Abstract
Sickle cell disease (SCD) is a clinically heterogeneous disease. Patients (pts) may present with severe symptoms in childhood, resulting in early morbidity and mortality, or may be asymptomatic until adulthood. Therapeutic goals include the prevention and treatment of complications across the spectrum of disease. We describe baseline and 12-mo assessments and therapies for pts in an ongoing registry documenting treatment patterns, natural history, and outcomes.
Pts ≥2 years old with HbSS, HbS/β-thalassemia, or HbSC were enrolled from 57 US centers and assessed every 6 mos for up to 3 years. Differences between pediatric (age <18) and adult (age ≥18) pts at 12 mos follow-up are reported. (ClinicalTrials.gov identifier, NCT01220115.)
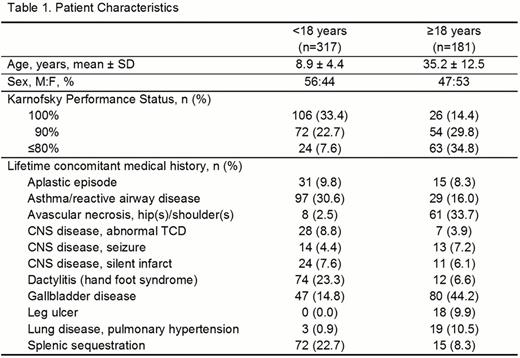
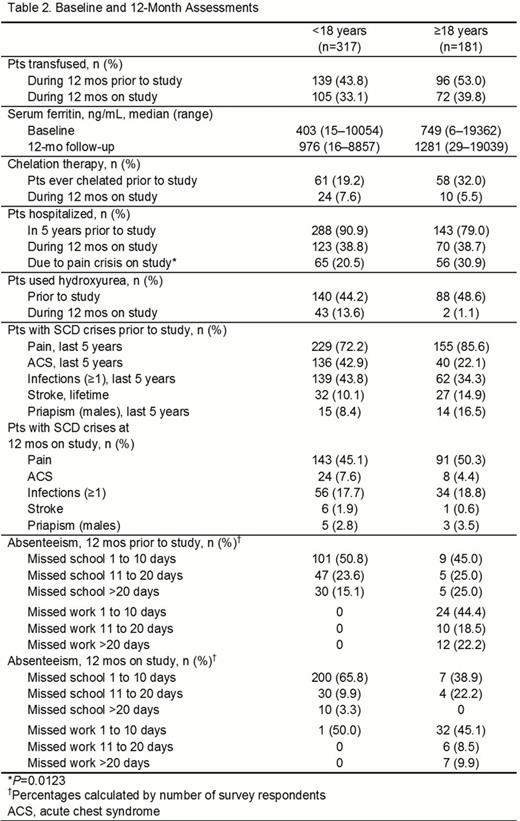
In all, 498 pts completed the baseline visit. Most pts had HbSS disease (age <18, 76.3%; age ≥18, 70.2%). Adult pts had worse performance status, and more frequent avascular necrosis, gallbladder disease, leg ulcers, and pulmonary hypertension at baseline (Table 1). They also had more transfusions, higher serum ferritin, more chelation exposure, more hospitalizations for pain, and potentially more strokes (Table 2). Pediatric pts had higher frequencies of asthma, abnormal transcranial Doppler (TCD) readings, dactylitis, splenic sequestration, acute chest syndrome, infections, hospitalizations at baseline, and more prevalent hydroxyurea use on study. Absenteeism from school (age <18, 1 to 10 d missed prior to study, 50.8%; 1 to 10 d missed on study, 65.8%) and work (age ≥18, 1 to 10 d missed prior to study, 44.4%; 1 to 10 d missed on study, 45.1%) was frequent.
This study showed differences in baseline characteristics and treatment patterns between adult and pediatric pts with SCD. Ongoing follow-up will provide further information on disease patterns, treatment practices, and outcomes, thus increasing our understanding of appropriate SCD management.
Heeney:Novartis: Consultancy, Research Funding; Eli Lilly: Research Funding. Off Label Use: Hydroxurea is indicated to reduce the frequency of painful crises and to reduce the need for blood transfusions in adult patients with sickle cell anemia. It is not approved for use in children. Mueller:Novartis: Research Funding. Adams-Graves:Novartis: Consultancy, Speakers Bureau. Paley:Novartis: Employment. Esposito:Novartis: Employment. Vichinsky:Novartis: Consultancy, Research Funding; ApoPharma: Consultancy, Research Funding; ARUP Research Laboratory: Research Funding.

This icon denotes a clinically relevant abstract
Author notes
Asterisk with author names denotes non-ASH members.