after CD4/CCR5 engagement,35 whereas anti-gp120 NAbs epitopes may have limited access to HIV already bound to MoDCs.
We also analyzed viral release into the supernatant. Similar curves were obtained for the inhibition of HIV-1 transfer and the inhibition of p24 release into the supernatant, thus confirming that overall productive infection was inhibited by NAbs (Figure 4A-D). The amount of viral p24 Ag released into the supernatant was also approximately 50% lower in the presence of the NNAbs 246-D and 4B3 (Figure 4E-F). Our results show that NAbs both efficiently inhibit HIV-1 transfer from MoDCs to CD4 T lymphocytes in trans and in cis and inhibit the infection of MoDCs.
IgG-mediated inhibition of HIV-1 replication in MoDCs is correlated with the maturation status of these cells
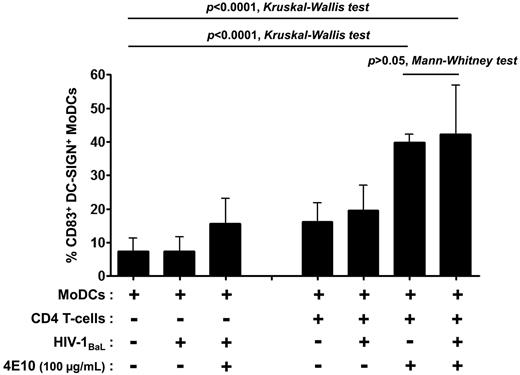
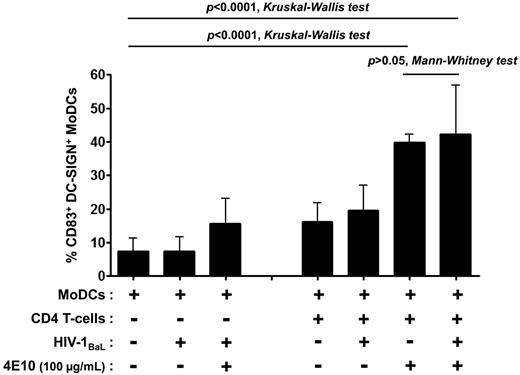
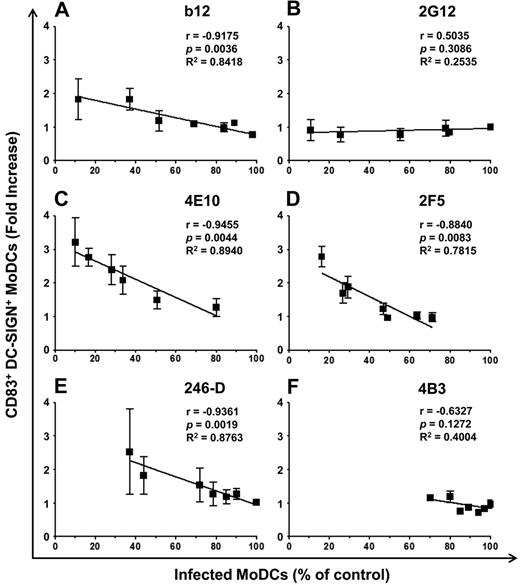
DC maturation decreases susceptibility to HIV-1 R5 infection.36 We therefore assessed the capacity of Abs to induce MoDC maturation. The expression of the DC maturation marker CD83 on the surface of infected MoDCs was determined in the coculture with various concentrations of Abs and compared with MoDC maturation in the absence of Abs (Figure 5A and Table 1). The anti-gp41 NAbs 4E10 and 2F5 induced a high percentage of mature MoDCs (50% and 42%, respectively, Figure 5A and Table 1). DC maturation was slightly lower in the presence of b12 and no CD83 expression was detected with 2G12, even when present at a concentration of 100 μg/mL (Table 1). The NNAbs 246-D and 5F3 induced MoDC maturation, whereas 4B3 and 1570-D did not increase the level of CD83 expression on DCs (Table 1). This maturation could not be because of the presence of endotoxins because endotoxin levels were found to be very low (between 0.0003 and 0.196 EU) at 100 μg/mL of Abs, the highest concentration used. In addition, DC maturation was inversely correlated with the relative percentage of infected MoDCs (Figure 6). MoDC infection did not induce MoDC maturation in the absence of CD4 T lymphocytes, and the 4E10 Ab alone had minimal effects on the maturation process (Figure 7). Efficient MoDC maturation was detected in the context of coculture with CD4 T lymphocytes in the presence of the 4E10 Ab independently of viral replication (P > .05 by t tests; Figure 7). No MoDC maturation was observed after incubation with purified polyclonal IgG from HIV-infected or HIV-negative subjects or with anti-dengue mAb (DEN-3; data not shown). Therefore, the MoDC maturation observed in the context of coculture was Ab dependent.
Induction of MoDC maturation by the anti–HIV-1 neutralizing mAb 4E10 in various culture conditions. The percentage of CD83+ DC-SIGN+ MoDCs was determined after 48 hours of culture in the presence or absence of virus, CD4 T lymphocytes, or Abs. Data are the means ± SD of at least 6 experiments performed with cells from different healthy blood donors. Groups were compared by 1-way ANOVA (Kruskal-Wallis test), and pairwise comparisons were based on t tests (Mann-Whitney test), with P < .05 considered significant.
Induction of MoDC maturation by the anti–HIV-1 neutralizing mAb 4E10 in various culture conditions. The percentage of CD83+ DC-SIGN+ MoDCs was determined after 48 hours of culture in the presence or absence of virus, CD4 T lymphocytes, or Abs. Data are the means ± SD of at least 6 experiments performed with cells from different healthy blood donors. Groups were compared by 1-way ANOVA (Kruskal-Wallis test), and pairwise comparisons were based on t tests (Mann-Whitney test), with P < .05 considered significant.
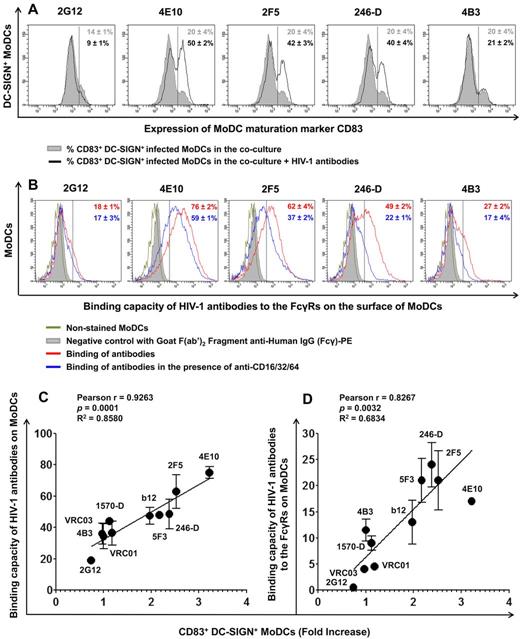
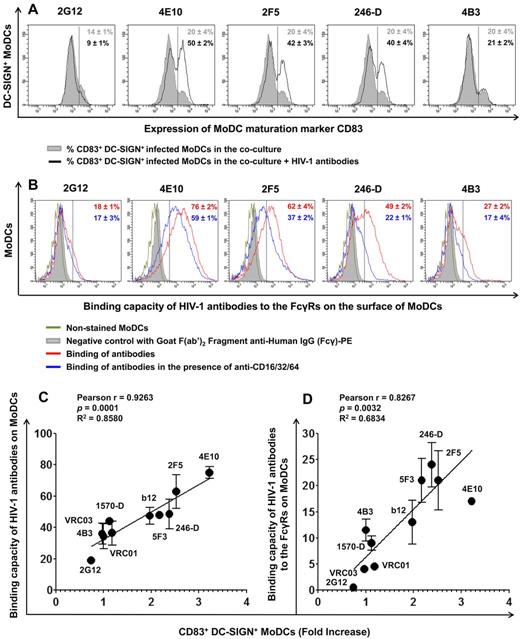
MoDC maturation, capacity of HIV-1 Abs to bind MoDCs, and their correlation. (A) The percentage of CD83+ DC-SIGN+ MoDCs in the coculture was determined in the presence or absence of HIV-1–specific Abs (100 μg/mL) after 48 hours of infection. All experiments were performed at least 5 times with cells from different healthy blood donors. Representative data are shown and are expressed as means ± SD. (B) The capacity of various anti–HIV-1 Abs to bind MoDCs was determined (red curve). FcγRs were blocked by adding 10 μg/mL of anti-FcγRI (CD64), anti-FcγRII (CD32), and anti-FcγRIII (CD16) to MoDCs for 30 minutes before adding the various anti–HIV-1 Abs (2G12, 4E10, 2F5, 246-D, and 4B3) at a concentration of 100 μg/mL (blue curve). The capacity of Abs to bind to FcγRs corresponds to the difference between the capacity of Abs to bind MoDCs (red value) and the capacity of Abs to bind MoDCs in the presence of anti-FcγRs Abs (blue value). Representative data are shown and are expressed as means ± SD. The correlation between the capacity of anti–HIV-1 Abs (at a concentration of 100 μg/mL, except for VRC01 and VRC03, which are 50 μg/mL) to bind to the surface of MoDCs (C) or to FcγRs (D) and MoDC maturation (fold increase in CD83+ DC-SIGN+ MoDCs) was analyzed. Data are the means ± SD of 2 independent experiments performed with cells from 2 healthy blood donors. Correlations were analyzed by calculating the Pearson correlation coefficient, with P < .05 considered significant.
MoDC maturation, capacity of HIV-1 Abs to bind MoDCs, and their correlation. (A) The percentage of CD83+ DC-SIGN+ MoDCs in the coculture was determined in the presence or absence of HIV-1–specific Abs (100 μg/mL) after 48 hours of infection. All experiments were performed at least 5 times with cells from different healthy blood donors. Representative data are shown and are expressed as means ± SD. (B) The capacity of various anti–HIV-1 Abs to bind MoDCs was determined (red curve). FcγRs were blocked by adding 10 μg/mL of anti-FcγRI (CD64), anti-FcγRII (CD32), and anti-FcγRIII (CD16) to MoDCs for 30 minutes before adding the various anti–HIV-1 Abs (2G12, 4E10, 2F5, 246-D, and 4B3) at a concentration of 100 μg/mL (blue curve). The capacity of Abs to bind to FcγRs corresponds to the difference between the capacity of Abs to bind MoDCs (red value) and the capacity of Abs to bind MoDCs in the presence of anti-FcγRs Abs (blue value). Representative data are shown and are expressed as means ± SD. The correlation between the capacity of anti–HIV-1 Abs (at a concentration of 100 μg/mL, except for VRC01 and VRC03, which are 50 μg/mL) to bind to the surface of MoDCs (C) or to FcγRs (D) and MoDC maturation (fold increase in CD83+ DC-SIGN+ MoDCs) was analyzed. Data are the means ± SD of 2 independent experiments performed with cells from 2 healthy blood donors. Correlations were analyzed by calculating the Pearson correlation coefficient, with P < .05 considered significant.
Correlation between the percentage of infected MoDCs and the percentage of mature MoDCs in the presence of HIV-1–specific Abs. Curve for the correlation between the percentage of infected MoDCs (with respect to control) and the fold increase in the percentage of CD83+ DC-SIGN+ MoDCs for each Ab concentration (100 μg/mL and serial 2-fold dilutions, as in Figure 4). Anti-gp120 NAbs b12 (A) and 2G12 (B); anti-gp41 NAbs 4E10 (C) and 2F5 (D); and NNAbs 246-D (E) and 4B3 (F). Data are the means ± SD of at least 5 independent experiments performed with cells from different healthy blood donors. Correlations were analyzed by calculating the Pearson correlation coefficient, with P < .05 considered significant.
Correlation between the percentage of infected MoDCs and the percentage of mature MoDCs in the presence of HIV-1–specific Abs. Curve for the correlation between the percentage of infected MoDCs (with respect to control) and the fold increase in the percentage of CD83+ DC-SIGN+ MoDCs for each Ab concentration (100 μg/mL and serial 2-fold dilutions, as in Figure 4). Anti-gp120 NAbs b12 (A) and 2G12 (B); anti-gp41 NAbs 4E10 (C) and 2F5 (D); and NNAbs 246-D (E) and 4B3 (F). Data are the means ± SD of at least 5 independent experiments performed with cells from different healthy blood donors. Correlations were analyzed by calculating the Pearson correlation coefficient, with P < .05 considered significant.
Binding of Abs to FcγRs on the surface of MoDCs
We demonstrated previously the involvement of Fcγ receptors (FcγRs) in the mechanism of HIV-1 inhibition by NAbs and NNAbs in MoDCs.29,30 In the present study, we investigated the mechanism of MoDC maturation by specific Abs against HIV-1 by analyzing the capacity of these Abs to bind to FcγRs present on the MoDC surface. NAbs 4E10 and 2F5 bound efficiently to the surface of MoDCs with approximately 76% and 62% Ab-positive cells, respectively. The NNAbs 246-D and 4B3 bound MoDCs, giving 49% and 27% positive cells, respectively. Ab 2G12 bound MoDCs only weakly, with approximately 18% positive MoDCs (Figure 5B). We investigated whether this binding was mediated by the Fc portions of these IgGs by blocking FcγRs with a mixture of Abs directed against FcγRI, FcγRII, and FcγRIII before adding specific Abs against HIV-1. We found that competition with anti-FcγRs Abs had no impact on NAb 2G12 binding to MoDCs, whereas the binding of the NNAbs 246-D and 4B3 to MoDCs decreased to 22% and 17%, respectively, after FcγRs blockade. The presence of anti-FcγR Abs also decreased the binding capacities of the NAbs 4E10 and 2F5 to MoDCs, by 17% and 25%, respectively, but 59% and 37% of MoDCs, respectively, remained positive despite the addition of anti-FcγRs Abs (Figure 5B). Therefore, the NAbs 4E10 and 2F5 bind FcγRs and other ligands on the surface of MoDCs. We then investigated the correlation between Ab binding and MoDC maturation. We found a strong association between the total binding capacity (Figure 5C) or FcγR–specific binding capacity (Figure 5D) of the various HIV-1–specific Abs and MoDC maturation. We therefore suggest that the binding of Abs to MoDCs induces the maturation of these cells, resulting in lower levels of HIV-1 replication.
Comparison between Ab-mediated inhibition of cell-to-cell HIV-1 transfer and conventional neutralization
The efficacy with which Abs inhibited HIV-1 transfer from MoDCs to CD4 T lymphocytes was compared with Ab-mediated inhibition in a conventional neutralization assay with cell-free viral particles infecting CD4 T lymphocytes (supplemental Figure 2). NAbs displayed similar levels of inhibition in both assays and the IC70 values were in the same range, indicating that inhibition of HIV-1 transfer by Abs is almost as efficient as inhibition of HIV-1 free virus particles. b12 inhibited free virus particles slightly more efficiently, and 2G12 inhibited HIV-1 cell-to-cell transmission better than it neutralized cell-free virus (supplemental Figure 2). Because only a few MoDCs were infected after 48 hours in the absence of CD4 T lymphocytes (Figure 2A), we did not assess the inhibitory activity of Abs in MoDCs alone.
Discussion
Immature DCs have been reported to capture virus and to transfer it efficiently to neighboring CD4 target cells through the formation of virological synapses.2,16,17 It has been suggested that this mode of cell-to-cell spread of HIV-1 is extremely efficient, allowing the rapid dissemination of the virus in the body.1 It is therefore essential to determine whether HIV-1–specific Abs block HIV-1 transfer, particularly transfer in trans, which is responsible for the initial transmission of the virus to CD4 T lymphocytes at the mucosal site.
We developed an HIV-1–transfer assay for analyzing early HIV-1 replication in immature MoDCs and CD4 T lymphocytes. With the protease inhibitor indinavir, we demonstrated that 48 hours of incubation corresponded to a single cycle of HIV-1 infection in MoDCs. We therefore used these conditions to assess the capacity of HIV-1–specific Abs to inhibit HIV-1 transfer in trans to CD4 T lymphocytes. We demonstrated that NAbs efficiently inhibited this transfer; the cell-to-cell spread of HIV is therefore susceptible to the inhibitory properties of Abs. An interesting trend was observed for 2G12, which inhibited HIV-1 cell-to-cell transmission slightly more efficiently than it neutralized cell-free virus. Indeed, 2G12 recognizes glycans with high mannose content on the surface of the viral envelope gp120.37 These glycans are also recognized by DC-SIGN and thus 2G12 interferes with the viral envelope gp120/DC-SIGN interaction.37 Because DC-SIGN is thought to play a critical role in HIV transfer,24 2G12 may have an additional effect by blocking the DC-SIGN/CD4 interaction.
Fluorescence microscopy and electron tomography have shown that inhibitors have access to the preformed virological synapses and can interfere with HIV-1 cell-to-cell infection.34 Consequently, the HIV-1 bound to the DC surface remains accessible to neutralizing mAbs. Moreover, the inhibitory effects of NAbs on HIV-1 transfer were of similar strength to those detected when free HIV-1 particles infect CD4 T lymphocytes (supplemental Figure 2) or in more classic neutralization assays involving PBMCs.29,30 In neutralizing conditions, PBMCs were first incubated with free virus particles, and then cultured to confluence. HIV-1 transfer from cell-to-cell probably occurs during this incubation phase, and Abs would also need to inhibit this cell-to-cell transmission. This may account for the lower overall inhibitory activity of NAbs in conventional primary PBMCs assays than in a single-cycle, T-cell line-based neutralization assay.38
Several studies have investigated the inhibition of HIV-1 transfer by Abs and have reported conflicting results, some showing HIV-1 transfer to be resistant to entry inhibitors or NAbs,8,25,39-43 and others reporting efficient inhibition of HIV-1 replication.12,18,27,28,33,34,44 All of these studies analyzed either the early step of viral entry or HIV-1 release into the supernatant; none considered HIV-1 replication in immature MoDCs. We show herein that immature MoDCs play a major role in both HIV-1 production and HIV-1 inhibition by Abs. A higher percentage of infected MoDCs was repeatedly observed 48 hours after infection after incubation in the presence of autologous activated CD4 T lymphocytes, as described previously.26 This higher rate of HIV-1 replication in DCs after interaction with CD4 T lymphocytes affects the NAb-mediated inhibition.
In addition to inhibiting HIV-1 transfer to CD4 T lymphocytes, NAbs decreased HIV-1 replication in MoDCs. By dissecting the early steps of HIV-1 replication during coculture, we showed that more than 80% of the virus had not yet fused with immature MoDCs 2 hours after infection. This suggests that HIV-1 bound to the surface of MoDCs remains accessible to anti–HIV-1 Abs. Moreover, some Abs, such as NNAb 246-D, did not affect HIV-1 transfer from infected MoDCs to CD4 T lymphocytes, but significantly decreased the percentage of infected MoDCs. Furthermore, the inhibition of HIV-1 replication in MoDCs was correlated with the expression of the DC maturation marker CD83.
In the context of DC–T-cell cross-talk, HIV/Abs immune complexes have been shown to be more efficient than virus alone for triggering Ag presentation by DCs.45 FcγR engagement on DCs induces maturation.45 We therefore assessed the capacity of Ab to bind receptors on the surface of DCs and found that some Abs, such as the NAbs 4E10 and 2F5, bound efficiently to MoDCs. This binding partially involved FcγRs, because lower levels of Ab binding were observed in the presence of anti-FcγR Abs. However, some FcγR-unrelated binding was also observed. This additional binding capacity of the NAbs 4E10 and 2F5 may be because of their polyspecific autoantibody properties46 because they bind other proteins such as phospholipids.47
Ab binding to FcγRs on the surface of MoDC was correlated with DC maturation (Figure 5C-D). Abs may therefore be involved in an additional mechanism leading to DC maturation that might protect DCs against infection with R5 viruses and contribute to the efficient induction of HIV-1–specific immune responses. This maturation may therefore be highly valuable in the context of a protective prophylactic vaccine, although it might lead to tolerance in the cases of chronic infections.45 For Ab 2G12, the binding to MoDCs was very low. We proposed that this low binding may be because of the very special dimeric form of this IgG,48 therefore minimizing the capacity of this Ab to bind to FcγRs and consequently preventing MoDC maturation. Our results suggest that, in the context of DC–T-cell coculture, the inhibition of DCs infection observed with some Abs involves at least 2 different mechanisms: the neutralization of virus particles still attached to the MoDC surface by the Fab part of the IgG and the maturation of MoDCs through IgG-FcγRs or other Ab-cell interactions.
HIV-1 replication is highly restricted in DCs. It has been suggested that SAMHD1 limits HIV-1 replication by decreasing dNTP pools in myeloid cells.49,50 Mature DCs are not very susceptible to R5 viral replication; the viral life cycle is blocked shortly after entry7 and the levels of the APOBEC3G restriction factor are high.36 In our experimental conditions for HIV-1 transfer, HIV-1 replication in DCs was strongly modulated, increasing on coculture with CD4 T lymphocytes and decreasing after the addition of HIV-1–specific Abs. Further investigations are required to determine the mechanisms involved in these modulations, which may have a major impact on HIV replication and dissemination in vivo.
Although favorable levels of HIV-1 replication were observed in the conditions used for the coculture of immature MoDCs and primary CD4 T lymphocytes in the present study, well-known NAbs were able to inhibit HIV-1 transfer and replication efficiently. These results are extremely encouraging and provide further evidence of the need for any vaccine to induce the production of such Abs directly at the mucosal site to prevent the early dissemination of HIV-1 after its sexual transmission. Moreover, although NNAbs inhibited HIV-1 transfer from DCs to CD4 T lymphocytes only inefficiently, some NNAbs decreased HIV-1 replication in MoDCs significantly, suggesting a possible contribution to protection against HIV-1.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by EuroNeut41 (FP7-HELTH-2007-A-201038), EuroPrise (LSHP-CT-2006-037611), Sidaction, Dormeur Investment Service Ltd, and Agence Nationale de Recherche sur le Sida grants. B.S. was supported by a EuroNeut41 grant and K.X. obtained a doctoral scholarship from EuroPrise.
Authorship
Contribution: B.S. and K.X. performed the experiments; B.S., A.L., M.P., V.H., and C.M. analyzed the data; B.S., A.L., M.E.B., G.L., S.S., T.D., A.P., M.L., V.H., and C.M. contributed reagents, materials, and analysis tools; and B.S., V.H. and C.M. conceived the study, designed the experiments, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for K.X. is State Key Laboratory for Infectious Disease Prevention and Control, National Institute for Viral Disease Control and Prevention, Beijing, China. The current affiliation for V.H. is Laboratory Science Department, Hematology and Flow Department, Covance CLS SA, Geneva, Switzerland.
Correspondence: Dr Vincent Holl, Laboratory Science Department, Hematology and Flow Department, Covance CLS SA, 7 Rue Moise-Marcinhes, CH-1217 Meyrin, Geneva, Switzerland; e-mail: vincent.holl@covance.com; or Dr Christiane Moog, Inserm U748, Institut de Virologie, Faculté de Médecine, Université de Strasbourg, 3 Rue Koeberlé, 67000 Strasbourg, France; e-mail: c.moog@unistra.fr.