Abstract
Abstract 1638
Hodgkin lymphoma (HL) represents the most common subtype of malignant lymphoma in young people in the Western world. Most of the patients can be cured with modern treatment strategies. However, about 20% of patients still die due to relapse or progressive disease. Recently, elevated numbers of tumor-associated macrophages in diagnostic pretreatment and relapse biopsies have been associated with poor long-term outcomes. Furthermore, high expression of CSF1R on the malignant Hodgkin Reed Sternberg cells has been linked to shorter progression-free survival, providing the rationale to test PLX3397, a highly selective inhibitor of CSF1R (also known as Fms) and Kit receptor tyrosine kinases, in HL patients.
Patients with relapsed or refractory HL were enrolled in this single-agent multicenter trial. All patients had progressed after or were ineligible for autologous stem cell transplantation, had radiographically measurable disease, and had discontinued all previous HL therapy. Patients were treated with oral PLX3397 at a dose of 900 mg/day continuously until occurrence of unacceptable toxicity or disease progression. The primary endpoint was the objective response rate according to the 2007 consensus criteria.
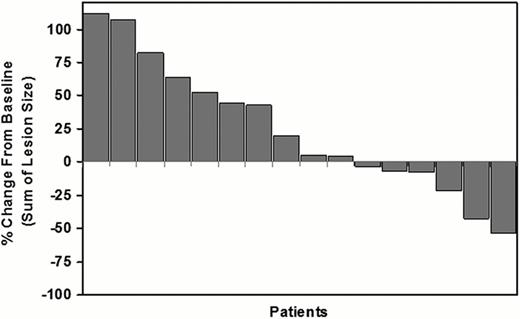
A total of 20 patients were enrolled (9 male, 11 female, median age 36 years). Six patients had tumor reduction ranging between 3% and 54% including one partial remission (overall response rate of 5%) (Figure). The median duration of progression-free survival was 56 days. The most common drug-related adverse effects were asthenia, fatigue, hair depigmentation, anemia, leukopenia, and increased LDH, all of which were grade 1 or 2 except for one grade 3 neutropenia. CD14dim/16+ labeled peripheral blood monocytes were markedly reduced in all patients at 4 weeks vs. Baseline (median=83% reduction, range of 44%-96% reduction), confirming inhibition of CSF1R signaling. Increases were observed for adiponectin in 82% of patients (median=2.6-fold, range 1.2 to 12-fold) and for CSF-1 in 94% of patients (median=4.0-fold, range 1.4 to 16.5-fold), also consistent with CSF1R inhibition. Archival formalin-fixed paraffin-embedded lymphoma tissues of sufficient quality were available for 12 patients. Biopsy material was obtained from either initial diagnosis or after relapse. CD68+ and CD163+ macrophages were highly elevated (>25% cellularity) in 42% and 58% of patients, respectively. The number of tumor-associated macrophages was not significantly associated with a reduction in lesion size after PLX3397 treatment in this small study cohort.
In this phase 2 clinical trial, PLX3397 at a dose of 900 mg/day showed limited activity in this heavily pretreated patient cohort. However, target inhibition of both Fms and Kit was clearly demonstrated. Although the efficacy of single agent PLX3397 in this study population was modest, the manageable safety profile and evidence of target inhibition may warrant further testing in combination therapy trials.
Younes:Seattle Genetics, Inc.: Consultancy, Research Funding; Millennium: Honoraria; Novartis: Honoraria, Research Funding; Celgene: Honoraria; Affimed: Research Funding; Gilead: Research Funding; Johnson & Johnson: Research Funding. Gascoyne:Plexxikon Inc.: Consultancy, Research Funding. West:Plexxikon Inc.: Employment. Nolop:Plexxikon Inc.: Employment. Steidl:Plexxikon Inc.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.