Abstract
The best conditioning regimen before allogeneic transplantation for high-risk diffuse large B-cell lymphoma (DLBCL) remains to be clarified. We analyzed data from 396 recipients of allotransplants for DLBCL receiving myeloablative (MAC; n = 165), reduced intensity (RIC; n = 143), or nonmyeloablative conditioning (NMAC; n = 88) regimens. Acute and chronic GVHD rates were similar across the groups. Five-year nonrelapse mortality (NRM) was higher in MAC than RIC and NMAC (56% vs 47% vs 36%; P = .007). Five-year relapse/progression was lower in MAC than in RIC/NMAC (26% vs 38% vs 40%; P = .031). Five-year progression-free survival (15%-25%) and overall survival (18%-26%) did not differ significantly between the cohorts. In multivariate analysis, NMAC and more recent transplant year were associated with lower NRM, whereas a lower Karnofsky performance score (< 90), prior relapse resistant to therapy, and use of unrelated donors were associated with higher NRM. NMAC transplants, no prior use of rituximab, and prior relapse resistant to therapy were associated with a greater risk of relapse/progression. In conclusion, allotransplantation with RIC or NMAC induces long-term progression-free survival in selected DLBCL patients with a lower risk of NRM but with higher risk of lymphoma progression or relapse.
Introduction
Approximately 80% of patients with diffuse large B-cell lymphoma (DLBCL) and other aggressive lymphomas may be cured by modern therapy.1-3 Some of those who do not achieve remission or who relapse can be rescued by high-dose chemotherapy and an autologous hematopoietic cell transplant (AHCT).4 Others, including those relapsing after an AHCT, are sometimes treated with an allogeneic transplant (alloHCT).5,6 However, because of the relatively low numbers of DLBCL patients included in published reports of alloHCT, the heterogeneity of histologic subtypes, differing conditioning protocols, and the short follow-up, the role of alloHCT for DLBCL patients still remains unclear. The use of myeloablative conditioning (MAC) was shown to achieve long-term survival of 40%-50%, but high transplant-related mortality up to 30%-40% seemed to limit this option to selected patients.7-9 It remains to be seen whether reduced-intensity conditioning (RIC) and nonmyeloablative conditioning (NMAC) may result in improved outcomes of lymphoma patients, as higher relapse rates between 30% and 80% were reported.10-15
We analyzed outcomes of 396 recipients of alloHCT for DLBCL reported to the Center for International Blood and Marrow Transplant Research (CIBMTR) to compare these conditioning approaches.
Methods
Subject selection
We reviewed all subjects with DLBCL reported to the CIBMTR 2000-2009 and included adult recipients of a first allogeneic HLA-matched related or unrelated T cell replete grafts for primary induction failure or relapse of DLBCL. All subjects whose data were included in this study provided institutional review board-approved consent to participate in the CIBMTR Research Database and have their data included in observational research studies. Each individual study does not receive institutional review board approval as these studies are not human subject research studies according to the OHRP Guidance on Research Involving Coded Private Information or Biologic Specimens (October 16, 2008). Individual studies, including this one, undergo administrative review by the institutional review board chair to ensure that the study, which was conducted in accordance with the Declaration of Helsinki, meets the criteria in the CIBMTR Research Database protocol. Subjects < 18 or ≥ 70 years of age (n = 19), twin transplants (n = 4), recipients with in vitro T cell–depleted transplants (n = 25), related mismatched donor (n = 13), complete response 1 status before transplantation (n = 15), recipients with < 6 months from autologous to allogeneic transplant (n = 12), recipients of cord blood cell grafts (n = 17), and recipients of second allogeneic transplants were excluded (n = 3). A total of 396 patients with DLBCL met the inclusion criteria; 228 were male. Median age was 54 years (range, 18-69 years). A total of 125 (32%) patients received a prior AHCT. A total of 129 patients received a related HLA-matched transplant, 267 received an unrelated donor alloHCT (HLA-matched: n = 168; partially HLA-matched: n = 68; HLA-mismatched: n = 31) after MAC (n = 165, 42%), RIC (n = 143, 36%), or NMAC (n = 88, 22%) regimens (see “Study endpoints and definitions”). Antithymocyte globulin was given to 88 subjects (Tables 1 and 2).
Study end points and definitions
Outcomes analyzed included engraftment rate, nonrelapse mortality (NRM), relapse/progression, progression-free survival (PFS), and overall survival (OS). The intensity of conditioning was categorized based on consensus criteria.16 Neutrophil recovery was defined as the first of 3 subsequent days with absolute neutrophil counts ≥ 0.5 × 109/L without growth factor support. Platelet recovery was defined as the first of 7 subsequent days with platelet counts ≥ 20 × 109/L without platelet transfusions. NRM was defined as death from any cause in the first 28 days or death without evidence of lymphoma progression/relapse. Progression was defined as an increase of ≥ 25% in the sites of lymphoma or development of new sites of lymphoma.17 Relapse was defined as recurrence of lymphoma after a complete response. For PFS, patients were considered treatment failures at the time of relapse/progression or death from any cause. Patients alive without evidence of disease relapse or progression were censored at last follow-up, and the PFS event was summarized by a survival curve. The OS interval variable was defined as the time from date of transplantation to date of death or last contact and summarized by a survival curve.
Other outcomes analyzed included acute GVHD (aGVHD)18 and chronic GVHD (cGVHD)19 graded by established criteria and cause of death. “Well matched” was defined as no known disparity between donor and recipient at HLA A, B, C, and DRB1 (8 of 8), partially matched as one known or one likely disparity, and mismatched as ≥ 2 disparities.20
Statistical analysis
Subject-, disease-, and transplant-related variables for the 3 cohorts based on conditioning intensity (MAC, RIC, and NMAC) were compared using the χ2 test for categorical and the Kruskal-Wallis test for continuous variables. Univariate probabilities of PFS and survival were calculated using the Kaplan-Meier estimator. Probabilities of aGVHD and cGVHD, NRM, and relapse/progression were calculated using cumulative incidence curves to accommodate competing risks. Conditioning regimens were compared using multivariate Cox proportional hazards regression models adjusting for risk factors significantly associated with outcomes. Factors significant at P = .05 level were retained in the final model.
Results
Changing use of pretransplant conditioning regimens
The use of MAC decreased over time (comparing 2000-2003 with 2004-2009; P < .001). In years 2000-2003, proportion of MAC was 53% versus 34% in 2004-2009, whereas RIC increased from 28% in 2000-2003 to 42% in 2004-2009. The proportion of NMAC-transplants was 19% in 2000-2003 versus 24% in 2004-2009.
Subjects
Subject-, disease-, and transplant-related variables of the MAC, RIC, and NMAC cohorts are summarized in Tables 1 and 2. Karnofsky performance score did not differ significantly. MAC subjects were significantly younger (medians 48 vs 54 vs 54 years; P < .001), more often had stage 3/4 disease (72% vs 59% vs 51%; P = .007) and B-symptoms at diagnosis (45% vs 27% vs 25%; P = .003) compared with the RIC and NMAC patients. MAC patients were less likely to receive a prior autotransplant (18% vs 36% vs 51%; P < .001) or radiation before transplantation (32% vs 48% vs 50%; P = .005). MAC subjects had a higher incidence of prior resistant lymphoma to therapy (42% vs 30% vs 26%; P < .001).
MAC regimens were based in most cases (128 of 165) on a combination of cyclophosphamide (120 mg/kg) with total body irradiation (TBI, 12 Gy) or with busulfan (> 9 mg/kg oral or equivalent intravenous). The majority of RIC regimens (118 of 143) were based on combination of fludarabine (150 mg/m2) with busulfan (≤ 9 mg/kg) or melphalan (< 150 mg/m2). NMAC regimens were based on low-dose TBI (2-5 Gy) combined with fludarabine (29 of 88 subjects) or on fludarabine with cyclophosphamide (42 of 88 subjects).
Transplant outcomes
Table 3 summarizes univariate probabilities of all outcomes of interest after transplantation. Supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article) displays variables tested in Cox proportional hazards regression models. Table 4 summarizes variables that had an impact on each study outcome.
Engraftment
Cumulative incidence of neutrophil recovery at days 28 and 100 after transplantation was significantly higher after RIC (day 28: 96%, 95% CI, 90%-98%; day 100: 99%, 95% CI, 94%-100%) and NMAC (day 28: 92%, 95% CI, 83%-96%; day 100: 97%, 95% CI, 90%-99%) compared with MAC regimens (day 28: 86%, 95% CI, 80%-90%; P = .012; day 100: 88%, 95% CI, 82%-92%; P < .001).
NRM
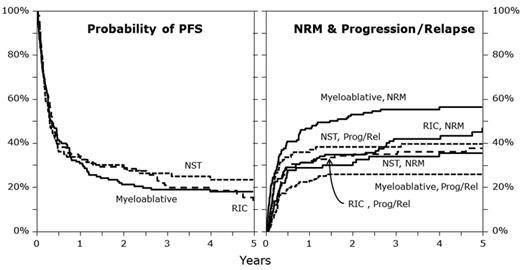
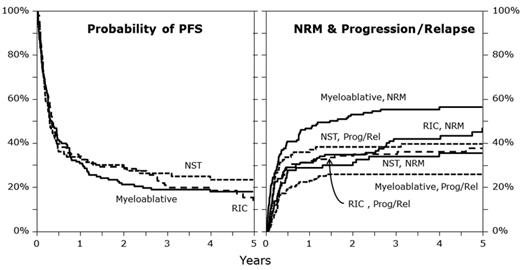
MAC regimens were associated with higher NRM compared with RIC and NMAC regimens (100-day NRM: 32% vs 24% vs 17%; P = .029; 1-year NRM: 47% vs 31% vs 29%; P = .004; 5-year NRM: 56% vs 47% vs 36%; P = .007; Figure 1). In multivariate analysis, NMAC (HR = 0.58, 95% CI, 0.36-0.97; P = .026) and RIC (HR = 0.71, 95% CI, 0.49-1.03; P = .069) were associated with lower NRM compared with MAC. Significant covariates associated with a higher NRM included Karnofsky performance score < 90% (HR = 1.51, 95% CI, 1.10-2.08; P = .011), and HLA-mismatched unrelated donor status (HR = 2.32, 95% CI, 1.57-3.43; P < .001). Transplants performed after year 2004 (HR = 0.49; P < .001) and chemotherapy-sensitive relapse before transplantation (HR = 0.5; 95% CI, 0.31-0.81; P = .005) were associated with lower NRM.
Illustration of PFS, progression/relapse, and NRM in the different conditioning groups. NST indicates nonmyeloablative stem cell transplantation; Prog, progression; and Rel, relapse.
Illustration of PFS, progression/relapse, and NRM in the different conditioning groups. NST indicates nonmyeloablative stem cell transplantation; Prog, progression; and Rel, relapse.
Relapse/progression
Cumulative incidence of relapse/progression at 1, 3, and 5 years after alloHCT was higher after NMAC and RIC than MAC regimens (at 1 year: 37% vs 32% vs 23%; P = .043; at 3 years: 38% vs 35% vs 26%; P = .078; at 5 years: 40% vs 38% vs 26%; P = .031; Figure 1). NMAC regimens (HR = 2.14, 95% CI, 1.29-3.54; P = .003) and RIC (HR = 1.45, 95% CI, 0.94-2.26; P = .096) were associated with a higher risk of relapse/progression in multivariate analysis compared with MAC. Significant covariates associated with a higher risk of relapse/progression were no therapy with rituximab before transplant (HR = 1.69, 95% CI, 1.15-2.48; P = .008), chemoresistant primary induction failure (HR = 3.23, 95% CI, 1.55-6.69; P = .002), and chemotherapy resistant relapse before transplant (HR = 3.6, 95% CI, 1.72-7.55; P < .001).
PFS
PFS was similar at 1, 3, and 5 years after transplantation between MAC, RIC, and NMAC regimens (at 1 year: 30% vs 37% vs 34%; P = .487; at 3 years: 19% vs 23% vs 27%; P = .309; at 5 years: 18% vs 23% vs 25%; P = .309; Figure 1). In multivariate analysis, PFS was similar for patients from RIC (HR = 1.05, 95% CI, 0.79-1.39; P = .74) and NMAC cohorts (HR = 1.05, 95% CI, 0.75-1.47; P = .788) compared with the MAC cohort. Significant covariates associated with lower PFS included lower Karnofsky performance status (HR = 1.43, 95% CI, 1.12-1.83; P = .004) and chemotherapy-resistant relapse (HR = 2.49, 95% CI, 1.74-3.57; P < .001). Transplantation performed in year 2004 or later was associated with superior PFS and lower risk of treatment failure (HR = 0.61, 95% CI, 0.48-0.78; P < .001).
Survival
Survival at 1, 3, and 5 years did not differ significantly between MAC, RIC, and NMAC regimens (at 1 year: 38% vs 46% vs 45%; at 3 years: 21% vs 27% vs 29%; at 5 years: 18% vs 20% vs 26%). Covariates significantly correlated with lower survival and higher mortality included Karnofsky performance score < 90 (HR = 1.48, 95% CI, 1.16-1.90; P = .002) and chemotherapy-resistant relapse (HR = 1.70, 95% CI, 1.14-2.56; P = .010). Transplants performed in 2004 or later were associated with lower mortality (HR = 0.60; 95% 0.47-0.76; P < .001).
Secondary outcomes
The incidence of grade ≥ 2 aGVHD (43%; 95% CI, 36%-51% both for MAC and RIC, 44%; 95% CI, 34%-55% for NMAC), and cGVHD at 5 years (37%; 95% CI, 30%-45% both for MAC and NMAC, 42%; 95% CI, 34%-51% for RIC) did not differ significantly between the cohorts.
Cause of death
The main cause of death among all 3 cohorts was relapsed/progressive lymphoma (MAC, 38%; RIC, 39%; NMAC, 48%). Other leading causes of deaths were sepsis/severe infection (MAC, 21%; RIC, 16%; NMAC, 16%), organ failure (MAC, 14%; RIC, 13%; NMAC, 6%), and GVHD (MAC, 11%; RIC, 8%; NMAC: 6%).
Discussion
In contrast to autologous HCT, which represents a standard second-line approach for relapsed/refractory patients with DLBCL, alloHCT that is performed in some centers as third-line approach is no standard option for this disease entity. A limited number of studies evaluating the outcomes of alloHCT in DLBCL have been published, and the heterogeneity of patients in these reports does not allow definite conclusions to be drawn regarding this approach.10,21-25 The current study is the largest report comparing outcomes after conditioning regimens of different intensity (including 164 MAC, 143 RIC, and 88 NMAC) in DLBCL patients. Patient-, disease-, and transplant-related differences observed between the different conditioning groups reflect the expected effect of patient selection, with the MAC cohort having lower median age, higher incidence of advanced and chemorefractory disease, and less use of radiotherapy or autotransplantation before allotransplantation. The patients in the 3 conditioning groups were different regarding disease characteristics as those patients with poor prognosis (eg, with chemorefractory disease) underwent more aggressive MAC transplantations. In this setting, the potential of the alloHCT based on the graft-versus-lymphoma effect might be insufficient because of still increased tumor burden after the conditioning.
In one major aspect, conditioning intensity affected the outcomes. Both the short- and long-term NRM was higher for MAC compared with the RIC and NMAC patients: The MAC cohort had a higher day-100 NRM (> 30%), and also 5-year NRM. Similarly, in an EBMT analysis studying 101 DLBCL patients, van Kampen et al observed a 3-year NRM of 41% after MAC compared with 20% in RIC.5 Lazarus et al reported a high NRM of 45% at 5 years in 79 DLBCL patients who received related donor alloHCT with MAC, primarily combinations of cyclophosphamide with 12 Gy TBI or with busulfan as second-line approach after initial chemotherapy.26 Regarding the RIC/NMAC transplants, we found a similar NRM rate compared with previous studies reporting on NRM rates between 23% and 28%.12,15,27 A lower Karnofsky performance status, a history of chemotherapy resistant relapse before alloHCT, or the use of unrelated donors impacted adversely on NRM in our study.
On the other hand, the cumulative incidence of relapse/progression at 5 years was higher after NMAC and RIC with 40% and 38% compared with MAC with 26% (P = .031) in our study. This is also similar to previous studies reporting relapse rates of 33%-41% after RIC regimens for DLBCL patients.12,14,15 After MAC alloHCT, Doocey et al documented a 5-year relapse rate of 32% in patients with different aggressive lymphoma entities, including DLBCL,8 and Lazarus et al observed a relapse rate of 33% at 5 years for DLBCL patients.26 Besides the negative impact of RIC regimens, nonreceipt of rituximab before transplantation and chemoresistant disease adversely impacted on relapse.
Finally, we found no difference in survival between the 3 conditioning groups. Although the OS and PFS after MAC (18% each at 5 years) were comparable with other studies,26 survival outcomes after RIC (5-year OS, 20%; PFS, 15%) and NMAC (5-year OS, 26%; PFS, 25%) in our study was lower compared with other studies (OS, 45%-52%; PFS, 35%-48%).5,12,14,15 Thomson et al observed a 4-year PFS of 48% after RIC,14 whereas Lazarus et al observed a 5-year PFS of 22% after MAC alloHCT.26 These differences between the results in this study and the previous literature are probably the result of different patient selection criteria. Karnofsky performance status, disease status before alloHCT, and year of transplantation were significant predictors regarding the PFS and OS.
As the present study was based on a retrospective multicenter registry analysis, limitations regarding the patients' demographics between 3 conditioning groups with possible bias should be taken into account. Nevertheless, the present data and the previous studies clearly demonstrate that allogeneic HCT may be curative for selected patients with relapsed, poor-risk DLBCL. The higher NRM after use of MAC regimens and the association of the relapse with the conditioning intensity provide an orientation for selecting the most appropriate conditioning strategies. The use of rituximab before HCT according to our study may reduce the relapse/progression rate after transplantation; however, it needs to be further investigated in large prospective studies. In summary, younger DLBCL patients and patients with little comorbidity may receive additional reduction in relapse risk from MAC regimens, whereas elderly patients or those with severe comorbidities may be selected for RIC regimens that are associated with lower NRM.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Asad Bashey, Maria L. Delioukina, James L. Gajewski, Brandon M. Hayes-Lattin, Roger Herzig, Osman Ilhan, Philip L. McCarthy, Alan M. Miller, Gustavo A. Milone, Silvia Montoto, Reinhold Munker, Gordon L. Phillips, and David A. Rizzieri for their helpful comments and insights as members of the study committee.
The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, or any other agency of the US government.
The CIBMTR is supported by the National Cancer Institute, the National Heart, Lung, and Blood Institute (NHLBI), and the National Institute of Allergy and Infectious Diseases (Public Health Service Grant/Cooperative Agreement U24-CA76518); NHLBI and National Cancer Institute (Grant/Cooperative Agreement 5U01HL069294); Health Resources and Services Administration (contract HHSH234200637015C); the Office of Naval Research (grants N00014-06-1-0704 and N00014-08-1-0058); and the following: Allos Inc; Amgen Inc; Angioblast; anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; Blue Cross and Blue Shield Association; Buchanan Family Foundation; CaridianBCT; Celgene Corporation; CellGenix, GmbH; Children's Leukemia Research Association; Fresenius-Biotech North America Inc; Gamida Cell Teva Joint Venture Ltd; Genentech Inc; Genzyme Corporation; GlaxoSmithKline; HistoGenetics Inc; Kiadis Pharma; Leukemia & Lymphoma Society; Medical College of Wisconsin; Merck & Co Inc; Millennium: Takeda Oncology Co; Milliman USA Inc; Miltenyi Biotec Inc; National Marrow Donor Program; Optum Healthcare Solutions Inc; Osiris Therapeutics Inc; Otsuka America Pharmaceutical Inc; RemedyMD; Sanofi; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix Inc; StemCyte, A Global Cord Blood Therapeutics Co; Stemsoft Software Inc; Swedish Orphan Biovitrum; Tarix Pharmaceuticals; Teva Neuroscience Inc; THERAKOS Inc; and Wellpoint Inc.
National Institutes of Health
Authorship
Contribution: U.B., E.K., and P.N.H. designed the study, interpreted data, prepared the manuscript, and gave approval of the final manuscript; J.C. and J.L.-R. performed the statistical analysis; and P.A., M.R.B., C.N.B., M.S.C., T.S.F., C.O.F., R.P.G., J.G., L.M.I., D.J.I., G.G.L., H.M.L., R.T.M., P.H.W., H.C.S., S.S., S.M.S., J.M.V., and E.K.W. participated in interpretation of data, manuscript preparation, and approval of the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ulrike Bacher, Department for Stem Cell Transplantation, University of Hamburg, Martinistrasse 52, 20246 Hamburg, Germany; e-mail: u.bacher@uke.de.
References
Author notes
U.B. and E.K. contributed equally to this study.