Abstract
In sickle cell disease, patients are predisposed to renal dysfunction and eventual renal failure as they reach adulthood. Many advances have been made within the field of sickle cell anemia, yet to this day sickle cell nephropathy remains an important cause of mortality in adult patients. Previous studies have determined that proteinuria and hematuria are two useful markers of sickle cell nephropathy. Currently, the best marker for detecting early renal dysfunction is proteinuria on urine dipstick due to its ease of use and efficiency. Our goal in this study is to determine the age at which the first signs of renal dysfunction appear.
Pediatric patients with sickle cell disease were selected for a retrospective chart review to determine age of onset for renal abnormalities. The sickle cell pediatric roster was used from the Children's Hospital University of Illinois to study a total of 175 patients within the age range of 0–31 years. Urinalysis was captured at patient's baseline when available and possible risk factors for glomerular dysfunction were studied. Factors such as urine protein and blood on dipstick were recorded and proteinuria was further quantified by using the urine protein to creatinine ratio. Blood on dipstick was further analyzed by red blood cells on microscopic urinalysis. Patients with positive urine for blood on dipstick with <5 RBCs on microscopic UA were marked as patients with hemoglobinuria. Other factors such as sickle cell hemoglobin type, LDH, reticulocyte count, HbF, and hydroxyurea treatment were also recorded to look for correlation with predictors of early renal dysfunction. The Fisher's exact test was used to compute the (two-tailed) probability.
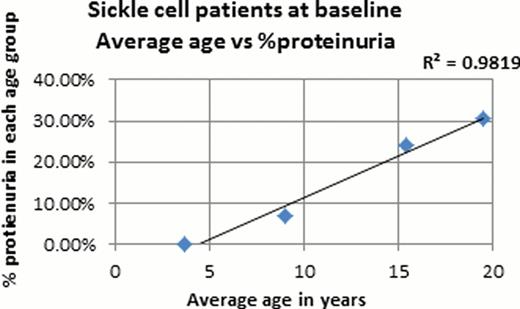
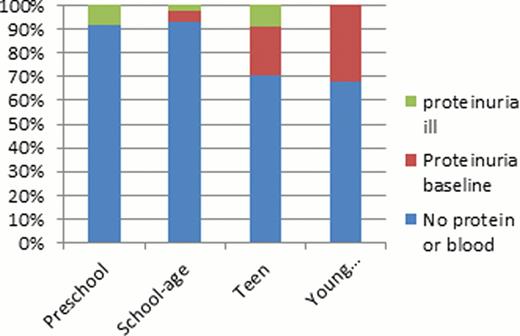
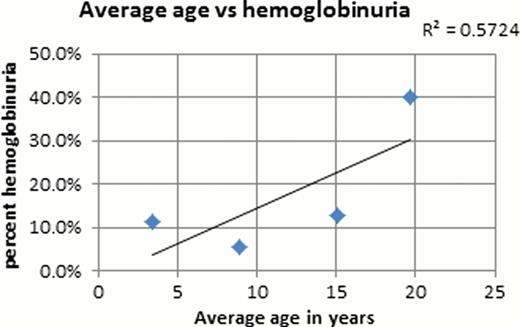
Urinalysis results were available for 141 of the 175 pediatric patients from the sickle cell roster. From the 141 patients that were studied 65% of the patients had urinalysis done at baseline, the other 35% had UA when acutely ill. The mean overall age was 9.9 years and a total of 25 of the 141 (17.7%) patients were observed to have proteinuria on dipstick. In the proteinuria group, 64% of the UA were obtained at baseline and the other 36% were during a sick visit. The majority (66%) of these ‘sick’ patients were febrile under the age of 7. Obtaining a UA during an acute illness could skew our results since pediatric patients who are acutely ill may have transient proteinuria but none at baseline. In our pediatric sickle cell population, about 14% had hemoglobinuria. Analysis of only baseline UA showed that no patients under the age of 5 at baseline had proteinuria and there is a strong correlation between age and proteinuria (R2 = 0.81, p<0.02). Similarly there is a correlation between age and hemoglobinuria (R2 = 0.57). Hemoglobinuria is occasionally observed in 11.4% of children <5 years of age, and at 12.9% in the teenage group. A significant increase in incidence of hemoglobinuria is noted in the young adults (40%). Of the 16 patients with hemoglobinuria only 8 had concurrent proteinuria. A larger sample size is needed to determine whether proteinuria and hemoglobinuria are independent versus correlated markers of early renal dysfunction. Preliminary analyses of baseline UA found no correlations between proteinuria and Hb level, LDH, reticulocytes, serum creatinine, or creatinine clearance.
From this retrospective chart review in this pediatric sickle cell disease population, it can be deduced that proteinuria becomes a concern in sickle patients in the adolescent years while hemoglobinuria appears in late teen to young adult years. It can be concluded that the first clinical signs of renal dysfunction which lead to nephropathy in sickle patients are more frequently seen in adolescent to late teen years and this is likely the marks the beginning of the deterioration of kidney function. Further studies are needed for multivariate analysis of other markers (GFR, Cr, Cr Clearance) of nephropathy and to improve early detection of renal dysfunction by conducting longitudinal studies. Our goal is to improve our current practice by routine screening in sickle patients to preserve renal function and improve the morbidity and mortality related to sickle cell nephropathy in the aging patient.
No relevant conflicts of interest to declare.

This icon denotes a clinically relevant abstract
Author notes
Asterisk with author names denotes non-ASH members.