Abstract
Myelodysplastic syndrome patients with renal impairment (RI) were excluded for the approval trials of azacitidine, thus the optimal use of the latter in such patients is currently undefined. Only one single center, retrospective study addressed the efficacy and toxicity of azacytidine in patients with RI (Batty GN et al), but no comparative analysis between patients with or without RI has been performed yet. We retrospectively analyzed 42 MDS patients treated with azacitidine in a non-trial clinical setting. Patients were classified according to WHO as RAEB-II (n=18), AML/MDS (n=5), CMML-II (n=8), MDS/MPN (n=4), RAEB-I (n=3) and RCMD (n=3). All patients had normal hepatic function and ECOG performance status of 0–2. Glomerular filtration rate (GFR) was estimated using the MRDM or the Cockroft-Gault formulas depending on patient's weight. Response to therapy and toxicity were evaluated using the IWG 2006 response criteria for MDS and CTCAE 3.0, respectively. In all patients azacitidine was started at 75mg/m2 SC for 7 days on 28-day cycles. Significance of differences was assessed by Kruskal-Wallis, Chi square or Fisher Exact tests as appropriate. Kaplan-Meier method with log-rank test was used for survival analysis. Overall survival (OS) was defined as the time from first azacitidine administration to death from any cause and Event-free survival (EFS) as the time from first azacitidine administration to leukemic transformation or death.
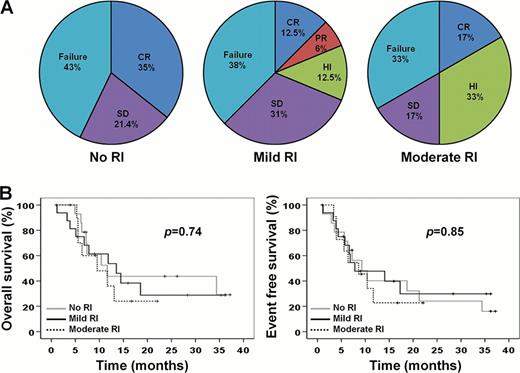
Table I lists patients' characteristics. Based on GFR levels 3 groups were defined: no RI (n=14), mild RI (n=16) and moderate RI (n=12). Median follow up and number of completed cycles for all patients were 9.5 (1–37.2) months and 5.5(1–29), respectively. No differences in response rates were observed among the 3 groups (Fig 1A), though hematological improvement was more frequent in patients with moderate RI (p=0.052). The median OS and EFS were similar among the no RI (20.2 and 15.5 months for OS and EFS, respectively), mild RI (17.1 and 16.3 months) and moderate RI (11.7 and 10.4 months) groups (Fig 1B). Also, patients in all groups experienced comparable rates of adverse events (Table II). No significant fluctuations in GFR were observed both during the first and the subsequent cycles of azacitidine, though patients with mild RI and two no RI patients displayed a transient decline in GFR on day 7 of the first cycle, which recovered afterwards without requiring dose and/or schedule adjustments (Fig 2).
Our results provide the first evidence that azacitidine is effective and well-tolerated in patients with mild and moderate RI and, if confirmed by prospective randomized studies, advocate that such patients can be managed in an analogous fashion to patients with normal renal function.
Patients' characteristics
| Parameter . | No RI (n=14) . | Mild RI (n=16) . | Moderate RI (n=12) . | p-value . |
|---|---|---|---|---|
| Age (median) | 70.3 (52–79) | 68.8 (59–79) | 77 (65–81) | 0.006 |
| Sex (Male/Female) | 10/4 | 13/3 | 8/4 | 0.665 |
| Median GFR (ml/min /1.73m2) | 93.5 (87–140) | 66.5 (57–76) | 40.5 (33–48) | >0.001 |
| Karyotype risk (Good/Intermediate/Poor) | 7/5/2 | 5/2/9 | 4/6/2 | 0.052 |
| IPSS risk group (Int-2/High) | 9/4 | 7/7 | 7/3 | 0.492 |
| WPSS risk group (High/Very high) | 7/7 | 7/7 | 7/3 | 0.6 |
| ANC(x 109/L) | 0.7 (0.08–13.3) | 1.99 (0.05–15.5) | 3.75 (0.74–6,3) | 0.115 |
| Hemoglobin (g/dl) | 8.35 (6.1–10.1) | 9.1 (6,8–11,5) | 9.35 (6.2–12.8) | 0.321 |
| Platelets (x 109/L) | 139.5 (11–311) | 64 (12–150) | 51.5 (15–160) | 0.178 |
| Heavily transfused (Yes/No) | 9/5 | 9/7 | 9/3 | 0.592 |
| Median number of cycles | 5.5 (1–29) | 5.5 (1–24) | 5 (1–21) | 0.48 |
| Median follow up time (months) | 8.9 (4.9–37,2) | 10.4 (1.2–35.3) | 7.8 (1–22) | 0.4 |
| Parameter . | No RI (n=14) . | Mild RI (n=16) . | Moderate RI (n=12) . | p-value . |
|---|---|---|---|---|
| Age (median) | 70.3 (52–79) | 68.8 (59–79) | 77 (65–81) | 0.006 |
| Sex (Male/Female) | 10/4 | 13/3 | 8/4 | 0.665 |
| Median GFR (ml/min /1.73m2) | 93.5 (87–140) | 66.5 (57–76) | 40.5 (33–48) | >0.001 |
| Karyotype risk (Good/Intermediate/Poor) | 7/5/2 | 5/2/9 | 4/6/2 | 0.052 |
| IPSS risk group (Int-2/High) | 9/4 | 7/7 | 7/3 | 0.492 |
| WPSS risk group (High/Very high) | 7/7 | 7/7 | 7/3 | 0.6 |
| ANC(x 109/L) | 0.7 (0.08–13.3) | 1.99 (0.05–15.5) | 3.75 (0.74–6,3) | 0.115 |
| Hemoglobin (g/dl) | 8.35 (6.1–10.1) | 9.1 (6,8–11,5) | 9.35 (6.2–12.8) | 0.321 |
| Platelets (x 109/L) | 139.5 (11–311) | 64 (12–150) | 51.5 (15–160) | 0.178 |
| Heavily transfused (Yes/No) | 9/5 | 9/7 | 9/3 | 0.592 |
| Median number of cycles | 5.5 (1–29) | 5.5 (1–24) | 5 (1–21) | 0.48 |
| Median follow up time (months) | 8.9 (4.9–37,2) | 10.4 (1.2–35.3) | 7.8 (1–22) | 0.4 |
Toxicity and adverse events
| Parameter . | No RI (n=14) . | Mild RI (n=16) . | Moderate RI (n=12) . | p-value . |
|---|---|---|---|---|
| Neutropenia (grade 3/4) | 8 (57%) | 10 (62.5%) | 6 (50%) | 0.8 |
| Thrombocytopenia (grade 3/4) | 7 (50%) | 12 (75%) | 9 (75%) | 0.269 |
| Infections (grade 1/2) | 3 (21.5%) | 4 (25%) | 3 (25%) | 0.269 |
| Infections (grade 3/4) | 5 (36%) | 9 (56%) | 7 (58%) | 0.535 |
| Bleeding (grade 1/2) | 1 (7%) | 0 (0%) | 1 (8%) | 0.513 |
| Bleeding (grade 3/4) | 3 (21.5%) | 7 (43%) | 7 (58%) | 0.152 |
| Dose adjustments | 3 (21.5%) | 5 (31%) | 4 (33%) | 0.832 |
| Discontinuation of treatment due to toxicity | 4 (28.5%) | 4 (25%) | 7 (58%) | 0.151 |
| Hospitalization (times) | 6 (0–16) | 9 (1–15) | 5.5 (1–10) | 0.141 |
| Hospitalization (days) | 16.5 (0–34) | 20 (10–43) | 16 (4–34) | 0.427 |
| Parameter . | No RI (n=14) . | Mild RI (n=16) . | Moderate RI (n=12) . | p-value . |
|---|---|---|---|---|
| Neutropenia (grade 3/4) | 8 (57%) | 10 (62.5%) | 6 (50%) | 0.8 |
| Thrombocytopenia (grade 3/4) | 7 (50%) | 12 (75%) | 9 (75%) | 0.269 |
| Infections (grade 1/2) | 3 (21.5%) | 4 (25%) | 3 (25%) | 0.269 |
| Infections (grade 3/4) | 5 (36%) | 9 (56%) | 7 (58%) | 0.535 |
| Bleeding (grade 1/2) | 1 (7%) | 0 (0%) | 1 (8%) | 0.513 |
| Bleeding (grade 3/4) | 3 (21.5%) | 7 (43%) | 7 (58%) | 0.152 |
| Dose adjustments | 3 (21.5%) | 5 (31%) | 4 (33%) | 0.832 |
| Discontinuation of treatment due to toxicity | 4 (28.5%) | 4 (25%) | 7 (58%) | 0.151 |
| Hospitalization (times) | 6 (0–16) | 9 (1–15) | 5.5 (1–10) | 0.141 |
| Hospitalization (days) | 16.5 (0–34) | 20 (10–43) | 16 (4–34) | 0.427 |
Response and outcomes
Kinetics of GFR in patients with moderate (A), mild (B) and no (C) RI.
Kinetics of GFR in patients with moderate (A), mild (B) and no (C) RI.
Kotsianidis:Genesis-Pharma: Honoraria, Research Funding.

This icon denotes a clinically relevant abstract
Author notes
Asterisk with author names denotes non-ASH members.