Abstract
BMP9 signaling has been implicated in hereditary hemorrhagic telangiectasia (HHT) and vascular remodeling, acting via the HHT target genes, endoglin and ALK1. This study sought to identify endothelial BMP9-regulated proteins that could affect the HHT phenotype. Gene ontology analysis of cDNA microarray data obtained after BMP9 treatment of primary human endothelial cells indicated regulation of chemokine, adhesion, and inflammation pathways. These responses included the up-regulation of the chemokine CXCL12/SDF1 and down-regulation of its receptor CXCR4. Quantitative mass spectrometry identified additional secreted proteins, including the chemokine CXCL10/IP10. RNA knockdown of endoglin and ALK1 impaired SDF1/CXCR4 regulation by BMP9. Because of the association of SDF1 with ischemia, we analyzed its expression under hypoxia in response to BMP9 in vitro, and during the response to hindlimb ischemia, in endoglin-deficient mice. BMP9 and hypoxia were additive inducers of SDF1 expression. Moreover, the data suggest that endoglin deficiency impaired SDF1 expression in endothelial cells in vivo. Our data implicate BMP9 in regulation of the SDF1/CXCR4 chemokine axis in endothelial cells and point to a role for BMP9 signaling via endoglin in a switch from an SDF1-responsive autocrine phenotype to an SDF1 nonresponsive paracrine state that represses endothelial cell migration and may promote vessel maturation.
Introduction
Endoglin directly interacts with the TGF-β receptors,1 including ALK1,2 and modulates TGF-β and bone morphogenetic protein (BMP) signaling.3 Mutations in either endoglin4 or ALK15 increase the risk of hereditary hemorrhagic telangiectasia (HHT1 and HHT2, respectively), whose symptoms include arteriovenous malformation, tissue ischemia, and reperfusion defects.6
The ALK1-endoglin signaling complex in endothelial cells is activated by BMP9,7 a circulating cytokine produced in the liver reticuloendothelium8 and endothelial cells, including those lining the mouse aorta.9 BMP9 interacts with endoglin and ALK1 to activate signaling pathways7 that promote endothelial cell quiescence10 and vessel maturation.11
Several endothelial cell–derived factors, including BMP9, are known to regulate vessel maturation via paracrine recruitment of other cell types.12 Moreover, our recent work using nonendothelial cells implicates endoglin in the regulation of tumor neoangiogenesis via the secreted insulin-like growth factor binding protein 4.13 Therefore, elucidation of the role of BMP9 signaling, specifically in terms of its effects on the expression of endothelial cell–secreted factors, is needed to better understand the mechanisms by which BMP9 affects vessel maturation, integrity, the vascular response to injury, and how deficiency in either endoglin or ALK1 impacts vessel integrity and cause HHT.
Stromal-derived factor 1 (SDF1, CXCL12) is a chemokine that signals via the chemokine receptor, CXCR4, to modulate hypoxia-induced angiogenesis.14 SDF1 regulates both endothelial cell–mediated paracrine signaling and endothelial cell-autonomous autocrine signaling. In endothelial cells, SDF1 is up-regulated by hypoxia14 and promotes recruitment, vascular remodeling, and differentiation15 of pericytes and their perivascular retention, reflecting its well known paracrine functions.
Although less studied, SDF1 expressed by endothelial cells promotes endothelial cell-autonomous phenotypic changes, including the regulation of branching morphogenesis, which is mediated by CXCR4 coexpression in the SDF1-expressing cells,16 indicating important autocrine functions for SDF1. CXCR4 exhibits complex time-dependent modulation of its cell surface expression, including loss of expression with change in endothelial cell morphology.16 Moreover, priming of endothelial progenitor cells with SDF1 increases their angiogenic potential.17 SDF1-dependent autocrine signals regulate postnatal vascular remodeling and promote vascular recovery in the hindlimb ischemia mouse model, suggesting that this factor plays a role in endothelial cell autocrine signaling relevant to vessel maturation.16
The present study demonstrates that BMP9 is a regulator of endothelial cell SDF1 expression, which is responsive to the level of endoglin expression and therefore is potentially relevant to the mechanism of endoglin haploinsufficiency leading to HHT. Conversely, BMP9 coordinately represses CXCR4 expression, thus potentially switching off endothelial cell responsiveness to SDF1. Moreover, data are provided suggesting that BMP9 and hypoxia reinforce the expression of SDF1 and that endoglin deficiency impairs the endothelial cell-autonomous capacity to up-regulate SDF1 expression in the vascular response to hindlimb ischemic injury in eng+/− mice, a mouse model of HHT.18 Thus, the present work sheds light on a spectrum of novel potential BMP9-mediated autocrine and paracrine effector proteins and provides insight into how haploinsufficiency in endoglin and ALK1 signaling causes HHT.
Methods
Cell culture
HUVECs (Lonza Walkersville, passage 2-7) and human arterial endothelial cells (HAECs, Lonza Walkersville, passage 2-7) were maintained in EGM-2 medium (Lonza Walkersville) at 37°C and 5% CO2. EGM-2 medium consists of EMB-2 with 1% FCS and other additives, including growth factors. Human microvascular endothelial cells-dermal (HMVEC-D) and human microvascular endothelial cells–coronary (HMVEC-C) were maintained in EGM-2-MV medium (Lonza Walkersville) at 37°C and 5% CO2. EGM-2-MV medium consists of EMB-2 with 1% FBS and other additives, including growth factors. SV40-transformed mouse microvascular endothelial cell line (SVECs,19 ) were maintained in DMEM with 10% FBS at 37°C and 5% CO2. Cells were treated with human recombinant BMP9 (R&D Systems) as indicated in the figure legends.
Affymetrix microarray analysis
Microarray expression analysis used single-use microarray chips (Affymetrix HG-U133_Plus_2_IVT chip) with 1-color (biotin-labeled) detection. HUVEC RNA (5 μg of total RNA at a concentration of at least 500 ng/μL) from a minimum of 3 independent biologic replicate experiments was pooled and was subjected to one-cycle (single amplification). Data analysis (x-ray/ANOVA) was conducted on a total of 54 683 genes in collaboration with the Biostatistics and Bioinformatics Resource Core Facility (Dartmouth College). Microarray data are available in the NCBI Gene Expression Omnibus Archives (accession no. GSE40960).
Protein and RNA analysis
For protein preparation, cells were disrupted in lysis buffer (150mM NaCl, 300mM sucrose, 1% Triton X-100, 0.5% sodium deoxycholate, 50mM Tris-HCl, pH 7.5) containing protease (Roche Diagnostics) and phosphatase (Calbiochem-EMD) inhibitors, used according to the manufacturer's instructions. Western blot analysis were performed with antiendoglin (BD Biosciences), and anti–β-actin (Sigma-Aldrich) as previously described.20 ELISA for the detection of human SDF1-α (QuantiKine; R&D Systems) was conducted as per the manufacturer's instructions. RNA isolation and RT-PCR were performed as previously described.13 Real-time quantitative RT-PCR was carried out essentially as described.13 For these experiments, cells plated on fibronectin were harvested, washed, and total RNA was isolated using RNeasy plus (QIAGEN). Quantitative RT-PCR reactions were run in triplicate on a BioRad iQ5 system. Primers used for standard RT- and quantitative RT-PCR are listed in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Statistical significance is presented as the SEM.
Viral transduction
Constructs expressing 21-nucleotide endoglin-specific short hairpin RNAs (shRNA) targeting human endoglin (shENG) or nontargeting control (shNT, Sigma-Aldrich, SHC002) were obtained from Sigma-Aldrich and used as described previously.13 Constructs were packaged into lentivirus pseudotyped with the vesicular stomatitis virus glycoprotein. Transduction was performed by incubating cells with lentivirus, and stably transduced cells were subsequently used for studies. All cell lines were verified by morphology, mouse and human endoglin-specific PCR, certified mycoplasma-negative by PCR (Lonza), and primary cell cultures used within the indicated passage numbers. Cells were transduced and selected using hygromycin as previously described.20
Cell migration
Migration assays were performed as follows: 1 × 106 cells/mL were suspended in migration buffer (RPMI, containing 1mM MgCl2, 0.2mM MnCl2, and 0.5% BSA), 100 μL (1 × 105 cells) plated in the upper chamber of transwell migration chambers (8.0 μm, Corning Life Sciences), and allowed to invade through a polycarbonate membrane, coated on the distal side with collagen, toward conditioned medium for 4-5 hours at 37°C.13 Cells remaining on the topside were removed, and cells that had migrated to the underside were stained with crystal violet. Cell migration was quantified in at least 3 independent experiments using triplicates, either by counting or by extraction of crystal violet and quantifying absorbance at 600 nm.
Preparation of conditioned media for cell migration and mass spectrometric analysis
Conditioned medium (50 mL from 5 confluent 15-cm plates of shNT- or shENG-treated HUVECs) was concentrated using an Amicon Ultracell 3-kDa centrifugal ultrafiltration cartridge, as previously described13 and used as described in “Cell migration.”
Endothelial cell hypoxia
HMVEC-D microvascular cells were incubated under standard conditions using normoxic (5% CO2, 95% air) or hypoxic conditions achieved by repeated purging a Billups-Rothenberg incubator every 30 minutes (3 times) with a mixture of 5% CO2, 1% O2, and 94% N2. Hypoxia was confirmed at 24 hours using the Hypoxyprobe-1 system (Chemicon International) as per the manufacturer's instructions. RNA preparations for the indicated conditions were obtained after 8 hours and 24 hours of hypoxia exposure. Additional methods are detailed in supplemental Methods.
Results
Endothelial cells up-regulate chemokine expression after BMP9 treatment
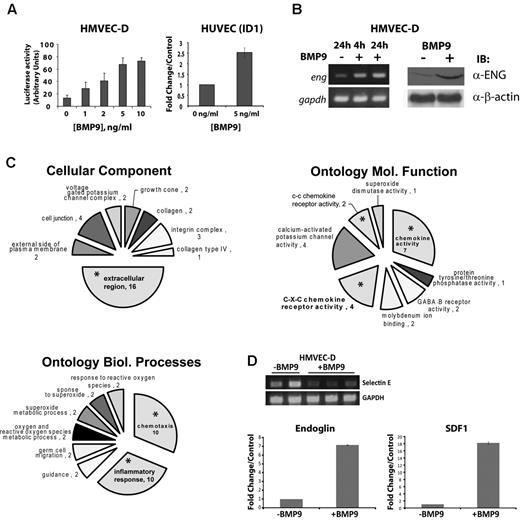
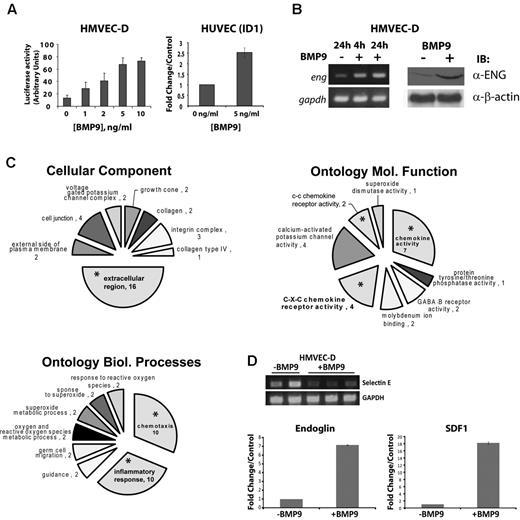
BMP9 is described as an autocrine and paracrine signaling factor.8 However, the downstream effectors that mediate the effects of BMP9 in endothelial cells are not well established, and the roles of BMP9 autocrine and paracrine functions require elucidation. Therefore, we examined the changes in gene expression in endothelial cells after BMP9 treatment. To determine the appropriate levels BMP9 needed for stimulation of Smad1/5-dependent signaling, we tested human dermal microvascular endothelial cells (HVMEC-D) with 0-10 ng/mL BMP9 after transfection with the BMP-response element Luciferase reporter (BRE-Luc), as previously described.21 This experiment indicated that, after 24 hours, 80% of maximal stimulation was achieved with 5 ng/mL (Figure 1A left panel), consistent with previously published data.7 This result was confirmed using quantitative RT-PCR to measure the BMP9-responsive gene ID122 mRNA levels in HUVECs treated with 5 ng/mL BMP9 (Figure 1A right panel). Under these conditions, we observed that endoglin mRNA and protein levels were increased (Figure 1B), indicating that endoglin is itself regulated by BMP9, consistent with other studies.23 Similar results were observed for other primary endothelial cell cultures, including human aortic (HAECs), microvascular carotid (HMVEC-C), and umbilical vein endothelial cells (HUVECs, data not shown).
BMP9 treatment of endothelial cells results in a prominent chemokine response. (A) HMVEC-D were transfected with BRE-Luciferase reporter construct and then treated with increasing doses of recombinant BMP9 for 24 hours. Cells were collected and analyzed for Luciferase activity. Data were normalized to the pGL3 empty Luciferase reporter. Data are mean ± SEM. Endothelial cells responded to physiologically relevant concentrations of recombinant BMP910 with dose-responsive increases in BRE-Luciferase promoter activity (left panel), which is confirmed by quantitative RT-PCR for BMP-responsive ID1 mRNA (right panel). (B) HMVEC-D were treated with 5 ng/mL BMP9 (+) or control (−), and total RNA and cell lysates were collected for analysis. RT-PCR (left panel, 4 or 24 hours after BMP9 treatment) and Western blotting (right panel, 24 hours after BMP9 treatment) show increased endoglin transcript and protein levels in response to BMP9 stimulation. (C) Cell component, Gene Ontology for biologic processes, and molecular function (x-ray/ANOVA) analyses suggest that BMP9 stimulation predominantly results in an alteration in endothelial cell extracellular protein expression and adhesion (upper left profile), chemokine receptor (right panel), and chemotaxis/inflammatory responses (lower left profile). Asterisks indicate emphasized extracellular, chemokine-related, and inflammatory pathways. (D) RT-PCR confirmation of the BMP9 down-regulated gene, E selection. Inset: Independent duplicate control untreated and triplicate cDNA preparations treated with 5 ng/mL BMP9. Quantitative real-time RT-PCR measurements indicate that BMP9 (5 ng/mL) produces 7- and 18-fold increases in endoglin and SDF1 mRNA expression at 24 hours after treatment, respectively. Bars represent the mean ± SE for 3 experiments.
BMP9 treatment of endothelial cells results in a prominent chemokine response. (A) HMVEC-D were transfected with BRE-Luciferase reporter construct and then treated with increasing doses of recombinant BMP9 for 24 hours. Cells were collected and analyzed for Luciferase activity. Data were normalized to the pGL3 empty Luciferase reporter. Data are mean ± SEM. Endothelial cells responded to physiologically relevant concentrations of recombinant BMP910 with dose-responsive increases in BRE-Luciferase promoter activity (left panel), which is confirmed by quantitative RT-PCR for BMP-responsive ID1 mRNA (right panel). (B) HMVEC-D were treated with 5 ng/mL BMP9 (+) or control (−), and total RNA and cell lysates were collected for analysis. RT-PCR (left panel, 4 or 24 hours after BMP9 treatment) and Western blotting (right panel, 24 hours after BMP9 treatment) show increased endoglin transcript and protein levels in response to BMP9 stimulation. (C) Cell component, Gene Ontology for biologic processes, and molecular function (x-ray/ANOVA) analyses suggest that BMP9 stimulation predominantly results in an alteration in endothelial cell extracellular protein expression and adhesion (upper left profile), chemokine receptor (right panel), and chemotaxis/inflammatory responses (lower left profile). Asterisks indicate emphasized extracellular, chemokine-related, and inflammatory pathways. (D) RT-PCR confirmation of the BMP9 down-regulated gene, E selection. Inset: Independent duplicate control untreated and triplicate cDNA preparations treated with 5 ng/mL BMP9. Quantitative real-time RT-PCR measurements indicate that BMP9 (5 ng/mL) produces 7- and 18-fold increases in endoglin and SDF1 mRNA expression at 24 hours after treatment, respectively. Bars represent the mean ± SE for 3 experiments.
HMVEC-D mRNA preparations (pooled from 3 independent experiments) were used for cDNA microarray analysis (5 ng/mL BMP9 treatment for 24 hours). Ontologic analysis (PANTHER,24 ) of the BMP9-regulated transcripts suggested that the most pronounced response observed in BMP9-treated endothelial cells reflected extracellular functions, including secreted markers of inflammatory, chemokine, and chemotactic responses (Figure 1C; supplemental Table 1.1). Prominent among BMP9 up-regulated genes was the chemokine SDF1 (supplemental Table 1.2), a mediator of endothelial cell angiogenic responses.25 Microarray data also indicated that E-selectin, a critical molecule for endothelial precursor homing to ischemic limb,26 and the SDF1 receptor CXCR4 were among the most down-regulated transcripts after BMP9 treatment (supplemental Table 1.2). Reduction of Selectin E expression was confirmed in by RT-PCR (Figure 1D inset panels).
Next, using quantitative RT-PCR, we established that endoglin mRNA was up-regulated ∼ 7-fold more than untreated cells (P < .05). Consistent with this result and the data shown in Figure 1B, quantitative isotope-coded affinity tag (ICAT) mass spectrometry independently confirmed that endoglin is a BMP9 target gene expressed in the endothelial cell membrane (supplemental Figure 1). Measurement of mRNA levels by quantitative RT-PCR indicated that SDF1 mRNA expression was potently increased after BMP9 treatment (> 18-fold, P < .01, Figure 1D). In addition, neither C3H10T1/2, which are BMP9-responsive mesenchymal stem cell and mesenchymal endothelial cell precursor models,27 nor human mesenchymal stem cells exhibited induction of SDF1, or CXCR4 repression, with BMP9 treatment (data not shown). Thus, our data indicate that endoglin and the chemokine SDF1 are prominent up-regulated transcriptional targets of BMP9 in endothelial cells, whereas multiple other genes, including CXCR4, are among a set of effectors whose expression is reduced after BMP9 treatment.
Expression of BMP9 in endothelial cells results in secretion of chemokines
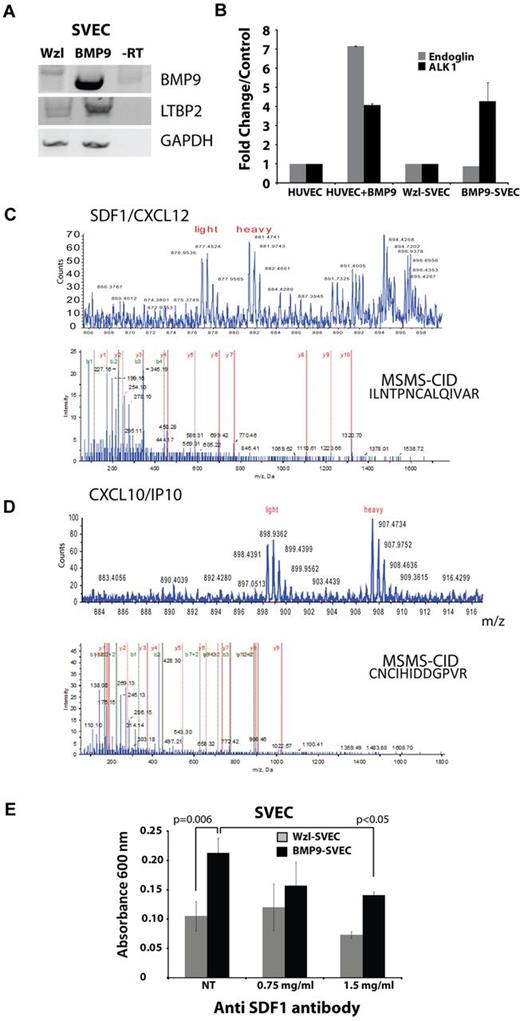
BMP9 is expressed in the mouse aortic endothelium,9 suggesting that it may function both as an endothelial cell-autonomous (ie, autocrine) as well as a circulating or local paracrine factor. Therefore, to gain insight into potential endothelial cell autocrine functions of BMP9, we modified a well-characterized SVEC19 to express BMP9. SVECs were a potentially useful endothelial cell model because our preliminary studies indicated they made little BMP9 mRNA, expressed ALK1, and tolerated serum-free culture conditions sufficiently to enable mass spectrometric analysis of secreted proteins absent excessive contamination with exogenous serum constituents necessary for primary cell culture.
The pWzl retrovirus was used20 to prepare SVECs that expressed BMP9, as determined by RT-PCR (BMP9-SVEC). RT-PCR confirmed that pWzl-SVECs expressed little BMP9, whereas the BMP9-SVEC–transduced cells expressed BMP9 mRNA (Figure 2A top panel).
BMP9 expression causes up-regulation of secreted regulatory factors. (A) RT-PCR of control and BMP9-transduced SV40-transformed SVECs. (B) Real-time quantitative RT-PCR measurement of endoglin and ALK1 expression in HUVECs (with and without BMP9 treatment for 24 hours) and SVECs (control and constitutively expressing BMP9). (C-D) Quantitative ICAT-based mass spectrometry (top panels) and collision-induced decay MSMS-based (MSMS-CID) protein sequence identification (bottom panels) of the chemokines CXCL12 (SDF1, C) and CXCL10 (IP10, D) in SVECs transduced with BMP9. (E) Transwell migration of BMP9-expressing and control SVECs, in the presence and absence of a neutralizing anti-SDF1 antibody. P values are from experiments conducted in triplicate.
BMP9 expression causes up-regulation of secreted regulatory factors. (A) RT-PCR of control and BMP9-transduced SV40-transformed SVECs. (B) Real-time quantitative RT-PCR measurement of endoglin and ALK1 expression in HUVECs (with and without BMP9 treatment for 24 hours) and SVECs (control and constitutively expressing BMP9). (C-D) Quantitative ICAT-based mass spectrometry (top panels) and collision-induced decay MSMS-based (MSMS-CID) protein sequence identification (bottom panels) of the chemokines CXCL12 (SDF1, C) and CXCL10 (IP10, D) in SVECs transduced with BMP9. (E) Transwell migration of BMP9-expressing and control SVECs, in the presence and absence of a neutralizing anti-SDF1 antibody. P values are from experiments conducted in triplicate.
Quantitative RT-PCR confirmed increased BMP9 expression in BMP9-SVECs (data not shown) and indicated that both BMP9-SVECs and control pWzl-SVECs expressed endoglin and ALK1 (Figure 2B). SVECs expressing BMP9 were similar to HUVECs in terms of up-regulation of ALK1 by BMP9 (Figure 2B), although endoglin levels did not show the increased characteristic of HMVEC-D (Figure 1B). Nonetheless, these data indicate that SVEC and derived SVEC-BMP9 cells are a useful adjunct model for identifying BMP9-dependent secreted proteins.
Serum-free conditioned media from BMP9-SVEC and pWzl-SVEC cells were compared using ICAT mass spectrometry,28 as described previously.13 The ICAT data suggested that latent TGF-β binding protein 2 (LTBP2) was among several up-regulated secreted proteins (supplemental Tables 3-11) observed in the BMP9-SVEC conditioned medium (supplemental Table 2.1). This observation was confirmed by RT-PCR (Figure 2A middle panel) and was represented in the HMVEC-D microarray dataset (LTBP2: 1.3-fold up-regulated after BMP9 treatment, P = .047). Together, these data identify novel, potentially endothelial cell–selective BMP9-regulated proteins (see supplemental Tables 1.2 and 2.1), including LTBP2, SDF1, E selectin, and CXCR4.
Analysis of the SVEC-conditioned medium by ICAT mass spectrometry13 also provided independent confirmation that SDF1 was secreted (Figure 2C; and diagnostic collision-induced decay mass spectrometric peptide sequence data, supplemental Table 2.2) in BMP9-expressing SVEC conditioned medium compared with the empty vector pWzl control conditioned medium. Consistent with the hypothesis of a broad induction of chemokinetic activities by BMP9, as suggested in Figure 1C, the chemokine CXCL10, a regulator of endothelial cell proliferation,29 was also detected as a prominent component in the BMP9-SVEC conditioned medium (Figure 2D; supplemental Table 2.3). These observations support the hypothesis that BMP9 promotes the secretion of multiple endothelial cell chemokine mediators.
Interestingly, transwell migration assay using BMP9-expressing or control SVECs showed that BMP9 expression was strongly promigratory and that this effect was significantly reduced in the presence of a neutralizing antibody for SDF1 (Figure 2E). Thus, these results support the hypothesis that BMP9 acts on endothelial cells to induce multiple autocrine chemokinetic responses,8 which include proangiogenic effects of SDF1 on endothelial cells in vitro.
Endoglin deficiency impairs the BMP9-dependent endothelial cell chemokine response
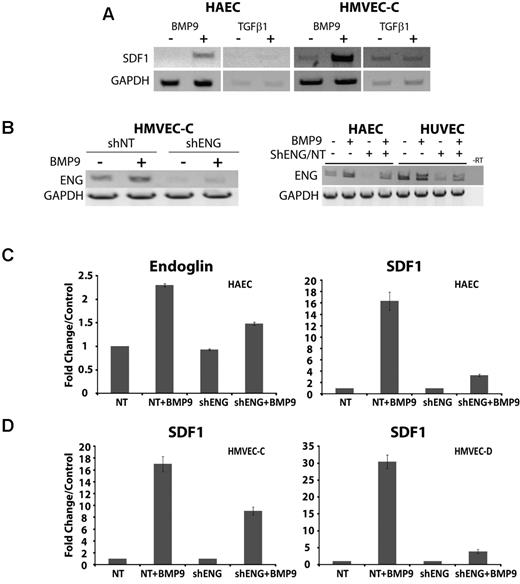
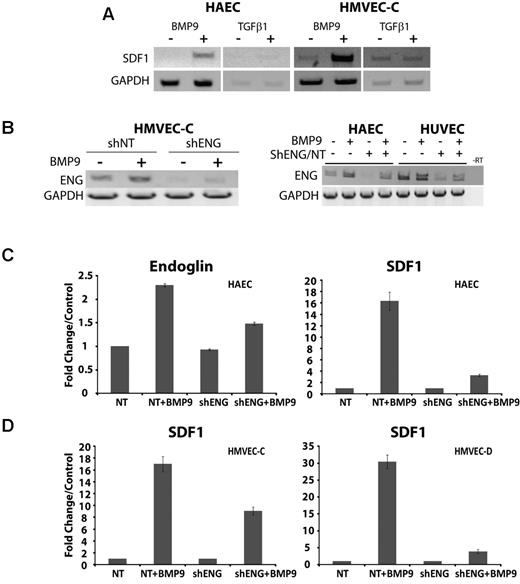
We examined the ligand specificity of SDF1 induction. Treatment of endothelial cells with either TGF-β1 or BMP9 indicated that, of these, only BMP9 promoted SDF1 expression (shown are HAEC and HMVEC-C, Figure 3A). HHT1 results from deficiency of endoglin, which participates in ALK1 signaling in response to BMP9.10 Therefore, we determined the consequences of endoglin knockdown on SDF1 expression and secretion. To do this, lentivirus was used to stably express control or anti-shENG in a variety of endothelial cells.13 RT-PCR data indicated that the shENG lentiviral constructs consistently achieved > 50% knockdown on endoglin mRNA (Figure 3B).
BMP9-dependent induction of SDF1 expression is promoted by endoglin and is dependent on endothelial cell type. (A) The ligand specificity for the up-regulation of SDF1 expression in HAEC and HMVEC-C was analyzed by RT-PCR after treatment with 5 ng/mL BMP9 or 10 ng/mL TGF-β1. (B) RT-PCR of HMVEC-C demonstrated up-regulation of endoglin in response to treatment with BMP9 (5 ng/mL, 24 hours) and is impaired by endoglin suppression. (C) Real-time quantitative RT-PCR of endoglin and SDF1 cDNA induction by BMP9 in HAECs, in the presence of control shNT or shENG suppression. (D) Quantitative RT-PCR of SDF1 cDNA induction by BMP9 in HMVEC-C and HMVEC-D cell culture in the presence of control shNT or shENG suppression. Error bars represent mean ± SE for 3 experiments.
BMP9-dependent induction of SDF1 expression is promoted by endoglin and is dependent on endothelial cell type. (A) The ligand specificity for the up-regulation of SDF1 expression in HAEC and HMVEC-C was analyzed by RT-PCR after treatment with 5 ng/mL BMP9 or 10 ng/mL TGF-β1. (B) RT-PCR of HMVEC-C demonstrated up-regulation of endoglin in response to treatment with BMP9 (5 ng/mL, 24 hours) and is impaired by endoglin suppression. (C) Real-time quantitative RT-PCR of endoglin and SDF1 cDNA induction by BMP9 in HAECs, in the presence of control shNT or shENG suppression. (D) Quantitative RT-PCR of SDF1 cDNA induction by BMP9 in HMVEC-C and HMVEC-D cell culture in the presence of control shNT or shENG suppression. Error bars represent mean ± SE for 3 experiments.
Consistent with RT-PCR data, real-time quantitative RT-PCR analysis indicated that endoglin knockdown approximately halved endoglin mRNA levels (shown for HAEC, Figure 3C) and resulted in about a halving of SDF1 expression in HMVEC-C (Figure 3D). Measurements using quantitative RT-PCR did not show effects of endoglin suppression on basal HAEC endoglin expression seen in Figure 3B. This discrepancy is probably attributable to the intrinsically semiquantitative nature of standard RT-PCR. However, in HAEC and HMVEC-D cells, endoglin knockdown disproportionately impaired SDF1 expression by ∼ 4- to 6-fold (Figure 3C-D). Quantitative variation between endothelial cell types (eg, HMVEC-C vs HMVEC-D and HAEC) may suggest anatomic differences to SDF1 induction by BMP9. However, overall the SDF1 response to BMP9 treatment and endoglin suppression support the view that endoglin promotes the induction of SDF1 by BMP9 in a variety of endothelial cell types.
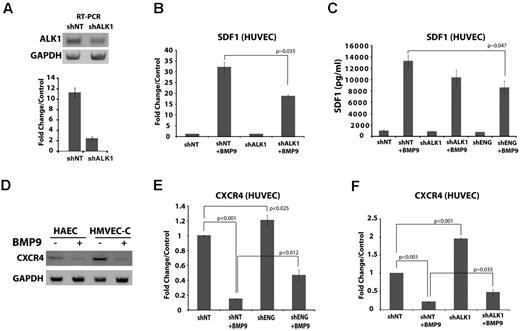
We next examined the involvement of the HHT2 target gene, ALK1, using shRNA. Treatment of HUVECs using ALK1 shRNA inhibited ALK1 mRNA expression as determined by RT-PCR (Figure 4A inset), and quantitative RT-PCR indicated that this suppression was > 4-fold (Figure 4A). ALK1 shRNA significantly suppressed BMP9-dependent induction of SDF1 as measured by quantitative RT-PCR (Figure 4B), consistent with the view that BMP9 acts via ALK1 to induce SDF1. ELISA was used to assess the contributions of ALK1 and endoglin to secretion of SDF1 protein into the conditioned medium from primary endothelial cells. SDF1 secretion was lower in response to BMP9 after ALK1 knockdown, but this trend did not achieve statistical significance (Figure 4C). However, ELISA revealed that shRNA-mediated suppression of endoglin caused significant reduction of SDF1 protein in the endothelial cell conditioned medium (Figure 4C) in response to BMP9. These results support the view that BMP9 promotes functional SDF1 secretion via ALK1 and endoglin signaling, and suggest that endoglin is a critical factor regulating SDF1 secretion in response to BMP9.
BMP9-dependent SDF1 induction is coordinated with CXCR4 repression. (A) RT-PCR (top panel) and quantitative RT-PCR (bottom panel) of HUVEC cDNA after ALK1 shRNA treatment. Bars represent the mean ± SEM; n = 3 experiments. (B) shRNA suppression of ALK1 inhibits the BMP9-dependent induction of SDF1 mRNA. (C) ELISA for CXCL12/SDF1 protein secreted into the conditioned medium of untreated control and BMP9-treated HUVECs, showing the effect of knockdown of endoglin and ALK1. Bars represent the mean ± SE for 3 experiments. (D-F) BMP9 repression of CXCR4 receptor mRNA expression. (D) RT-PCR indicated that the SDF1 chemokine receptor, CXCR4, was repressed by BMP9 in primary endothelial cells; shown are HAECs and HMVEC-C. (E-F) Quantitative RT-PCR indicating that BMP9 (5 ng/mL, 24 hours) repression of CXCR4 mRNA HUVECs requires endoglin and ALK1. BMP9-dependent repression was relieved after shRNA suppression of either endoglin (E; P = .012) or ALK1 (F; P ∼ .033).
BMP9-dependent SDF1 induction is coordinated with CXCR4 repression. (A) RT-PCR (top panel) and quantitative RT-PCR (bottom panel) of HUVEC cDNA after ALK1 shRNA treatment. Bars represent the mean ± SEM; n = 3 experiments. (B) shRNA suppression of ALK1 inhibits the BMP9-dependent induction of SDF1 mRNA. (C) ELISA for CXCL12/SDF1 protein secreted into the conditioned medium of untreated control and BMP9-treated HUVECs, showing the effect of knockdown of endoglin and ALK1. Bars represent the mean ± SE for 3 experiments. (D-F) BMP9 repression of CXCR4 receptor mRNA expression. (D) RT-PCR indicated that the SDF1 chemokine receptor, CXCR4, was repressed by BMP9 in primary endothelial cells; shown are HAECs and HMVEC-C. (E-F) Quantitative RT-PCR indicating that BMP9 (5 ng/mL, 24 hours) repression of CXCR4 mRNA HUVECs requires endoglin and ALK1. BMP9-dependent repression was relieved after shRNA suppression of either endoglin (E; P = .012) or ALK1 (F; P ∼ .033).
Finally, we examined the effect of BMP9 treatment on the levels of the SDF1 receptor, CXCR4. In agreement with HMVEC-D microarray data (supplemental Table 1.2), BMP9 potently reduced CXCR4 mRNA levels in endothelial cells as determined by RT-PCR (shown are HAEC, HMVEC-C, Figure 4D). By quantitative RT-PCR, HUVEC CXCR4 basal expression was significantly elevated and BMP9-dependent repression was significantly relieved in the endoglin knockdown HUVECs (Figure 4E). Similarly, shRNA suppression of ALK1 markedly raised basal CXCR4 expression and significantly relieved the repression of CXCR4 expression by BMP9 (Figure 4F). These data suggest that ALK1 and endoglin potentiate BMP9-mediated repression CXCR4 expression. Moreover, this result suggests that CXCR4 repression could inhibit endothelial cell migration to SDF1. Indeed, in contrast to SVECs (Figure 2E), attempts to demonstrate BMP9-dependent endothelial cell migration that could be inhibited by anti-SDF1 antibody were not statistically significant in primary endothelial cells, consistent with the potent repression of CXCR4 by BMP9 (data not shown). Together, these results suggest that endoglin levels regulate BMP9-dependent endothelial cell–autonomous SDF1 expression and CXCR4 repression, and thus may contribute to a switch from endothelial cell autocrine to a paracrine function.
BMP9 potentiates endothelial cell SDF1 expression in hypoxia
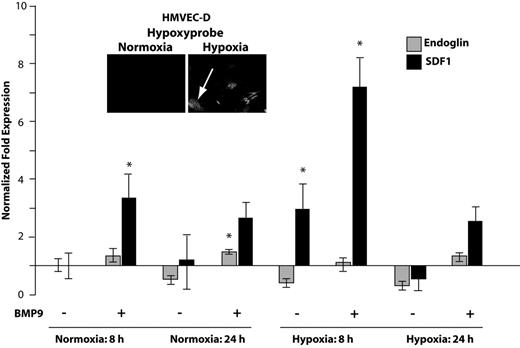
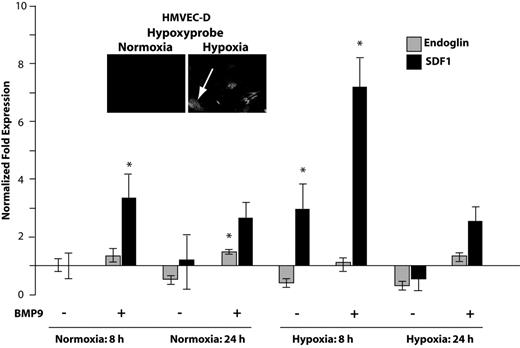
Hypoxia generates elevated levels of hypoxia-inducible factor-α (HIF1α) that in endothelial cells up-regulates SDF1.14 Endoglin and ALK1 are both responsive to hypoxia.30 Therefore, we asked whether hypoxia affects the ability of BMP9 to regulate SDF1 expression. HMVEC-D cells, which are highly hypoxia-responsive (data not shown),31 were grown under normoxic or hypoxic conditions. Hypoxia was achieved by 24 hours as indicated by staining cells with the oxygen-sensitive dye pimonidazole (Figure 5 inset panels). As early as 8 hours of hypoxia, BMP9 potentiated the induction of SDF1 seen in the absence of BMP9. By 24 hours, these trends persisted but were attenuated. The relative contributions of BMP9 and hypoxia to the induction of SDF1 were ∼ 3-fold at 8 hours of hypoxia (Figure 5). These data suggest that circulating BMP9 may potentiate the early phase endothelial cell response to hypoxic induction of SDF1.
BMP9 potentiates early endothelial cell response to hypoxia. HMVEC-D microvascular cells were incubated under standard normoxic (5% CO2, 95% air) or hypoxic conditions achieved by repeated purging with a mixture of 5% CO2, 1% O2, and 94% N2. Cells were treated with 5 ng/mL BMP9 as indicated, and quantitative RT-PCR was used to quantify endoglin and SDF1 mRNA levels. The normalizing control was normoxia in the absence of added BMP9. Error bars represent SEM for duplicate experiments. Establishment of hypoxia was independently judged effective by staining cells with the oxygen-sensing dye pimonidazole (Hypoxyprobe51 ). The dye, which is not detectable under normoxia, becomes fluorescent in at levels of oxygen approaching 1% (arrow, inset panels). *P < .05.
BMP9 potentiates early endothelial cell response to hypoxia. HMVEC-D microvascular cells were incubated under standard normoxic (5% CO2, 95% air) or hypoxic conditions achieved by repeated purging with a mixture of 5% CO2, 1% O2, and 94% N2. Cells were treated with 5 ng/mL BMP9 as indicated, and quantitative RT-PCR was used to quantify endoglin and SDF1 mRNA levels. The normalizing control was normoxia in the absence of added BMP9. Error bars represent SEM for duplicate experiments. Establishment of hypoxia was independently judged effective by staining cells with the oxygen-sensing dye pimonidazole (Hypoxyprobe51 ). The dye, which is not detectable under normoxia, becomes fluorescent in at levels of oxygen approaching 1% (arrow, inset panels). *P < .05.
Endoglin deficiency promotes vascular tortuosity and impairs reperfusion in response to ischemic injury
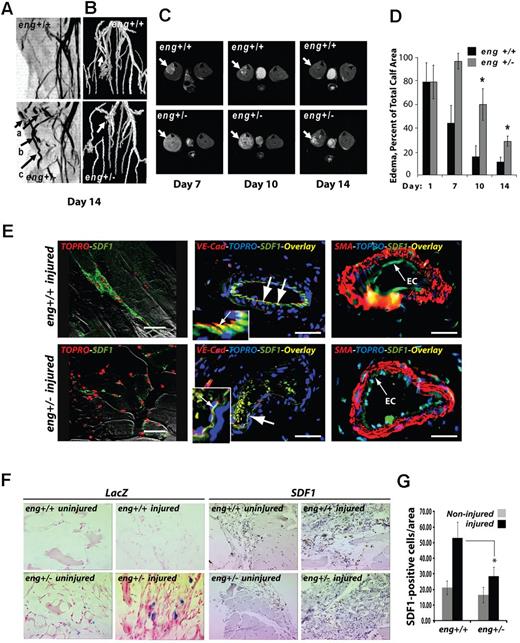
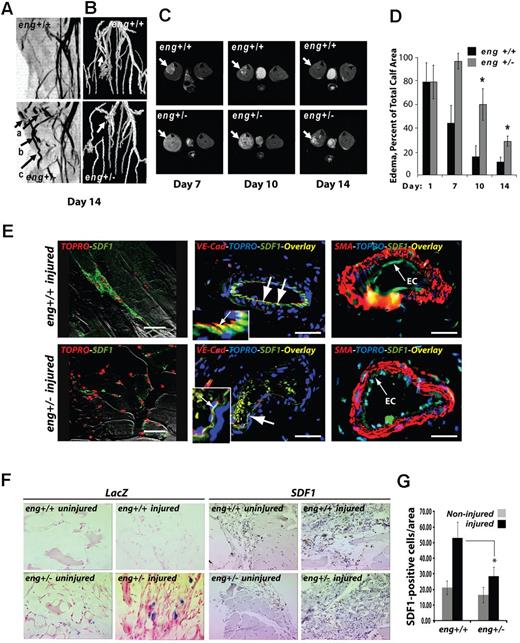
SDF1 is a well-established hypoxia response in the mouse hindlimb ischemia model of ischemic-reperfusion injury32 as well as in acute human limb muscle ischemia.33 Therefore, to determine whether SDF1 expression during hypoxia occurred in an endothelial cell–autonomous fashion in vivo and whether endoglin levels regulated this response, endoglin-deficient and wild-type mice were subjected to right hindlimb femoral artery ligation.34 Injured and control contralateral limbs of FVB strain eng+/− and wild-type eng+/+ mice were monitored at 24 hours, one week, and 2 weeks after surgery by magnetic resonance (MR) imaging. The MR multi-intensity plot (Figure 6A) revealed the presence of vascular malformations in eng+/− vessels that were not characteristic of wild-type eng+/+ mice. These malformations included focal dilatations thickened vessels, and tortuous vessels (Figure 6A arrows). The MR surface projection reconstruction confirmed impaired collateral vessel formation in eng+/− versus wild-type mice in response to injury (Figure 6B arrows). These results suggested that vascular tortuosity was increased in new vessels formed in response to ischemic reperfusion.
Eng+/− HHT-like vascular malformation and perfusion defects in hindlimb after ischemic injury. (A) MR multi-intensity plot reveals the presence of vascular malformations. Eng+/− vascular malformations not seen in wild-type eng+/+ vessels include (arrows): (a) focal dilatations; (b) dilated and thickened vessels; and (c) tortuous vessels. (B) MR surface projection shows collateral vessels formed in response to injury (arrows). (C) A representative axial view of 8-week-old eng+/+ and heterozygous endoglin-targeted (eng+/−) mice (n = 5/group) imaged using MR imaging (RARE8 images). Arrows indicate ischemic muscle with edematous water accumulation; brightness is proportional to degree of residual injury. (D) Percentage of muscle area with edema. *P < .05. n = 4 animals. (E) Anti–VE-cadherin and anti-SDF1 immunofluorescence show colocalization of SDF1 expression in endothelial cells (day 10 after injury; overlay color: yellow, arrows). Smooth muscle cell staining (red, SMA) highlights adjacent endothelial cell location of anti-SDF1 staining (green). SDF1 staining appeared less prominent in eng+/−–injured tissues. EC indicates endothelial cells. Nuclei were stained with TOPRO. (F) Mutant eng+/− and wild-type eng+/+ mice subjected to hindlimb ischemic injury; sections at day 10 were stained using anti-SDF1 antibody (or IgG-matched control) and visualized by horseradish peroxidase–conjugated secondary antibody. LacZ response is the result of the mutated eng allele,52 which is absent in wild-type sections. Strongest SDF1 response is in injured eng+/+ tissues (arrows). (G) SDF1-positive stained cell counts/field for noninjured and injured eng+/+ and eng+/− hindlimb ischemia tissue sections measured as previously described.35 *P < .05. n = 3 fields counted/section.
Eng+/− HHT-like vascular malformation and perfusion defects in hindlimb after ischemic injury. (A) MR multi-intensity plot reveals the presence of vascular malformations. Eng+/− vascular malformations not seen in wild-type eng+/+ vessels include (arrows): (a) focal dilatations; (b) dilated and thickened vessels; and (c) tortuous vessels. (B) MR surface projection shows collateral vessels formed in response to injury (arrows). (C) A representative axial view of 8-week-old eng+/+ and heterozygous endoglin-targeted (eng+/−) mice (n = 5/group) imaged using MR imaging (RARE8 images). Arrows indicate ischemic muscle with edematous water accumulation; brightness is proportional to degree of residual injury. (D) Percentage of muscle area with edema. *P < .05. n = 4 animals. (E) Anti–VE-cadherin and anti-SDF1 immunofluorescence show colocalization of SDF1 expression in endothelial cells (day 10 after injury; overlay color: yellow, arrows). Smooth muscle cell staining (red, SMA) highlights adjacent endothelial cell location of anti-SDF1 staining (green). SDF1 staining appeared less prominent in eng+/−–injured tissues. EC indicates endothelial cells. Nuclei were stained with TOPRO. (F) Mutant eng+/− and wild-type eng+/+ mice subjected to hindlimb ischemic injury; sections at day 10 were stained using anti-SDF1 antibody (or IgG-matched control) and visualized by horseradish peroxidase–conjugated secondary antibody. LacZ response is the result of the mutated eng allele,52 which is absent in wild-type sections. Strongest SDF1 response is in injured eng+/+ tissues (arrows). (G) SDF1-positive stained cell counts/field for noninjured and injured eng+/+ and eng+/− hindlimb ischemia tissue sections measured as previously described.35 *P < .05. n = 3 fields counted/section.
Endoglin deficiency impairs endothelial cell SDF1 expression and leads to enhanced edema after ischemic injury
To further explore how endoglin haploinsufficiency affects the remodeling response after ischemia, we first examined the sites of injury for indications of impaired revascularization. Endoglin heterozygous (eng+/−) and control mice were maintained and experimentation was conducted according to the Public Health Service standards established in the Guidelines for the Care and Use of Experimental Animals, and all studies were approved by the Maine Medical Center Research Institute's Institutional Animal Care and Use, and Institutional Biosafety Committees. MR axial views of the injured hindlimbs in eng+/+ versus eng+/− mice suggested an impaired vascular response with loss of endoglin (Figure 6C). The level of edema and residual injury can be correlated by MR image brightness in the muscle, which reflects water accumulation (Figure 6C arrows). The data showed evidence of diminished perfusion in eng+/− mice, with sustained residual injury at 7, 10, and 14 days after injury, compared with the wild-type mice. Measurement of the difference in percent area involvement, determined as previously described,35 reached significance by day 10 and beyond (Figure 6D). These data suggest that eng deficiency impaired the vascular response to ischemic injury as reflected by tissue edema.
We further examined the eng+/− hindlimb ischemia model to assess the endothelial SDF1 response to ischemic injury. At 2 weeks after injury, the right and left (contralateral uninjured) muscles of the lower limbs, including gastrocnemius and tibialis, were harvested from eng+/+ and the eng+/− mice. Double immunofluorescence of day 10 postinjury vessels in the area of ischemic injury revealed localization of SDF1 expression in wild-type endothelial cells, which is consistent with prior studies showing BMP9 expression in the mouse aortic endothelium.9 Finally, SDF1 expression was reduced or absent in endoglin-deficient eng+/− endothelial cells (Figure 6E). These in vivo data suggest that an endoglin-dependent endothelial cell-autonomous increase of SDF1 expression occurs in response to ischemic injury.
For eng+/− tissues, β-galactosidase is expressed from the eng locus, providing an indicator of endoglin expression.36 Therefore, control uninjured and ligated tissues were tested for β-galactosidase activity as a proxy for endoglin expression. β-galactosidase staining in eng+/− sections confirmed that endoglin is locally up-regulated by day 10 after hindlimb ischemic injury (Figure 6F LacZ panels). Consistent with this observation, no β-galactosidase staining was observed without injury, confirming a role for endoglin in the ischemic injury response.
SDF1 immunostaining of muscle sections near the site of injury were compared between groups (Figure 6F SDF1 panels). Immunostaining suggested that SDF1 was increased in many cell types during the ischemic response.14 This result is consistent with a previous study that showed that, although BMP9 is detected in the endothelium, its signaling operates via SMAD activation to activate nonendothelial cells, supporting the idea that endothelial BMP9 promotes both autocrine and paracrine effects.9
Quantitation of the number of SDF1-positive cells per area in the injury area indicates that endoglin deficiency significantly reduced the levels of SDF1 expression in response to ischemic injury (Figure 6G). These results are consistent with the view that hypoxia induces an endoglin-dependent up-regulation of SDF1, which is mediated by circulating BMP9 and is impaired in the case of endoglin haploinsufficiency in vivo.
Discussion
Angiogenesis is a multistep process that results in newly formed blood vessels from preexisting vessels that occurs in adults commonly as the result of vascular injury and remodeling. Disturbances in this process at sprouting, migration, proliferation, or maturation can lead to aberrant vascular structures, such as that seen in HHT. HHT1 and HHT2 are rare vascular diseases caused by mutations in endoglin and ALK1, respectively. HHT1 and HHT2 are characterized by vascular abnormalities, including cutaneous telangiectasias, epistaxis, and arteriovenous malformations. Recent studies suggest that HHT is not principally a TGF-β signaling-dependent disease37 but results, at least in part, from defects in BMP signaling. This view is supported by studies indicating that BMP9 is a primary ligand for ALK1 and endoglin that promotes vascular quiescence.7,10 Evidence suggests that BMP9 may achieve vascular quiescence by regulating vascular remodeling in the maturation phase of angiogenesis.38 Vascular remodeling is a complex process, and insight into the downstream modulators that mediate BMP9 signaling in endothelial cells is needed.
In this study, we combined cDNA microarray, mass spectrometry, endothelial cell model, and genetic approaches to identify candidate BMP9 target genes. Microarray and quantitative mass spectrometry data strongly implicated BMP9 in the up-regulation of chemokine, adhesion, and extracellular matrix-associated remodeling pathways, consistent with previous work that implicated BMP9 signaling in vessel maturation11 and endothelial cell quiescence.10 Among the most prevalent BMP9 target gene functions were chemokine response/chemotaxis secreted factors, including SDF1 and CXCR4.
It is often problematic to infer protein levels from changes in mRNA expression data alone, and broad unbiased analysis of protein levels is hampered by the availability of immune reagents. Therefore, quantitative mass spectrometry of endothelial cell-conditioned medium was use to address changes in secreted protein expression in the context of in vitro endothelial cell culture models. The stable-expressing SVEC-BMP9 in vitro system supports this approach because it is an endothelial cell model that survives to secrete factors in the absence of confounding protein supplementation. However, this model does not come without its limitations. For example, we were not able to reliably compare the activity of BMP9 expressed by BMP9-SVEC with exogenously added BMP9. Determination of BMP9 activity includes consideration of the level, processing, and secretion to ensure they compare with the range of observed circulating levels. In addition, SVECs did not show up-regulation of endoglin with BMP9 stimulation, as was seen in primary endothelial cells. A related limitation is that the ELISA for SDF1-α does not detect mouse SDF1. Moreover, Western blotting either did not work or was highly variable, mostly because of the shortcomings of the available antibodies, precluding comparisons between the SVEC-BMP and BMP9-treated endothelial cells. However, the up-regulation of SDF1, LTBP2, and ALK1 in both transduced SVEC mass spectrometric and primary endothelial cell microarray data suggest that the SVEC model is informative but underscore the necessity of primary endothelial cell culture to verify data obtained in the mass spectrometry studies of cell lines. Interestingly, mass spectrometric interrogation of conditioned medium obtained from BMP9-expressing SVECs demonstrated that a variety of potential BMP9-dependent secreted angiogenic factors were prominent. In addition to SDF1, identified proteins included biglycan, galectin-3, SPARC, CXCL10, LTBP2, and ECM1 (supplemental Table 2.1). These proteins represent potential novel effectors of BMP9 signaling that suggest roles in myofibroblast proliferation (biglycan), endothelial cell morphogenesis (Galectin-3), inflammatory responses (CXCL10), and cell matrix regulatory responses (ECM1, see supplemental Discussion for observed secreted protein information). Taken together, these data suggest that BMP9 signaling in endothelial cells elicits a qualitative shift in the secretome of endothelial cells that contributes to a rebalancing of proangiogenic, inflammatory, and maturation events during vascular remodeling.
The response of the vasculature to ischemic injury and the reestablishment of homeostasis during vascular remodeling involve the hypoxia-dependent up-regulation of endoglin.30,39 Along with endoglin's up-regulation after ischemic injury, the reperfusion of tissues is initiated by hypoxia-dependent induction of SDF1 in endothelial cells.32 During tissue repair, SDF1 provides an endothelial cell-derived signal that promotes recruitment of bone marrow-derived progenitor cells40 and their differentiation into pericytes.15 These events contribute to endothelial cell quiescence and vessel maturation. Hypoxia-dependent HIF1α-mediated induction of SDF1 expression is well described in several vascular disease settings.32 For example, in a model of burn wound healing, heterozygous HIF1α-deficient mice exhibited diminished SDF1 levels and mounted an impaired burn wound response. This result suggests that SDF1 levels are critical to the healing process. These studies and our present data support a role for ALK1 and endoglin in the regulation of SDF1 in response to BMP9 as an integral part of the vascular wound healing response.
Our data indicate a BMP9-specific role for SDF1 induction as TGF-β1 treatment of endothelial cells did not affect SDF1 expression. This result supports the view that BMP9-dependent regulation of SDF1 is relevant to HHT. DNA sequence analysis of RT-PCR products indicated that the SDF1α isoform was the predominant BMP9-regulated SDF1 isoform. The SDF1 isoforms have been shown to be functionally distinct and SDF1α has been associated with the brain response to ischemia.41 Just as striking as the endoglin-dependent SDF1 induction by BMP9 is the coordinated attenuation of endothelial cell CXCR4 expression. Suppression of endoglin or ALK1 was sufficient to decrease the BMP9 repression of CXCR4 in endothelial cells. Interestingly, the latter result is consistent with studies in zebrafish, which show up-regulation of CXCR4 in ALK1-mutant zebrafish.42 This insight supports the hypothesis that ALK1 is a conserved upstream component of CXCR4 regulation and implicates endoglin and BMP9 in its regulation.
The SDF1/CXCR4 signaling complex has been shown to be up-regulated in response to hypoxic stimuli.33 Our study suggests that the endothelial cell response to BMP9 may be biphasic, with initial repression of CXCR4 and the subsequent up-regulation of SDF1. This view suggests that BMP9 effects a shift from SDF1 autocrine regulation of endothelial cell phenotype, via CXCR4 repression, to a paracrine endothelial cell-autonomous SDF1 signal. Thus, the down-regulation of CXCR4 contributes to SDF1 acting as a chemoattractant for the recruitment of progenitor cells to the site of vascular injury. These data are consistent with a critical role for BMP9 in orchestrating the vessel maturation and remodeling phases of angiogenesis. To our knowledge, this is the first time a signaling mechanism has affected the opposing regulation of SDF1 and CXCR4 in endothelial cells. More experimentation is required to determine the balance of endothelial cell BMP9-dependent autocrine and paracrine functions in ischemia and the process of vessel remodeling and maturation.
Recently, studies in BMP9 signaling have implicated an important role for crosstalk between the BMP and Notch signaling pathways.43 Ricard et al showed that multiple Notch target genes are also BMP9 target genes38 ; BMP9 treatment caused an early and transient up-regulation of the Notch ligand, Dll4, and the Notch effector, Hey1, in endothelial cells followed by a down-regulation at 6 hours. We have repeated and extended the time course to 24 hours and have shown continuous down-regulation of Dll4 after the initial increase in expression (data not shown). Interestingly, it has been shown that Dll4 expression is sufficient to down-regulate CXCR4 in HUVECs without affecting SDF1 expression.44 We hypothesize that the early up-regulation of Dll4 by BMP9 followed by its signaling through the Notch receptors may be the mechanism for the inhibition of CXCR4 in HUVECs in this in vitro analysis. Finally, angiogenic sprouting is a carefully orchestrated event that involves the interplay of tip-and-stalk cells and their interaction with the extracellular matrix. CXCR4 has been identified as a gene enriched in tips cells,45 and Dll4 is well established marker of tip cells.46 Thus, BMP9 may play a role in regulating 2 tip cell markers, suggesting its role in the tip cell/stalk cell identity and vascular quiescence.
Loss of endoglin expression in the mouse is embryonic lethal.36 Despite the presence of an apparently normal endothelium, there is a failure of recruitment and differentiation of vascular smooth muscle cell precursors.47 Restoration of endoglin expression in endoglin-null embryonic endothelial cells rescues the capacity of smooth muscle precursors to invest vessels and differentiate into vascular smooth muscle cells.48 Our data suggest that endoglin expression in endothelial cells promotes smooth muscle precursor cell recruitment and vessel maturation via regulation of the SDF1/CXCR4 signaling axis. Recent data indicating that SDF1 can promote myogenic differentiation49 support the candidacy of the endoglin/BMP9-dependent endothelial cell–secreted SDF1 as a signal for maturation of the vessel. Therefore, we hypothesize that endoglin haploinsufficiency contributes to the HHT phenotype via dysregulation of SDF1 and CXCR4 expression. Interestingly, others have recently shown that mononuclear cells from HHT1 patients have increased expression of CXCR4, providing further evidence that regulation of the SDF1/CXCR4 signal axis is important in HHT.50 The possible reduction in SDF1 and the increase of CXCR4 in endothelial cells suggests not only a lack of vessel of maturation by recruitment of pericytes via paracrine signaling but also the increase of autocrine SDF1/CXCR4 signaling. This increase in autocrine signaling may lead to aberrant sprouting and proliferation that contributes to the suggested hypervascularized state of HHT. Changes in CXCR4 expression may also leave endothelial cells diminished in their ability to establish tip/stalk cell identity, leading to issues in cell migration and organization in angiogenic sprouting. More studies will be required to provide an integrated picture of how the BMP9-dependent secretome may regulate vascular development and vessel maturation and repair and modify the HHT vascular phenotype.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Dartmouth Genomics and Microarray Facility for Affymetrix analyses, the MMCRI Nucleic Acid, Protein Analysis and Cell Imaging Core for mass spectrometry, and Dr A. Terzic, Maine Medical Center Research Institute, for expert assistance.
This work was supported by the National Institutes of Health (grants HL065301 and P30RR030927/P30GM103392; transgenic mouse, confocal microscopy, DNA sequencing, histology, and viral vector cores [R. Friesel, PI]; HL070865, L.L.; HL69182, V.L.; and P20RR181789/P20GM103465, FACS, bioinformatics, and histology cores [D. M. Wojchowski, PI]) and the Maine Medical Center. C.P.H.V. was supported by the National Institutes of Health (grant HL083151) and the Maine Medical Center.
National Institutes of Health
Authorship
Contribution: K.Y. designed and executed experiments, including short hairpin RNA endoglin interference; B.C. performed endothelial cell culture, RNA preparation, virus preparation, and RT-PCR, and initially observed BMP9-SDF regulation and SVEC characterization studies; D.R. performed microarray analyses and selectin and CXCR4 RT-PCR studies; E.T. performed quantitative RT-PCR development and implementation and cell migration studies; C.O. performed RT-PCR and initial hypoxia experiments; I.P. performed MRI studies; L.B. performed endothelial cell BMP9 hypoxia studies; V.L. performed carotid ligation studies; L.L. performed MR and carotid ligation data analysis; and C.P.H.V. performed mass spectrometry and SVEC stable line production.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for D.R. is Imperial College, London, United Kingdom. The current affiliation for C.O. is Central New Mexico Community College, Albuquerque, NM.
Correspondence: Calvin P. H. Vary, Maine Medical Center Research Institute, 81 Research Dr, Scarborough, ME 04074; e-mail: varyc@mmc.org.