Abstract
Abstract 1169
New oral anticoagulants (NOACs) have been recently approved by the European Medicine Agency (EMA) and the Food and Drug Administration (FDA) for several indications. New oral anticoagulants include anti-IIa agent (dabigatran etexilate) and anti-Xa agents (rivaroxaban, apixaban and edoxaban). NOACs do not require monitoring nor frequent dose adjustment. However, searching for the optimal dose in the individual patient may be useful in some situations. Recent studies have shown that aPTT, HTI and ECT could be used to monitor dabigatran whereas PT and anti-Xa chromogenic assays could be used to monitor anti-Xa agents, while standardizing the time between the last intake of rivaroxaban and the sampling is mandatory. These tests only measure the initiation phase of the coagulation cascade. Thrombin generation assay (TGA) which measures the entire thrombin generation process could be used to better discriminate the inhibitory profile of the NOACs in patients.
To study the impact of dabigatran and rivaroxaban in patients by thrombin generation assay compared to other traditional coagulometric and chromogenic assays.
Five patients under dabigatran and 5 patients under rivaroxaban for atrial fibrillation were included in this study. Blood samples were taken at different intervals: at Ctrough, 2h and 3h after drug administration. The following tests were performed at each timepoint Rivaroxaban:
Prothrombin Time (PT) using the following reagents: Triniclot PT Excel S.. and Innovin.., Thrombin Generation Assay (TGA) using PPP-Reagent and PPP-Reagent High, Biophen Direct Factor-Xa Inhibitor.. (DiXaI). Dabigatran: Activated Partial Thromboplastin Time (aPTT) using CK-Prest.. and Synthasil.., Hemoclot Thrombin Inhibitor.. (HTI) and Thrombin Generation Assay (TGA) using PPP-Reagent and PPP-Reagent Low. All the tests were calibrated by spiking rivaroxaban or dabigatran at increasing concentrations in pooled citrated normal human platelet poor plasma (PPP).
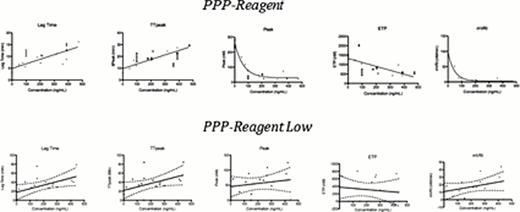
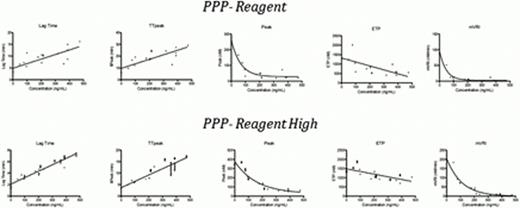
The Peak and mVRI were the most sensitive CAT parameters with a high sensitivity (Peak IC50 was 3ng/mL with PPP-Reagent Low and PPP-Reagent and 14ng/mL with PPP-Reagent High; mVRI IC50 was 1ng/mL with PPP-Reagent Low and PPP-Reagent and 3ng/mL with PPP-Reagent High) and both reagent showed a low variability (CV<1.0%).
The PPP-reagent Low does not allow resolving TGA profiles with different rivaroxaban concentrations. Consequently, PPP-Reagent and PPP-Reagent High were only tested ex vivo.
The low sensitivity of the lag time explains the lack of sensitivity of PT, even with the more sensitive reagent (i.e. Triniclot PT Excel S..).
Dabigatran mainly delayed the initiation phase with a strong dose-dependent increase of lag time and Tmax and a slight dose-dependent decrease of Cmax and ETP.
The concentration in dabigatran needed to double the lag time was 66 ng/mL and 70ng/mL with the PPP-Reagent and the PPP-Reagent Low respectively. The PPP-reagent High is less sensitive in comparison to PPP-Reagent and PPP-Reagent Low.
Similar concentrations of rivarovaban or dabigatran in different patients provided with Biophen DiXaI.. and HTI are associated with differences in TGA profile (Figure 1 & 2 showing the dispersion of the different measurement). Therefore, anti-Xa tests and dilute thrombin time are not the most accurate tests to use to propose cut-off associated with a bleeding or thrombosis risk. This illustrates the interest to have tests able to evaluate the entire thrombin generation process to propose cut-off in one or more parameters associated with a bleeding or thrombosis risk.
Thrombin generation assay could be superior to traditional coagulometric and chromogenic assays to monitor new oral anticoagulants in terms of prediction of bleeding or thrombosis risk.
Impact of dabigatran on the different CAT parameters using PPP-Reagent and PPP-Reagent Low and comparison with the plasma drug concentration
Impact of dabigatran on the different CAT parameters using PPP-Reagent and PPP-Reagent Low and comparison with the plasma drug concentration
Impact of rivaroxaban on the different CAT parameters using the PPP-Reagent and PPP-Reagent High and comparison with the plasma drug concentration
Impact of rivaroxaban on the different CAT parameters using the PPP-Reagent and PPP-Reagent High and comparison with the plasma drug concentration
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.