Abstract
The presence of VRE bacteremia in hematopoietic stem cell transplant recipients portends a worsening clinical course and poor overall survival (Avery et al; BMT, 2005). The impact of VRE bacteremia on outcome in AML patients (pts) receiving induction chemotherapy (IC) has not been established.
We conducted an IRB-approved retrospective study of AML pts who received cytarabine-based IC at Cleveland Clinic between 2000–2008 to determine VRE rates and effect on complete remission (CR) and overall survival (OS). Data on age at AML diagnosis (dx), gender, diabetes, smoking history, history of antecedent hematological disorder, pathologic classification, hematologic parameters at dx and at VRE occurrence, metaphase cytogenetics (per CALGB/Alliance 8461), precedingnon-VRE bacteremias, invasive fungal infection (IFI), time from dx and induction to VRE and number of VRE infections, complete remission (CR) and overall survival (OS) were collected from our AML database.
The association of these factors with VRE bacteremia was assessed using Fisher's exact test, the Cochran-Armitage trend test and Wilcoxon rank sum test. The impact of VRE bacteremia on OS was assessed using a 2:1 matched-pairs analysis based on gender and year of dx (+ 3 years), and factors known to influence outcome: age at dx (+ 5 years), etiology, and cytogenetic risk. The timing of VRE was also accounted for in the matching. Frailty models, which included a term for WBC at dx, were use to assess the impact of VRE bacteremia while taking into account both the impact of WBC and the paired nature of the data.
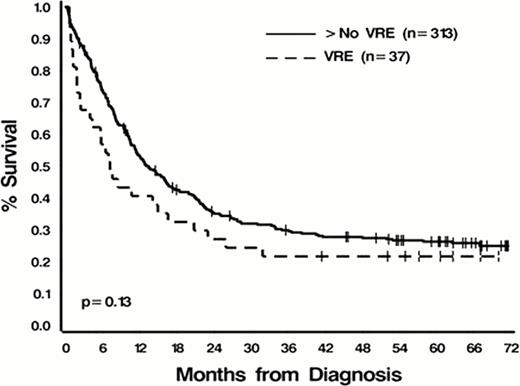
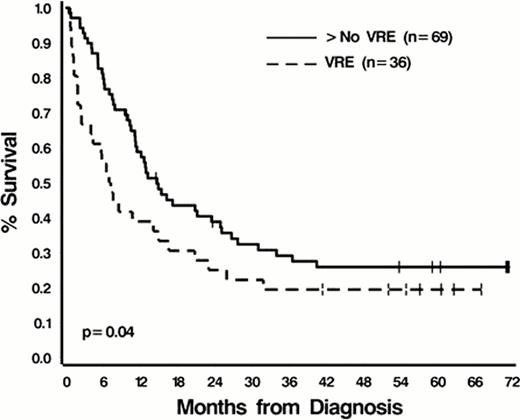
Of 350 pts evaluable for analysis, the median age at dx was 57 years (range 19–88); 192 (55%) were male; 114 (33%) had secondary AML; median baseline WBC was 10.4K/mL (range, 0.48–550); cytogenetic risk distribution was favorable (14%), intermediate (54%), and unfavorable (32%); 45% were current or former smokers; 17% had a history of diabetes; and 7% had IFI. With the exception of IFI (16% in pts with VRE versus 6% in non-VRE pts, p=.04) there were no significant differences in these factors between the two groups (all p>.08). Of 37 pts (9.8%) who had documented VRE bacteremias during IC, the median interval from the start of IC to VRE infection was 17 days (range, 9–58). The majority (89%) of VRE bacteremias occurred in pts receiving IC between 2005 and 2008 (infection rate of 22%, 33/152) while only 4 infections occurred in 198 pts treated between 2000 and 2004 (infection rate of 2%). One plausible explanation for this epidemiologic shift could be the frequent use of fluoroquinolone prohylaxis to prevent neutropenic fever, which became routine in 2004. The overall CR rate for the cohort was 73%; 70% in VRE pts and 73% in non-VRE pts (p=0.7). Median follow-up was 72.2 months (range 1.1–145.4). Unadjusted median OS for the entire cohort was 12.8 months (95% C.I. 10.6–15.9); 7.1 months (95% C.I. 3.9–16.5) for VRE pts and 13.1 months (95% C.I. 11.2–16.3) for non-VRE pts (p=0.13, Figure 1A). Using the 2:1 matching to adjust for the impact of age, etiology, and cytogenetics, VRE pts had a significantly inferior OS compared to non-VRE pts even after adjusting for WBC at dx (p=0.04 and.80, respectively, Figure 1B). Mutivariableanalyses confirmed this association.
In conclusion, VRE bacteremia in pts undergoing IC for AML is an independent risk factor for worse OS. The routine use of fluoroquinoloneprophylaxis is likely contributing to the increased prevalence of VRE bacteremia. Consideration should therefore be given to escalating VRE appropriate antibiotic care in these patients sooner and in the post-remission setting.
Survival from Diagnosis
A. All patients
B. VRE cases and matched controls
Saunthararajah:Cleveland Clinic Innovation: patent application for oral THU-decitabine., patent application for oral THU-decitabine. Patents & Royalties. Advani:Genzyme: Honoraria, Research Funding; Immunomedics: Research Funding. Maciejewski:NIH: Research Funding; Aplastic Anemia&MDS International Foundation: Research Funding.

This icon denotes a clinically relevant abstract
Author notes
Asterisk with author names denotes non-ASH members.