Abstract
Abstract 1091
In the presence of thrombocytopenia, antithrombotic therapy in patients with thrombosis is a challenge for the managing physicians. Current guidelines are based on anecdotal data and expert opinion. Hereby, we used an in-vitro model with thrombelastography (TEG) to study the interactions of anticoagulants with plasma clotting proteins and varying concentrations of platelets. The objective of this study is to better elucidate the range of platelet concentrations in plasma which will permit clot formation in the presence of anticoagulant.
Fresh human platelet-rich plasma and platelet-poor plasma were obtained from the same donors to produce plasma samples with predefined platelet counts. For each experiment, these samples were incubated with a reaction mixture containing 30 μg/mL corn trypsin inhibitor and one of the following anticoagulants at therapeutic concentrations: heparin (0.3 IU/mL), dalteparin (1.0 IU/mL), fondaparinux (1.25 mg/L), rivaroxaban (150 ng/mL) or dabigatran (180 ng/mL). Clotting was initiated with 10 mM CaCl2 and tissue factor (TF) (Thromborel® S). The amount of tissue factor for each anticoagulant was pre-optimized so that the plasma did not clot in the absence of platelets but the clotting time would return to baseline when platelet count increased to 150 x109/L, corresponding to the expected clinical profile. All parameters for TEG (R,a, MA, TMA) were monitored for 180 min. The area under the curve for each TEG tracing in the first 15 min (AUC15) after clot initiation was estimated as it represents a global measurement of clot strength during its formation. Williams' t-test was used to compare multiple data points with its corresponding baseline control. A p < 0.05 was considered statistically significant.
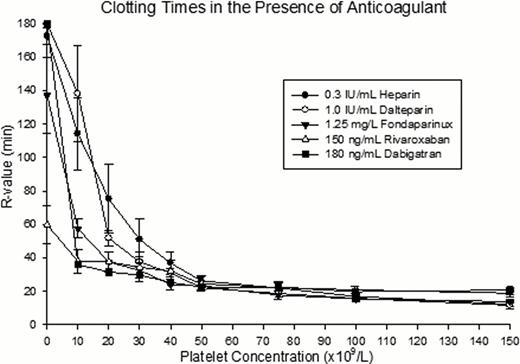
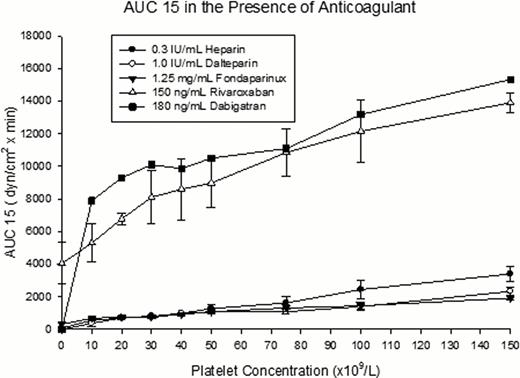
The TF concentration in Thromborel S® was 3140 pM as determined by ELISA. We found that the optimal TF concentrations required for each anticoagulant were 1.2 pM, 0.7 pM, 0.07 pM for heparin, dalteparin, and fondaparinux respectively. No extrinsic tissue factor was required for rivaroxaban and dabigatran. In the presence of an anticoagulant, clot formation was significantly delayed when platelet counts were below 50 x109/L (fig.1). In contrast, when platelet counts were between 50–150 x109/L, there were no significant differences in all TEG parameters. The AUC15 linearly decreased when platelet counts fell below 150 x109/L. In the presence of heparinoids, the overall AUCs are reduced by an average of 6-fold comparing to the controls without anticoagulants (fig.2). In the presence of rivaroxaban or dabigatran the reduction in the overall AUCs was minimal compared to the heparinoids. The slopes of AUC15 against platelet count in the heparinoids were similar, with an average slope of 15. In contrast, the direct factor specific anticoagulants had distinctly different slopes that averaged at 56.
Our findings suggest that, in the presence of therapeutic concentration of an anticoagulant, coagulation is delayed when platelet count is below 50 x109/L and clot formation is globally attenuated with lower platelet counts. Due to the fact that the clotting time is significantly prolonged when platelet counts fall below the threshold of 50 x109/L, we recommend withholding or reducing anticoagulants when patients with thrombocytopenia have platelet counts lower than this level. This data is consistent with the current clinical practice in adult population. Furthermore, since rivaroxaban and dabigatran required no extrinsic TF to initiate clot formation in our model, these new anticoagulants may have a wider safety margins for the treatment of thrombosis in thrombocytopenic patients. Yet, without the availability of specific antidote for these new anticoagulants, their use in patients with high bleeding risk warrants further evaluation.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.